COVID-19 was declared pandemic by March 2020. Clinical, analytical, and radiological findings have been reported. Detailed different evolution of patients of the same local outbreak has been scarcely reported. We report 6 selected cases of such an evolution.
Clinical casesThe clinical, radiological, analytical evolution of 6 patients is reported. Patients were selected as it were epidemiological close contacts, and showed particular different clinical evolution.
ResultsThe clinical course at the start of infection (first week) was similar among patients. In relationship with clinical evolution, middle to severe course were related with inflammation markers levels evolution (D-dimer, IL-6, ferritin, lymphocytes count, etc.). Specially lung alterations were observed, but neurological/neuropsychiatric findings are still common. In evolution, 2 patients showed middle symptoms, but the 2 most severely affected died.
ConclusionsIt remains to be elucidated the different evolutive pathways and outcomes of COVD-19. In our 6 patients of the same local outbreak, clinical, laboratory and radiological features were different. We discuss some aspects of the pathophysiology of the disease, other than the widely described of the respiratory system.
La COVID-19 fue declarada pandemia en marzo del 2020. Han sido comunicados los hallazgos clínicos, analíticos y radiológicos. Sin embargo, la diversa evolución de pacientes del mismo brote local lo ha sido escasamente. Comunicamos en este trabajo 6 casos seleccionados de tal evolución.
Casos clínicosSe expone la evolución clínica, radiológica y analítica de 6 pacientes. Estos fueron seleccionados ya que fueron epidemiológicamente contactos estrechos y mostraron una evolución clínica particularmente diferente.
ResultadosEl curso clínico al inicio de la infección (primera semana) fue similar entre los pacientes. En relación con la evolución clínica, un curso moderado a severo se relacionó evolutivamente con marcadores elevados de inflamación (dímero D, IL-6, ferritina, linfopenia, etc.). Fueron observadas alteraciones pulmonares típicas, pero fueron comunes también hallazgos neurológicos y neuropsiquiátricos. En la evolución 2 pacientes mostraron síntomas moderados, pero los 2 más gravemente afectados murieron.
ConclusionesEstá por elucidar las diferentes vías evolutivas y resultados finales de los pacientes con COVID-19. En nuestros 6 pacientes del mismo brote local, las características clínicas, de laboratorio y radiológicas fueron diferentes. Discutimos aspectos de la fisiopatología de la enfermedad distintos de los ampliamente descritos del sistema respiratorio.
The World Health Organization declared COVID-19 to be a pandemic on March 11, 2020. Spain continues to be one of the most highly affected countries in the world in terms of total cases, total cases per million inhabitants, deaths and excess deaths. March was the month with the greatest increase in COVID-19 cases, with Intensive Care Units (ICU) and hospital beds at the limit of their capacity. In Spain, as in other countries, patients presented a wide range of clinical symptoms, 14%–30% patients required ICU, and the mortality rate was around 3%–10%1.
In this series of selected cases, we have attempted to highlight the different aspects of the disease in close contact cases, and emphasize the different evolution of these patients and the widely described respiratory deterioration.
MethodsWe present a case series of COVID-19 patients selected from the same local outbreak. The local Ethics Committee waived the need for informed consent.
In all patients, symptoms onset around the third week of March 2020. COVID-19 was clinically suspected and diagnosed by reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2. The demographic and clinical characteristics of the patients, the chronology of the diagnostic process, and the outcomes obtained are shown in Table 1.
Demographic characteristics, symptoms, results of RT-PCR for SARS-CoV-2, and outcome.
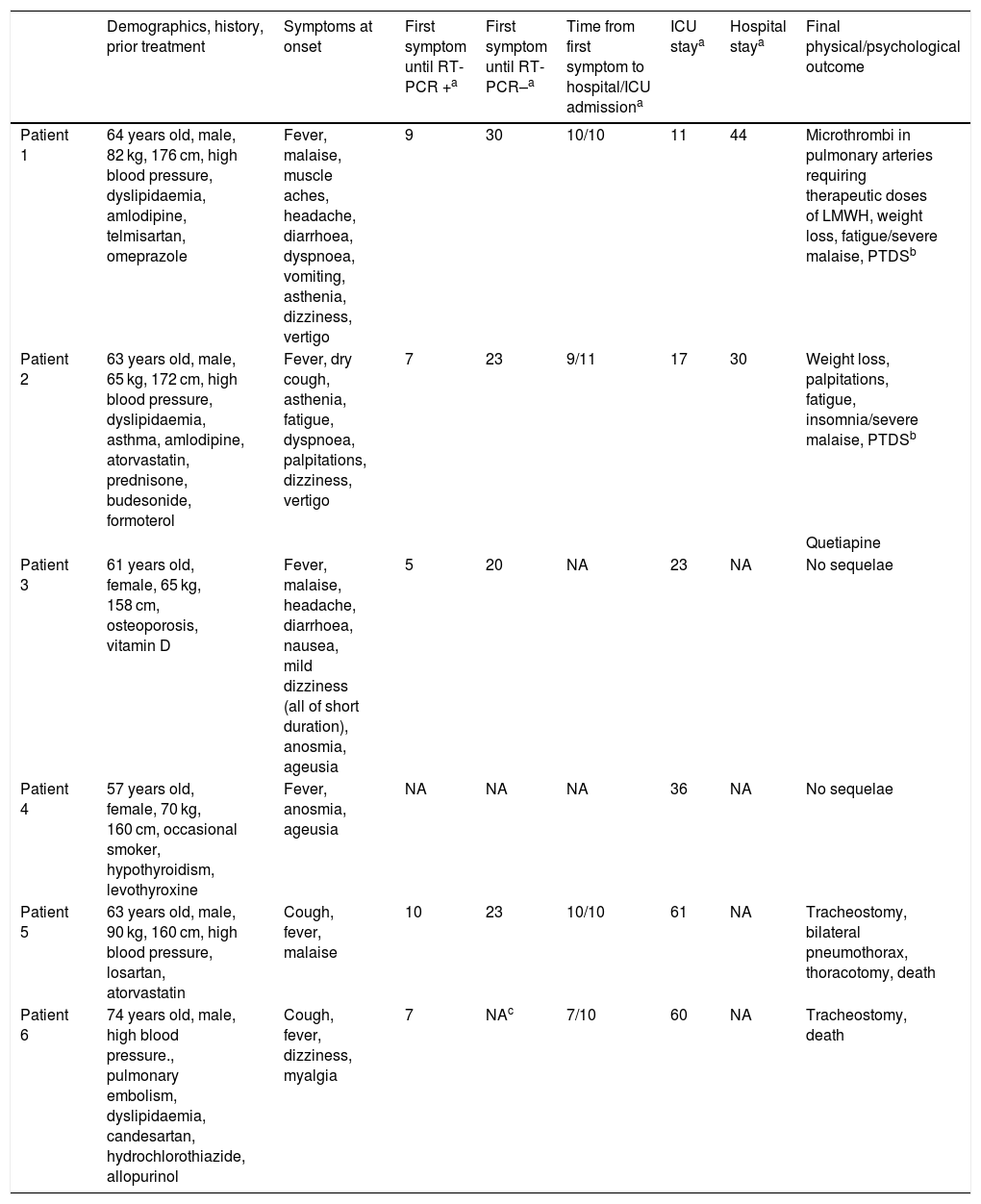
| Demographics, history, prior treatment | Symptoms at onset | First symptom until RT-PCR +a | First symptom until RT-PCR–a | Time from first symptom to hospital/ICU admissiona | ICU staya | Hospital staya | Final physical/psychological outcome | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 64 years old, male, 82 kg, 176 cm, high blood pressure, dyslipidaemia, amlodipine, telmisartan, omeprazole | Fever, malaise, muscle aches, headache, diarrhoea, dyspnoea, vomiting, asthenia, dizziness, vertigo | 9 | 30 | 10/10 | 11 | 44 | Microthrombi in pulmonary arteries requiring therapeutic doses of LMWH, weight loss, fatigue/severe malaise, PTDSb |
| Patient 2 | 63 years old, male, 65 kg, 172 cm, high blood pressure, dyslipidaemia, asthma, amlodipine, atorvastatin, prednisone, budesonide, formoterol | Fever, dry cough, asthenia, fatigue, dyspnoea, palpitations, dizziness, vertigo | 7 | 23 | 9/11 | 17 | 30 | Weight loss, palpitations, fatigue, insomnia/severe malaise, PTDSb |
| Quetiapine | ||||||||
| Patient 3 | 61 years old, female, 65 kg, 158 cm, osteoporosis, vitamin D | Fever, malaise, headache, diarrhoea, nausea, mild dizziness (all of short duration), anosmia, ageusia | 5 | 20 | NA | 23 | NA | No sequelae |
| Patient 4 | 57 years old, female, 70 kg, 160 cm, occasional smoker, hypothyroidism, levothyroxine | Fever, anosmia, ageusia | NA | NA | NA | 36 | NA | No sequelae |
| Patient 5 | 63 years old, male, 90 kg, 160 cm, high blood pressure, losartan, atorvastatin | Cough, fever, malaise | 10 | 23 | 10/10 | 61 | NA | Tracheostomy, bilateral pneumothorax, thoracotomy, death |
| Patient 6 | 74 years old, male, high blood pressure., pulmonary embolism, dyslipidaemia, candesartan, hydrochlorothiazide, allopurinol | Cough, fever, dizziness, myalgia | 7 | NAc | 7/10 | 60 | NA | Tracheostomy, death |
Patients 1 and 3 and patients 2 and 4 were couples, patients 5 and 6 died in the ICU.
LMWH: low molecular weight heparin; NA: not applicable; PTDS: post-traumatic distress syndrome; RT-PCR: reverse transcriptase-polymerase chain reaction.
Patients 1 and 3, and 2 and 4, respectively, were couples. Patient 6 was a close friend of patient 1, with whom he was in contact in the days prior to the onset of symptoms. The clinical course and the findings of patient 5 are included due to their particular severity and the fact that he attended a party with several other people, some of whom had mild or moderate forms of COVID-19.
ResultsTable 2 shows the evolutionary characteristics of the 4 patients admitted to the ICU. Table 3 shows some of the procedures performed and the clinical course in the ICU, and Table 4 shows a short description of the diagnostic imaging procedures performed (which are detailed in Figs. 1–7).
Laboratory data from the 4 patients admitted to the ICU. Patients 1 and 2 survived, patients 5 and 6 died in the ICU.
| Patient 1 | Patient 2 | Patient 5 | Patient 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haemoglobin (g/dl) | 12.9 | 9.7 | <*a | 15 | 12.2 | = | 14.3 | 6.5 | < | 12.6 | 7.6 | < |
| Leukocytes (×109/l) | 2.3 | 20.4 | > | 6.6 | 10.2 | > | 6.2 | 23.5 | > | 9.8 | 14.1 | = |
| Neutrophils (×109/l) | 1.9 | 18 | > < | 4.8 | 1.8 | > | 4.3 | 10.5 | > | 9.2 | 13 | = |
| Lymphocytes (×*109/l) | 0.29 | 0.52 | < > | 1.3 | 0.2 | < | 1.4 | 0.2 | < | 1.4 | 0.1 | < |
| Platelets (×109/l) | 128 | 84 | = | 129 | 147 | = | 212 | 67 | = | 209 | 77 | <a |
| Quick Index (%) | – | 81 | = | 93 | 65 | = | 73 | 69 | = | 90 | 55 | = |
| INR | – | 1.15 | = | 1.06 | – | = | 1.22 | 1.23 | = | 1 | 1.46 | = |
| Fibrinogen (mg/dl) | – | 354 | = | 700 | 147 | = | 568 | 734 | > | 400 | 93 | < > |
| D-dimer (ng/ml) | 643 | 15,331 | > | 284 | 912 | > | 200 | 8548 | > | 258 | 6100 | > |
| Glucose (mg/dl) | 117 | 117 | = | 117 | 140 | = | 186 | 325 | > | 148 | 270 | > |
| NA (mEq/l) | 136 | 145 | = | 130 | 140 | = | 136 | 149 | = | 140 | 162 | > |
| K (mEq/l) | 3.7 | 4 | = | 4.1 | 3.8 | = | 3.3 | 3.1 | < | 3.9 | 5.1 | = |
| Bilirubin (mg/dl) | – | 0.67 | = | 0.49 | 1 | = | 0.63 | 1.11 | = | 0.59 | 0.98 | = |
| AST (I/U) | 32 | 28 | = | 22 | 150 | = | 41 | 2587 | >b | 82 | 111 | >a |
| ALT (I/U) | 99 | 160 | >a | – | 81 | = | 39 | 2679 | >b | 100 | 100 | > < |
| GGT (I/U) | – | – | – | – | – | – | 231 | 438 | > | 67 | 300 | > |
| Urea (mg/dl) | 46 | 66 | = | 29 | 86 | = | 16 | 58 | >a | 119 | 164 | = |
| Creatinine (mg/dl) | 1.22 | 0.86 | = | 0.94 | 0.7 | = | 0.71 | 1.67 | >a | 1.3 | 1.37 | = |
| Ferritin (μg/l) | 675 | 207 | – | 721 | 683 | > | 200 | 16,775 | > | 394 | 2000 | > |
| LDH (I/U) | 584 | 505 | > | 532 | 618 | >a | 488 | 1135 | > | 737 | 208 | > |
| Albumin (g/dl) | 3.6 | 3 | <a | – | 3 | <a | 4.2 | 2 | < | 2.8 | 2 | < |
| Lactate (mEq/l) | 1 | 1.1 | = | 0.8 | 0.7 | = | 0.8 | 2 | = | 2 | 2.8 | = |
| Procalcitonin (ng/ml) | 0.16 | 0.11 | = | 0.54 | 0.51 | = | 0 | > | >b | 0.33 | 1.77 | >b |
| HS troponin I (mg/dl) | 6.6 | 9.78 | > | 7 | 60 | >a | 20 | 1251 | = | 12 | 121 | > |
| C-reactive protein (mg/l) | 2.1 | 5.1 | = | 7.4 | 1.7 | >a | 9 | 45.2 | = | 28.3 | 38 | > |
| Interleukin 6 (ng/ml) | 9.8 | 20.8 | > | – | – | – | – | 9c | – | – | 7c | – |
Patients 3 and 4 were not admitted to the hospital and no blood tests or radiology were performed.
The data shown are at admission to the hospital, worst result (nadir) and general trend (if the result was maintained for more than 5 consecutive days). INR: international normalized ratio; Na: sodium; K: potassium; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutaryltranspeptidase; LDH: lactate dehydrogenase; HS troponin I: high sensitivity troponin I.
=: In normal range; >: predominantly higher than the normal range; <: predominantly below the normal range.
Details of ICU stay.
| Date of admission | PaFi 1 | Date intubated | PaFi 2 | Prone positioning | PaFi 3 | Date extubated | PaFi 4 | Date discharge from ICU (discharge from hospital)/deatha | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 14/04/20 | 60 | 28/03/20 | 169 | 0 | 274 | 07/04/20 | 459 | 22/04/20 (29/04/20) |
| Patient 2 | 23/03/20 | 86 | 23/03/20 | 97 | 4 | 368 | 01/04/20 | 442 | 08/04/20 (20/04/20) |
| Patient 5 | 23/03/20 | 132 | 23/03/20 | 328 | 9 | NA | NA | 66b | 14/05/20a |
| Patient 6 | 14/03/20 | 79 | 15/03/20 | 260 | 1 | NA | NA | 56b | 18/05/20a |
NA: not applicable; PaFi: PaO2/FiO2 (1: pre-tracheal intubation; 2: immediate post-tracheal intubation; 3: pre-tracheal extubation; 4: immediate post-tracheal extubation or pre-death); prone positioning: number of times the patient was pronated for ventilation (pronation usually lasted 12–16 h and was indicated when oxygenation did not improve with the usual ventilation manoeuvres, e.g., PaO2/FiO2 < 150–200).
Description of ECG and radiological findings in patients admitted to the ICU.
| ECG | Rx 1 (date) | Rx 2 (date) | Other imaging studies | |
|---|---|---|---|---|
| Patient 1 | Sinus rhythm, 82 bpm, narrow QRS, QTc 491 ms. No change during ICU stay | Bilateral parenchymal opacities (25/03/20) | Parenchymal opacities in both lungs, bilateral pleural effusion (30/03/20) | CT: pulmonary thromboembolism in the right upper lobe and several segmental arteries, as well as segmental arteries of the left upper lobe; diffuse bilateral ground glass opacifications (16/04/20) |
| Patient 2 | Sinus rhythm, 85 bpm, narrow QRS QT 393 ms. No change during ICU stay | Bilateral opacities (23/03/20) | No opacities (03/04/20) | NA |
| Laminar atelectasis (17/04/20) | ||||
| Patient 5 | Sinus rhythm, 90 bpm, narrow QRS QT 500 ms | Interstitial reticular opacifications in the middle lobe (25/03/20) | Interstitial reticular pattern, bilateral subpleural opacities, discrete interstitial alveolar pattern (10/04/20) | US: no findings of pulmonary thromboembolism (10/04/20) |
| Bilateral pneumothorax (not simultaneous) and subcutaneous emphysema (22/04/20) | ||||
| Patient 6 | Sinus rhythm, 40 bpm, narrow QRS, QTc 357 ms. Developed atrial fibrillation during ICU staya | Interstitial reticular opacification, condensation of the upper lobe of the left lung (14/03/20) | Progressive alveolar condensations, pleural collection in the right hemithorax (haemothorax) (16/05/20) | CT: bilateral ground glass opacifications, peripheral opacities in both upper lobes, middle lobe, and lingula. Consolidations in the right lower lobe. Bilateral pleural effusion. No findings of pulmonary thromboembolism (19/04/20) |
| Insertion of chest drains and left thoracotomy |
CT: computed tomography; ECG: electrocardiographic findings; NA: not available; QRS: type of QRS complex; QT and QTc: QT interval duration (normal range 400–440 ms); US: lung examination with ultrasound; X-ray 1: chest X-ray on admission to the ICU; X-ray 2: worse chest X-ray findings (both with date of study).
A 64-year-old man with moderate fever for 7 days before admission, followed by the symptoms summarized in Table 1. He was admitted to the ICU after a diagnosis of COVID-19 with pneumonia and clinical worsening. Intubation and mechanical ventilation began on the second day of admission to the ICU. The specific COVID-19 treatments and local protocols in place at that time are summarized in Table 5 (Appendix, additional material online). The patient was extubated 10 days later following clinical and analytical improvement. He presented episodes of agitation, drowsiness, disorientation to time, place and person, and incoherent speech in the ICU after extubation and in the Pulmonology ward (where he remained in contact and respiratory isolation, accompanied by his wife, who had also been previously diagnosed with SARS-CoV-2, patient 3). A late computed tomography scan showed pulmonary thromboembolism (Table 4). He was discharged with enoxaparin. The RT-PCR for SARS-CoV-2 was positive before hospital admission and became negative 21 days later.
Patient 2A 63-year-old man who reported a 5-day history of fever and productive cough. His clinical course is shown in Table 4. After 7 days in the Pulmonology Unit, he required intubation and mechanical ventilation in the ICU. He was extubated 8 days later.
He developed agitation, disorientation requiring low-dose propofol infusion, dexmedetomidine and lorazepam infusion, followed by risperidone. In the Pulmonology ward, where he remained in isolation, he presented delirium, confusion, moderate agitation and euphoria, which progressively improved. He was discharged home (where he lived with his wife, patient 4) with quetiapine and ordered to self-isolate for a further 14 days. The RT-PCR for SARS-CoV-2 was positive before hospital admission and became negative 21 days later.
Patients 3 and 4Before hospitalization, these patients had been living with their respective partners (patients 1 and 2) for at least 7 days while they both had symptoms of COVID-19. Their symptoms were anosmia and dysgeusia/ageusia, lasting more than 10 days. Patient 3 also reported mild fever for 3 days. They quarantined at home for 2 weeks after diagnosis.
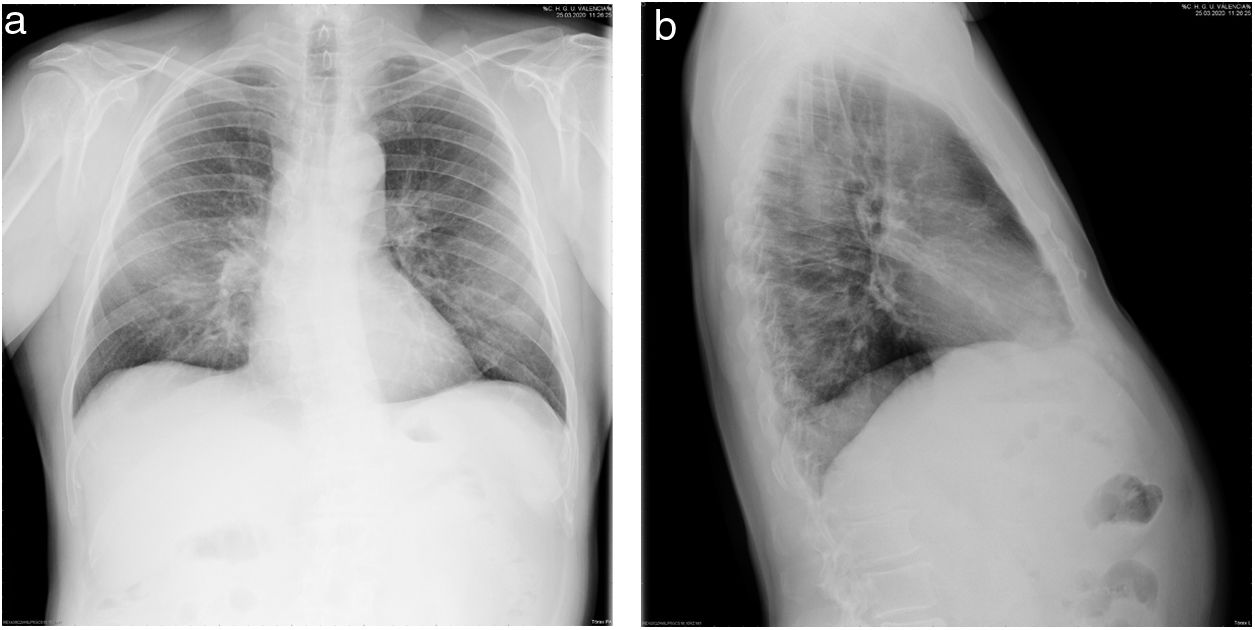
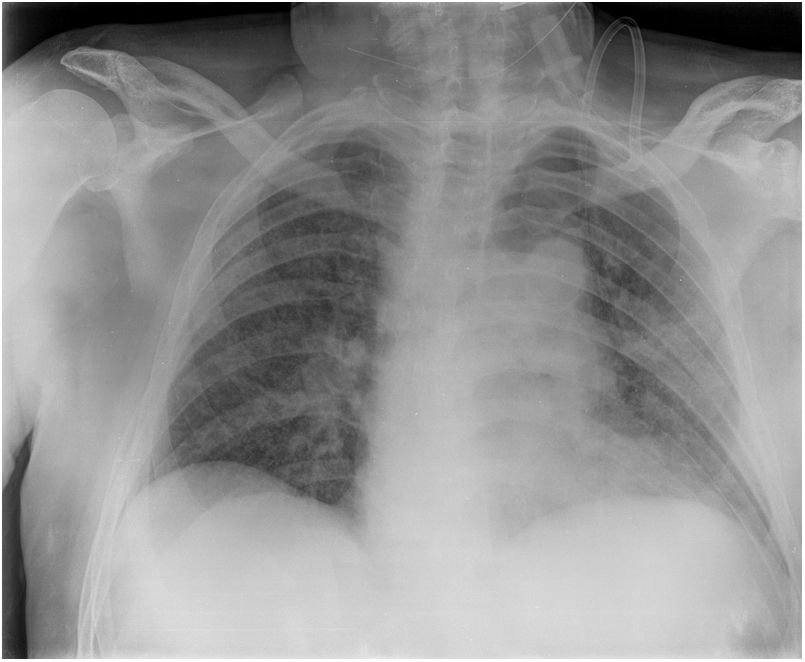
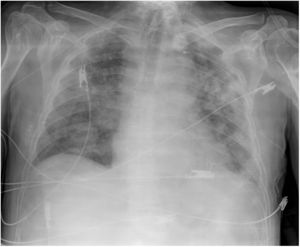
Patient 5Male patient with fever (37.9 °C), tachypnoea (40 rpm), cyanosis and SaO2 77% breathing room air, blood pressure 140/70 mmHg and heart rate 110 beats per minute. He was diagnosed with bilateral interstitial pneumonia (Fig. 1). After presenting neurological deterioration, he was admitted to the ICU where he was intubated and started on mechanical ventilation.
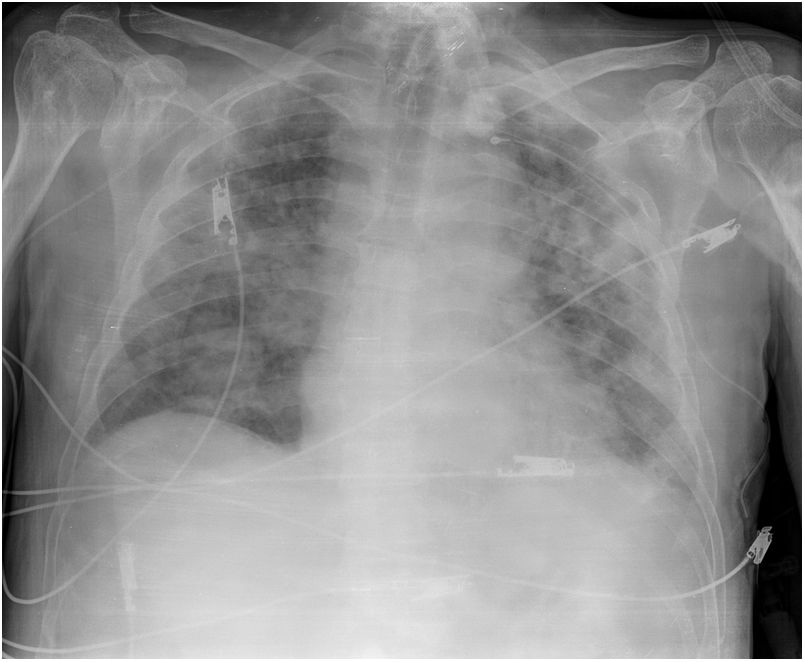
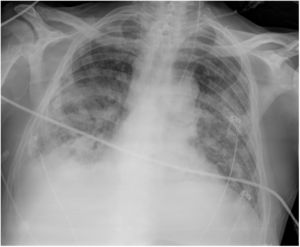
The treatment was administered according to protocol (Appendix, Table 5, additional material online). An early tracheostomy was performed. His labs showed elevated D-dimer, ferritin, and LDH, which remained elevated (Table 2). Chest radiology showed deterioration (Fig. 2).
Inhaled nitric oxide was started for refractory hypoxaemia. Due to persistence of the inflammatory process, anakinra (IL-1 receptor antagonist) was introduced, but oxygenation did not improve despite this measure, and was followed by Pseudomonas aeruginosa, streptococci and fungal coinfections, treated according to antibiogram and local protocol. He eventually developed haemodynamic instability, which was treated with low doses of norepinephrine and dobutamine. Full-dose low-molecular-weight heparin (LMWH) anticoagulation was added, followed by fondaparinux.
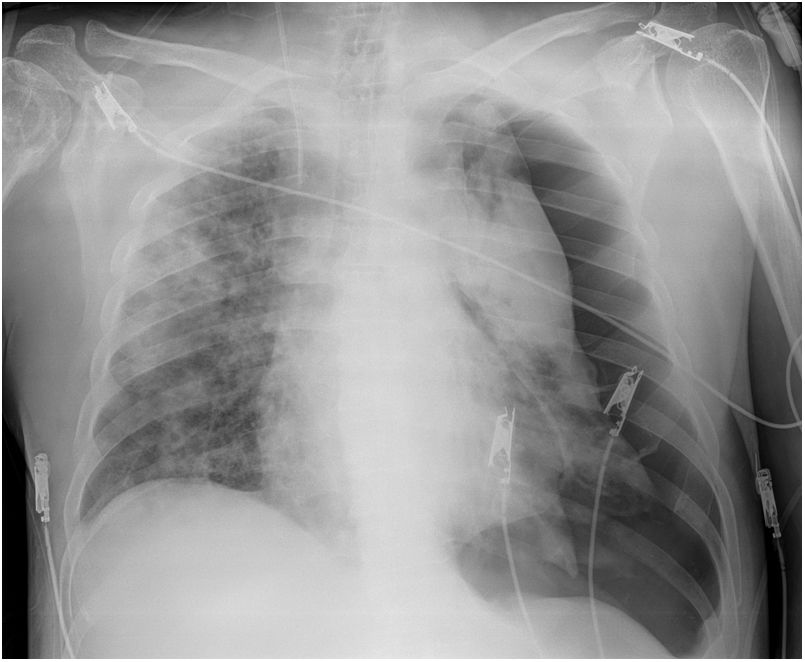
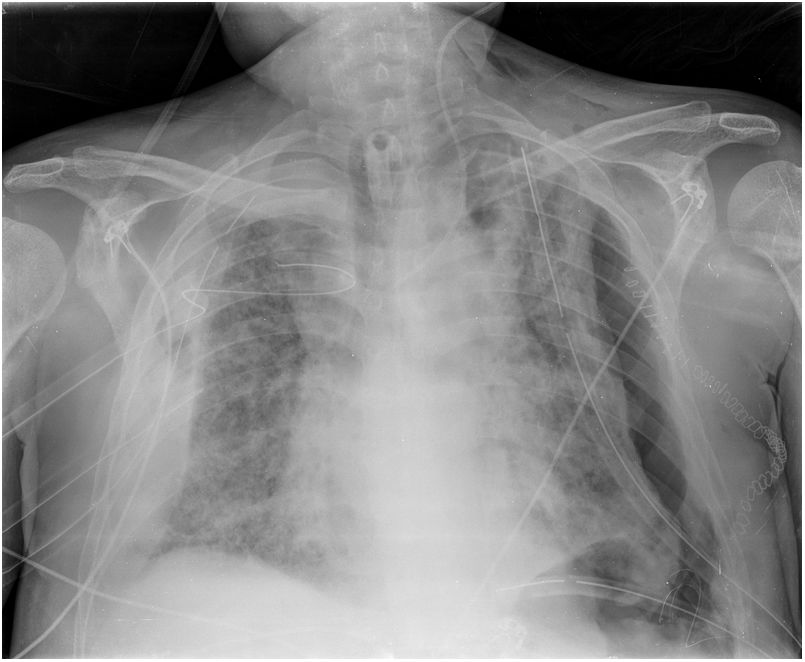
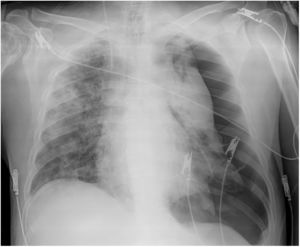
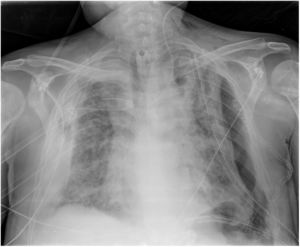
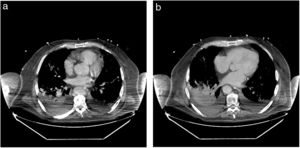
He presented the following complications after the fourth week in the ICU: left pneumothorax (Fig. 3), kidney failure, increased markers of myocardial injury (with normal echocardiogram), right pneumothorax (which required thoracotomy for haemothorax, Fig. 4), blood culture positive for Candida auris, and liver failure.
In the days before death, ruxolitinib (JAK1 and JAK2 kinase inhibitor) was introduced through a compassionate use programme.
The RT-PCR for SARS-CoV-2 was positive before hospital admission and became negative 25 days later.
Patient 6A 74-year-old man who was admitted to the emergency room for fever of 39 °C, cough and dizziness. He had attended a meeting with friends who had been diagnosed with SARS-CoV-2 by RT-PCR several days later (including his wife).
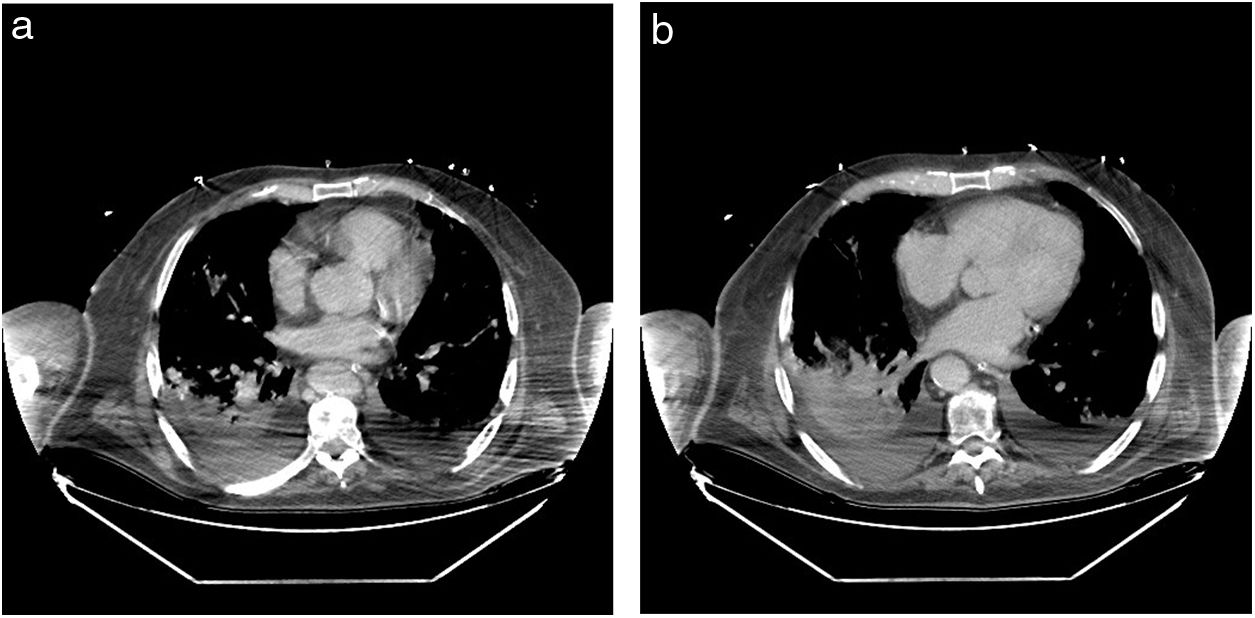
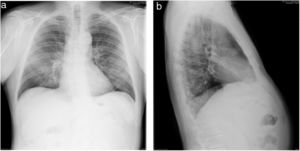
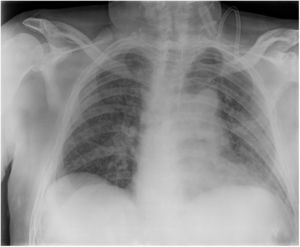
SaO2 was 84% breathing room air, improving to 99% with a mask, but he presented high blood pressure (80/50 mmHg, 65 beats per minute), tachypnoea, and cyanosis. Admission to the ICU and tracheal intubation 6 days later was indicated, and low-dose norepinephrine infusion was started. He was diagnosed with bilateral atypical pneumonia (Fig. 5), and was treated according to protocol (Appendix, Table 5, additional material online). Prophylactic administration of enoxaparin was followed by therapeutic doses (then switched to fondaparinux) due to abnormal coagulation parameters. A tracheostomy was performed on the 17th day of his stay in the ICU. A computed tomography scan showed bilateral consolidations and pleural effusion (Fig. 6a and b).
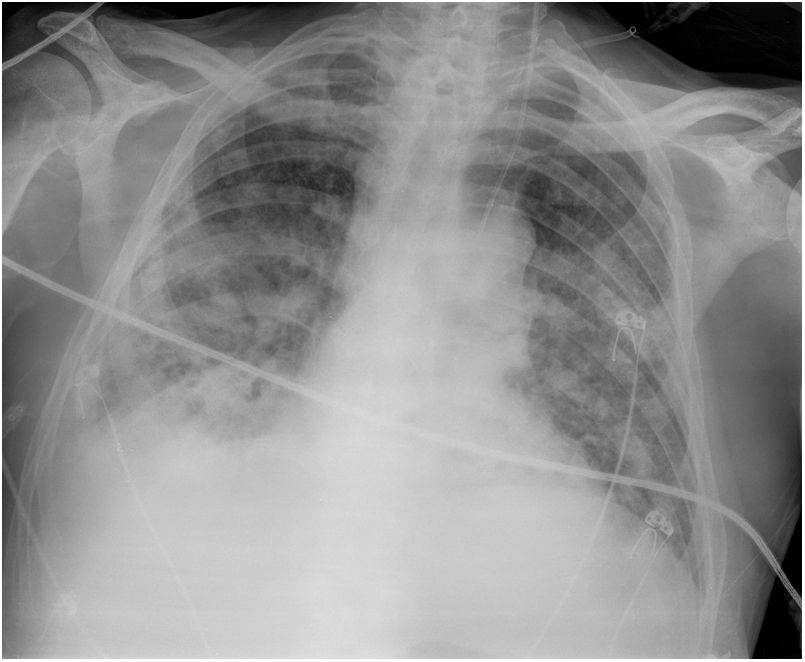
In the final days, he presented polyneuropathy, myopathy and kidney failure. Several transthoracic and transoesophageal echocardiograms were performed, but showed no abnormalities. Chest radiology showed deterioration (Fig. 7).
A series of coinfections and colonizations appeared (Staphylococcus aureus sensitive to methicillin, Acinetobacter baumanii, Enterobacter cloacae, Enterococcus faecium and C. auris) that were treated according to antibiogram.
An attempt at weaning from mechanical ventilation was unsuccessful. Following multiple organ failure, the patient died 44 days after admission to the ICU.
DiscussionIn our hospital (Appendix Banex, Tables 6 and 7 additional online material), 1226 patients were diagnosed with SARS-CoV-2 infection, 371 (30.26%) were hospitalized, and 66 (5.38%, 43 men) were admitted to the ICU. The ICU mortality rate was 44% (29 patients) in this first wave of the pandemic.
In this article, we describe the clinical, laboratory and radiological findings in 6 selected patients diagnosed with COVID-19 in a tertiary public hospital in Valencia, Spain. The patients were admitted during the 2nd and 3rd weeks of the pandemic in Spain.
The reasons for the different clinical course presented by patients infected at the same time or after the index cases are unknown. The mean incubation period before the onset of symptoms is approximately 4–5 days, and almost all symptomatic patients develop symptoms within 11.5 days2. Important factors are the viral load, the patient’s own characteristics (advanced age, men, genetic factors) and comorbidities (arterial hypertension, diabetes, etc.)3.
In our cases, symptoms were similar in both mild and severe patients, but the latter worsened over the following 5 days. This finding is consistent with other studies4.
Reports based on close contacts between confirmed COVID-19 patients in Wuhan5 have shown that asymptomatic patients were younger, usually women, with a higher CD4 + T lymphocyte count during recovery, more rapid radiological improvement, and a shorter duration of viral shedding (samples from nasopharyngeal swabs; mean duration [interquartile range], 8 [3–12] days versus 19 [16–24] days; p = 0.001) compared to symptomatic cases. These finding suggest that patients with milder infections might have fewer immune system alterations.
It should be noted that the 4 severely affected patients had been treated with antihypertensives, and 3 of them with angiotensin II receptor antagonists or angiotensin converting enzyme inhibitors. Opinions vary on the clinical course of the disease in patients treated with both drugs, and several laboratory parameters have been associated with poorer outcomes, including higher mortality4,6. Interestingly, the patients who developed severe clotting disorders, mainly in the pulmonary circulation (patients 1 and 5), had higher concentrations of D-dimer (Table 2), a finding previously reported elsewhere4. Recent evidence has shown that this involved a hypercoagulable state in most patients, and more than 60% presented clinical evidence of thrombotic events, particularly critically ill patients. This may explain the finding of pulmonary embolism in addition to pulmonary vasculitis and endothelialitis. The pulmonary artery thrombi in patient 1 were diagnosed relatively late (close to hospital discharge) due to persistent hypoxaemia.
We obtained few laboratory determinations of IL-6, but this inflammatory marker also appeared to be increased, and has also been described as an independent marker of in-hospital mortality4.
Therefore, D-dimer and IL-6 concentrations at admission appeared to be predictors of mortality. In our patients, both D-dimer and IL-6, in addition to ferritin, increased from the early stages of the disease, and were an expression of the hyperinflammatory syndrome that has been suggested to cause multiorgan involvement in COVID-19.
Lymphopenia is another common finding in COVID-19 patients2, and can also be of prognostic value. A recent study reviewed the role of immune system cells in the immunopathogenicity of lung damage and general hyperinflammation7.
None of our patients developed important organ deterioration in the first days/weeks, except for pulmonary involvement and signs of generalized inflammation (Table 3). In this regard, ferritin, a marker (or mediator) of inflammation8, increased and remained elevated in patients with longer ICU stays (patients 2, 5 and 6) and more severe evolution (patients 5 and 6). Despite increased high sensitivity troponin i levels, serial imaging scans in all patients during their ICU stay showed no evidence of heart disease. We, unlike other authors, only observed a few electrocardiogram changes, such as bradycardia, slight QT or QTc prolongation, and atrial fibrillation in some cases.
The patients that made poorer progress presented several coinfections that were rarely found at the time of presentation. This meant that changes in late laboratory parameters coincided with an increase in lactate, procalcitonin, C-reactive protein, etc. (Table 2), similar to typical acute respiratory distress syndrome aggravated by concurrent coinfections. It has been reported that systemic viral infections are often accompanied or immediately followed by bacterial and fungal infections, and that these can affect outcomes6.
In addition to anosmia and dysgeusia, recent studies have reviewed the issue of central nervous system (CNS) involvement9. We would draw attention to the neurological alterations consistently observed in these patients. Although this was not specifically defined in the first major studies performed in China, the USA, and Italy, we have observed neurological and psychiatric alterations in our patient since the first cases were admitted to our hospital and the ICU. Patients in the ICU presented confusion, dizziness, drowsiness and appeared disoriented and bradypsychic on admission (with normal to moderately low SaO2 and normocapnia), and exhibited the same symptoms plus agitation, delirium, euphoria, confusion, etc. when sedation was stopped and they were extubated. The symptoms continued after transfer to the ward10. CNS involvement could be explained by hypoxia or the side effects of drugs (sedatives, muscle relaxants), but some patients with a short stay in the ICU also presented these symptoms. We could speculate that this is due to various mechanisms of CNS involvement, because other coronaviruses are also neurotropic and neurological involvement was initially poorly reported despite an incidence of between 36.4%11 and 67%12. However, it is still unclear whether this is a result of viral invasion or general reaction to inflammation.
Changes in blood–brain barrier (BBB) permeability, theoretically a prerequisite for CNS invasion, are caused by several mechanisms and factors, including advanced age, inflammation and viriasis13. We could also hypothesise on the prevalence of a functional alteration caused by changes in the cholinergic anti-inflammatory pathway, without the need for direct viral invasion14. A potential invasion by SARS-CoV2 of some CNS nuclei could explain, at least in part, the origin of the respiratory failure observed in some COVID-19 patients15, and could have therapeutic implications. It remains to be seen whether this damage persists over time.
Critical patients were treated according to institutional protocols. However, these were changing day by day (following the recommendations of the Ministry of Health and the local administration), so the timing of the administration of some drugs differed in some patients. For example, anti-inflammatory drugs were started more than 1 week after the onset of symptoms in all cases, depending on the clinical response and laboratory findings, and drugs such as anakinra or sedatives were in short supply. This partly precluded a staged approach (therapeutic window).
This case series has several limitations. The number of cases is low, but our aim is to show the characteristics of selected typical COVID-19 cases. We naturally did not attempt to find a causal relationship; however, the descriptive information might be of interest to the reader because it shows the presentation of most patients during the pandemic.
In conclusion, we present the different evolutionary pathways and outcomes observed in 6 closely-related patients diagnosed with COVID-19. We discuss the typical findings, the clinical, laboratory and radiological characteristics, and the pathophysiology of the disease in addition to the widely described respiratory characteristics.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Errando CL, Romero-García CS, Hernández-Cádiz MJ, Pallardó-López MÁ, Puig J. Infección por SARS-CoV-2. Análisis descriptivo de una serie de casos seleccionada. Rev Esp Anestesiol Reanim. 2022;69:34–42.