Following the appearance of the first severe cases of COVID-19, it was observed that most deaths were associated with lung involvement. The atypical presentation of this new viral pneumonia – initially characterised by severe hypoxemia with mis-matched blood gas and radiological findings – created a great deal of confusion. Pneumonia can rapidly progress to acute respiratory failure requiring ventilatory support in approximately 5–15% of cases. Many mechanically ventilated patients progress to more aggressive forms of pneumonia that can culminate in typical acute respiratory distress syndrome (ARDS).
The atypical, rapidly evolving presentation with significant pulmonary vascular involvement in patients with COVID-19 has led several groups to suggest that this entity is different from ARDS.1,2 In this context, Gattinoni et al. suggested that COVID-19 patients present 2 different phenotypes: the L (low) phenotype and the H (high) phenotype, based on clinical, radiological and respiratory mechanic manifestations.3 In summary, type L patients do not show the typical signs and symptoms of ARDS, since they have high tolerance to hypoxaemia, few radiological infiltrates, and low lung elastance. Type H patients, meanwhile, present the typical symptoms of ARDS, severe radiological involvement and high elastance. The authors suggested two different ventilatory strategies for treating these phenotypes.
Many physicians with experience in the management of these patients expressed their doubts regarding the frequency with which both phenotypes presented in such a pure form, and also disagreed on separating ventilatory treatment so pragmatically and categorically. This raises some obvious questions: Is the L phenotype pneumonia and the H phenotype progression to ARDS? Are there intermediate forms of presentation? Should ventilation be delivered according to a fixed protocol, or should it be personalized regardless of the phenotype?
It seems obvious, aside from opinions not backed by conclusive evidence, that we need markers, diagnostic methods and monitoring systems to determine the stage of the disease in a particular patient and the pathophysiological mechanism of hypoxaemia/hypercapnia. Only in this way can objective, personalized, lung-protective ventilation be delivered. In other words, we do not need new ARDS classifications or simplistic theories that divide the grey ranges of diseases, including COVID-19, into black and white.
One of the diagnostic and monitoring methods available, namely, volumetric capnography (Fig. 1) has certain interesting advantages in this type of patient. This non-invasive tool measures the volume of expired carbon dioxide in intubated patients, giving direct information on ventilatory dead space (VD=ventilated areas with no blood flow) and indirect information about the shunt effect (perfused but poorly and/or non-ventilated areas).4
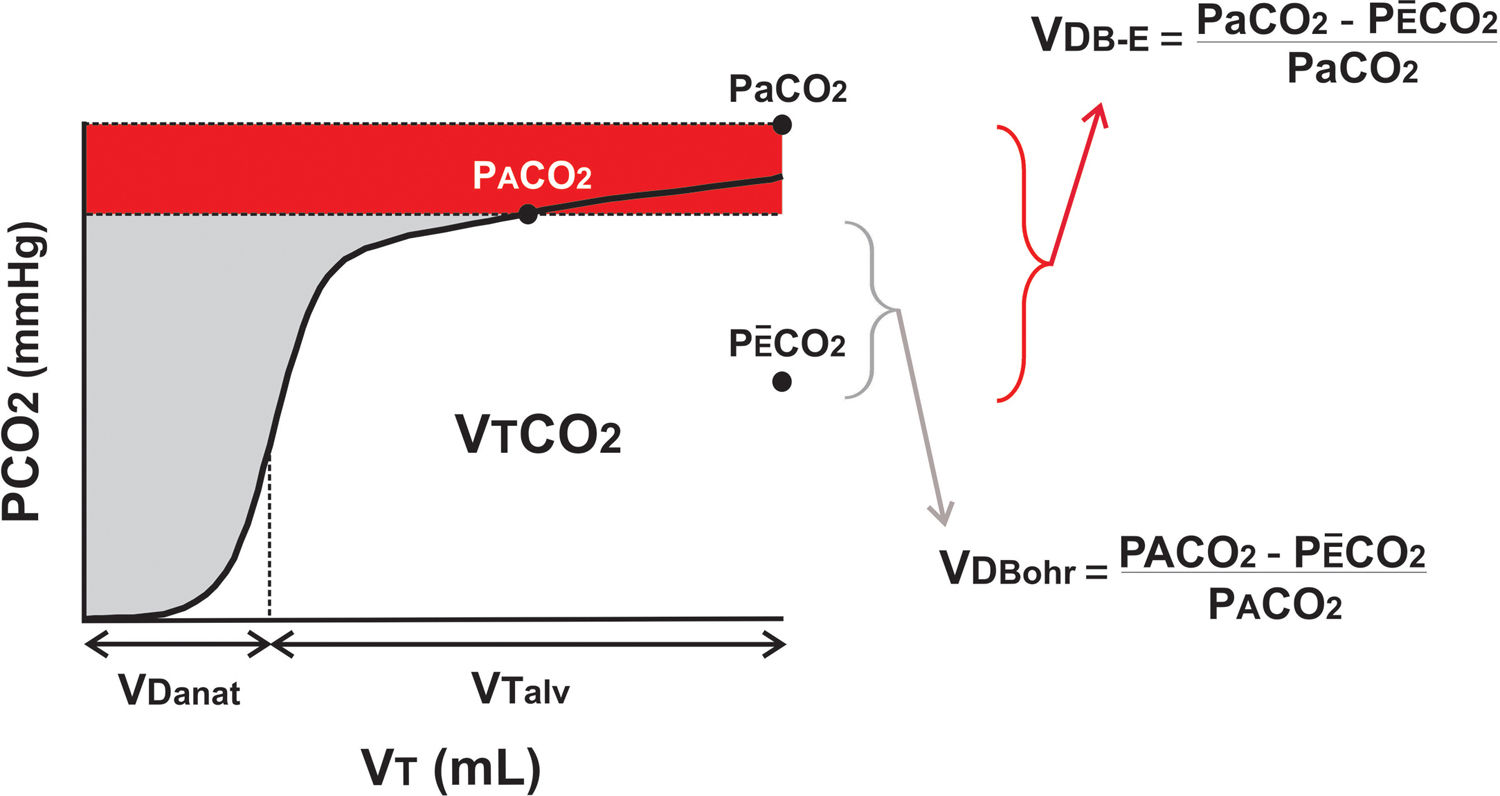
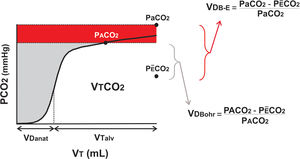
Volumetric capnography and calculation of dead space.
Volumetric capnography expresses the amount of CO2 expired per breath (VTCO2). It separates tidal volume (VT) in the anatomical dead space (VDanat) from alveolar gas (VTalv). The difference between Bohr's formula and Enghoff's modification is given by the shunt effect, shown as the difference between arterial and alveolar pressure of CO2 (Pa-ACO2; in red).
Patients with severe COVID-19 present significant pulmonary vascular involvement (endothelial damage, microthrombi/emboli, hypoxic pulmonary vasoconstriction) with major ventilation–perfusion ratio changes due to the presence of heterogeneous pulmonary zones of VD and shunt. This is why volumetric capnography not only diagnoses the presence of pathological VD and shunt, but can also be used to adjust protective ventilation to minimize overdistention and impaired oxygenation. In other words, continuous monitoring using this technique has important clinical connotations in terms of minimising the risk of ventilator-induced lung injury.
How does volumetric capnography measure VD and estimate the degree of shunt? VD is measured non-invasively using the Bohr equation (Fig. 1), which quantifies the difference between the alveolar partial pressure (PACO2) and the partial pressure of mean expired carbon dioxide (PÊCO2) obtained in each breath. In the past, the impossibility of measuring PACO2 led to the use of Enghoff's modification of Bohr's formula, which replaces alveolar pressure with partial pressure of CO2 in arterial blood (PaCO2). This invasive and intermittent measurement includes not only the VD but also the right-left shunt (red area in Fig. 1).
Each of these formulas, therefore, quantify different aspects of the ventilation–perfusion ratio: the exaggerated increase in VDBohr expresses “pure” pulmonary overdistention and VDBohr-Enghoff expresses the shunt effect.5
In conclusion, volumetric capnography gives us a tool to objectively establish: (1) the predominant pathophysiological phenomenon in patients with COVID-19, (2) the best mechanical ventilation setting for each patients, and (3) how to monitor the evolution of the disease based on physiological data.
This might lead to a more rational use of ventilation and may show whether the L and H phenotypes are true forms of presentation of a specific lesion or only temporary manifestations of the same disease.
FundingNo author has received any income or grants related to this manuscript.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Turchetto ES, Tusman G, Makinistian RL. Ventilación mecánica en daño pulmonar por SARS-CoV-2: ¿qué puede aportar la capnografía volumétrica? Rev Esp Anestesiol Reanim. 2021;68:116–118.