Just as the appearance of the human immunodeficiency virus in the 80s led to a paradigm shift in the protection measures used by health personnel (use of gloves, management of venous lines, etc.), so will the appearance of the SARS-CoV-2 virus change our action protocols, particularly when there is a high risk of contamination (manual ventilation, orotracheal intubation, etc.).
Since it was first detected in Wuhan in late 2019, the virus has spread rapidly around the world and was eventually classified by the WHO as a public health emergency of international concern.1 SARS-CoV-2 is known to spread through direct contact, respiratory droplets, and aerosols from infected individuals.
The SARS-CoV-2 virus, with a basic reproduction number of around 2.5, is highly contagious,2,3 even during the 14-day incubation period.4 Symptoms can appear 4 or 5 days after exposure.5,6 Asymptomatic carriers of SARS-CoV-2, around 1% of confirmed cases,7 can potentially transmit the virus during the incubation period,8 a factor that makes the identification and prevention of SARS-CoV-2 infection a major challenge.
According to the only study published so far on the impact of surgery performed during the COVID-19 incubation period in patients with no known infection9 (retrospective, multicentre study performed in hospitals in China), 44% of cases were ultimately admitted to the Intensive Care Unit (normal rates do not exceed 26%), and 20.6% died (the normal mortality rate is 1%). In other words, mortality in patients undergoing surgery during the incubation period was 10–20 times higher than normal figures. A prospective, multicentre, cohort study is currently underway to determine the optimal timing for surgical interventions after SARS-CoV-2 infection, and to evaluate key surgical indicators. The study, which has been carried out in 116 countries and 1644 hospitals, had recruited 137,169 patients once data collection had finalised.10
Although the use of nasal or pharyngeal swab PCR and serology to detect antibodies is now standard practice, some patients have developed clinical and radiological signs and symptoms of COVID-19 between 2 and 4 days after surgery.9
There are several unanswered questions regarding immunization and its duration. Even after we have succeeded in effectively controlling the virus, we will have to be prepared for further infection and outbreaks that will occur. We will have to be particularly vigilant in the context of scheduled surgeries performed on a wide range of patients.
A number of new protocols and recommendations to minimize the perioperative transmission of pathogens and create a safe environment for surgical interventions have appeared during the current COVID-19 pandemic,11,12 and can be added to existing protocols for the surveillance and control of surgical site infections.13 In this study, we present a critical review of existing recommendations, highlighting those that should be implemented from now on.
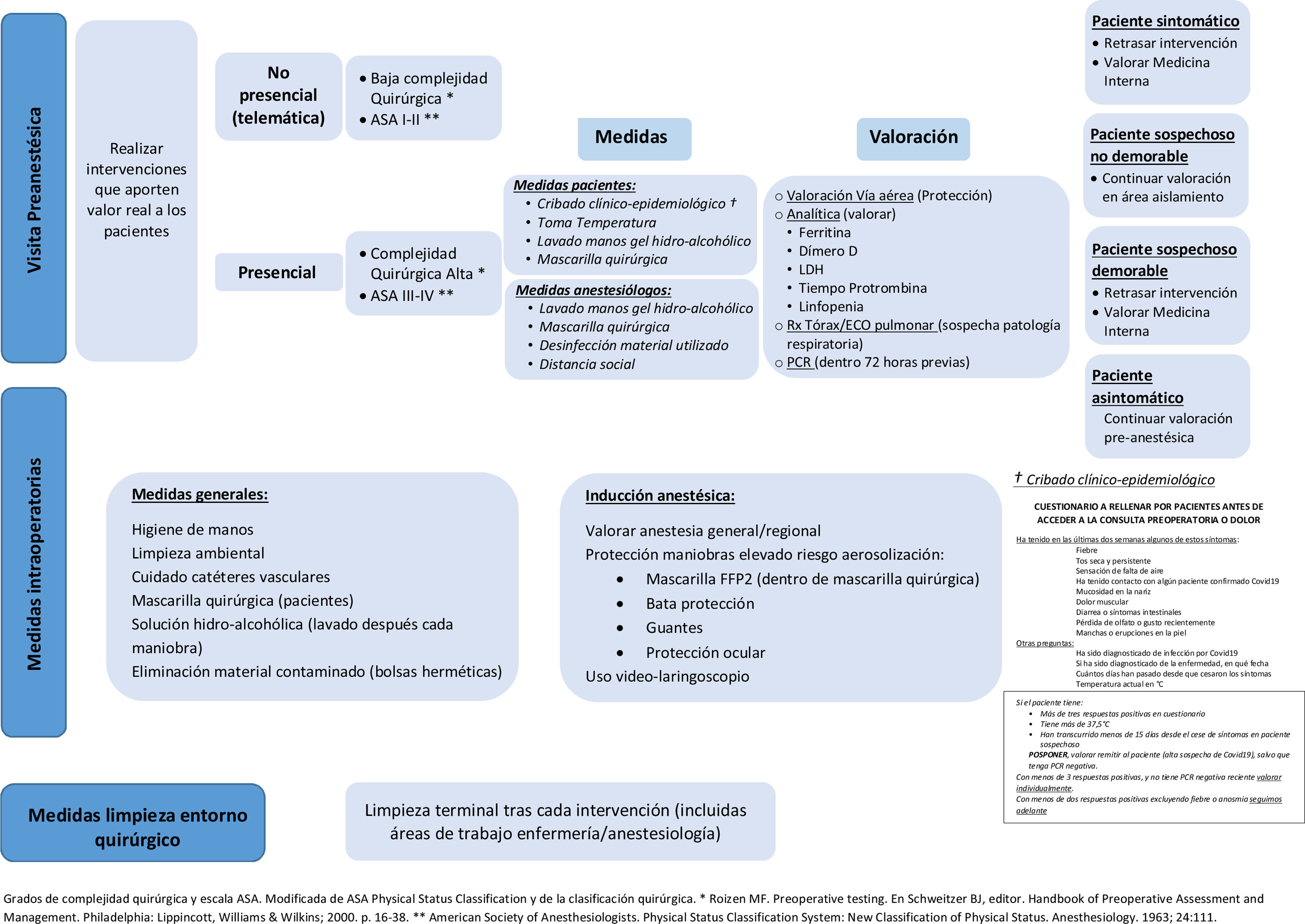
Pre-anaesthesia assessmentAfter having adjusted our surgical activity to the various possible scenarios during different virus alert phases,14 we must take advantage of the situation to reorganize surgical waiting lists to prioritize interventions that represent a real, obvious benefit for patients. Following the enforced hiatus in the surgery schedule caused by the SARS-CoV-2 pandemic, we must be careful not to make the same mistakes when we resume our activity. We need to confer with the surgical team to define the interventions that will provide a clear benefit in terms of quality of life and in which the surgical risk index and the risk posed by the patient's comorbidities is acceptable compared with the risk of postponing surgery. It is essential to include patients in this decision-making process. This will allow the team to agree on the most appropriate treatment after discussing with the patient the advantages and disadvantages of each option available, the benefits and risks, taking into consideration the patient's preferences and personal values and existing scientific knowledge (shared clinical decision-making).15 We should only perform interventions that really benefit our patients.
Preoperative consultationOne of the tools implemented during the initial phases of the pandemic has been the tele-medicine preoperative consultation. This minimizes patient visits to hospitals and reduces the risk of infection, and is used to either re-evaluate patients that have already been seen or to perform a pre-anaesthesia evaluation. The feasibility of this system has been assessed in various studies, and the conclusion has been that the tele-medicine pre-anaesthesia evaluation was equivalent to a face-to-face assessment insofar as it did not result in more cancellations and was equally effective in predicting difficult airway management.16 Tele-medicine visits must be clearly defined and protocolised in a manner that ensures compliance with the Organic Law on Data Protection. They must be use to agree on a procedure with the patient, with all legal safeguards, and must be assigned a slot on the clinician's schedule in order to guarantee that they are conducted according to good clinical practice.17 Each hospital should evaluate the feasibility of implementing an electronic signature system, a record of informed consent for surgical interventions, or a system for emailing patients a consent form for them to sign and return by the same means.
This type of telematic evaluation should be implemented above all in patients with low surgical complexity, in those who are familiar with digital technologies, who have non-limiting comorbidities (ASA I or II), and who consent to this type of evaluation (Fig. 1).
In face-to-face preoperative consultations a series of measures must be implemented to minimize the risk of doctor-patient contamination. Before scheduling the consultation, patients must undergo clinical epidemiological screening in which they fill out a questionnaire that includes questions on their epidemiological history (whether they have had contact with a positive case in the last 14 days and/or whether the live in a nursing home or other kind of institution) and their signs or symptoms of potential SARS-CoV-2 infection (fever, dry cough, mild constitutional symptoms, anosmia, diarrhoea, headache). The patient's temperature must be measured (infrared thermometers) and they must be given hydroalcoholic gel for hand washing and a surgical mask as soon as they enter the hospital. The anaesthesiologist, in turn, must use strict aseptic technique (mask, hand washing with hydroalcoholic solution, hydroalcoholic wipes for disinfecting elements that can be touched during the visit, computers, keyboards, mice, stethoscope, etc.) and practice safe physical distancing.
During airway assessment the clinician must evaluate the degree of ventilation difficulty and identify the factors predicting difficult mask ventilation described by Langeron et al.18. They must also evaluate intubation difficulty, for example, using the El-Ganzouri et al.19 difficult intubation index, assess mouth opening, mandibular subluxation, and determine the patient's Mallampati class. All these evaluations must be carried out under strict protective measures.
The lab workup must include biochemical parameters that can be elevated early in cases of COVID-19 disease, such as ferritin, which is the earliest and starts to increase from the fourth day, D-dimer, which is elevated after the first week and progressively increases, and lactate dehydrogenase. It is also extremely important to evaluate prothrombin time and the presence of lymphocytopenia in the hemogram.
Regarding chest X-ray, it is important to bear in mind that virus will not have reached the alveoli in the initial phase of infection, so the radiological image will be normal. However, in the phase of pulmonary infection (peripheral opacities, increased diffuse consolidations, focal interstitial pattern, diffuse interstitial pattern, alveolar interstitial pattern) chest radiography has a sensitive of 68% a specificity of 95% for detecting consolidation, and a sensitivity of 60% and a specificity of 100% in the case of alveolar-interstitial syndrome.20 We also have another useful preoperative tool that should not be overlooked – lung ultrasound, which has a sensitivity and specificity of 93% and 100% for detecting alveolar consolidation, and of 98% and 88% for detecting alveolo-interstitial syndrome, respectively.20 Bear in mind, however, that in the absence of symptoms, chest radiographs produce few diagnoses and many false positives. They do not improve risk prediction or stratification, and should be reserved for patients with symptoms consistent with acute cardiopulmonary disease (dyspnoea, wheezing, productive cough, etc.).21 Therefore, routine radiological studies are not recommended, and are only indicated in patients with symptoms, history, or examination indicative of pulmonary involvement.
Patients requiring more comprehensive pre-anaesthesia preparation due to surgical complexity or the presence of limiting comorbidities (ASA III or IV) should be scheduled for a face-to-face consultation (Fig. 1).
Nasopharyngeal swab PCR is the test of choice for diagnosing active SARS-CoV-2 infection. It is estimated to have a sensitivity of between 70% and 80%, depending on the evolutionary stage of the infection and the quality of the sample. Specificity is greater than 95%. The test should be performed within 48–72h of surgery, and the patient must take measures to avoid infection in the days leading up to surgery.
When ordering nasal or pharyngeal swab PCR it is important to bear in mind the limitations of these techniques and remember that the result will depend on the timing of sampling, the quality of the sample, and the sensitivity and specificity of the test. A positive PCR can guide the decision to delay the intervention for at least 15 days, if the surgical procedure is not urgent. However, a negative PCR (which can be a false negative or an active infection in a very early phase) is no reason to relax the universal protection measures that must be implemented during surgery.
A strategy must be defined to cope with the different scenarios that may be encountered during the preoperative evaluation (symptomatic patient, asymptomatic patient with fever, laboratory abnormalities with/without symptoms): continue the preoperative visit, but in an isolation area (suspected infection, surgery cannot be delayed), delay the surgery and refer to Internal Medicine for evaluation (suspected infection, surgery can be delayed), etc.
At the end of the preoperative assessment, the patient must be give clear, concise information on the risks of surgical site infections and how to prevent, control, identify or treat them; advise showering the day before the intervention, brushing teeth, rinsing mouth and no routine hair removal22; schedule admission for the same day as the surgical intervention, if possible.
The Recommendations for scheduling safe surgery during the COVID-19 pandemic prepared by different scientific societies and endorsed by the Ministry of Health and published on 2 June 202012 recommends clinical epidemiological screening at least 14 days before surgery and observing strict physical distancing and protection measures for 2 weeks prior to surgery. It recommends a second clinical evaluation visit 72h before surgery, which includes a PCR test. These recommendations are difficult to follow in routine practice, and must be adapted to the workload of each hospital. In our case, the complexity of the surgical intervention, the scheduling priority and the patient's comorbidity (defined by the surgeon in the surgery order form) determine the timing of the preoperative visit – the greater the surgical complexity and the patient's comorbidity burden, the earlier the date of the preoperative consultation. The surgery can be scheduled once the patient's surgical risk has been assessed, their clinical status has been optimized, the patient has been fully informed (including the need for protective measures and physical distancing), and informed consent has been obtained. Preoperative PCR screening is performed 72h before the intervention, at which time the patient is again urged to observe strict protective and physical distancing until they are admitted for surgery.
Intraoperative measuresSARS-CoV-2 can survive for at least 3 days on a variety of materials commonly found in operating rooms (stainless steel, plastic, etc.).23 Solid evidence obtained from studies conducted over the past 12 years has shown that a multimodal approach is needed to minimize the high risk of intraoperative pathogen transmission. Hand hygiene, environmental cleanliness, care of catheters (both arterial and venous) must be improved, patients must be decolonised, and surveillance optimized. All these factors must be combined to improve the perioperative control of infections by bacterial and viral pathogens.11
All patients entering the surgical area should wear a surgical mask for reverse protection.
Essential measures to maintain a safe and decontaminated work environment include placing hydroalcoholic dispensers for hand disinfection near the anaesthesiologist's work area, placing contaminated material used during induction in hermetically sealed bags, and systematically cleaning the work area with disinfectant wipes after anaesthesia induction and each manoeuvre.
During induction, particularly in emergency procedures, several patient manoeuvres are performed that carry a high risk of viral or bacterial transmission; some 350 possible contaminating activities have been quantified during routine intraoperative practice.24 Risk of infection during interventions can be reduced up to 20-fold by simply placing bottles of hydroalcoholic solution or hydroalcoholic-based surface wipes in the anaesthesiologist's work area and by washing hands after each intervention.25
Anaesthesia inductionThe type of anaesthesia used will depend on the respiratory status of the patient and the type of surgery performed. Regional anaesthesia should prevail, whenever possible, with the patient wearing a surgical mask throughout the process.
Microbes, viruses and bacteria colonize our skin.26 The mouth is the largest reservoir of bacteria, which are dispersed through the air to the immediate surroundings when we speak. Respiratory secretions, either from aerosolization during intubation or from contaminated surfaces and subsequent contact with the eyes, nose and/or mouth are potential causes of infection. Universal protection measures should be implemented during procedures with a high risk of aerosolization (protective masks [at least FFP2] under a surgical mask, gown, gloves and goggles). Nasal decontamination with topical antimicrobials is not routinely recommended to eliminate staphylococcus aureus and reduce the risk of surgical site infection.22
Disposable or sterilizable video laryngoscopes should always be used for intubation, as this will reduce proximity to the potential source of aerosolization.
Particular care must be taken with intravascular catheters (arterial or venous), since they are in direct contact with the patient's circulatory system and can cause repeated contamination and increase mortality directly associated with the infection. Cleaning all lines before each injection and placing disinfecting caps on catheters during and after the procedure are essential measures.27
After anaesthesia induction, all surfaces in the anaesthesiologist's work area should be swabbed with hydroalcoholic or quaternary ammonium-based wipes.
Cleaning measures in the surgical environmentThe current situation compels us to improve the organization and quality of cleaning to substantially reduce possible contamination of our work area.
Routine and/or terminal cleaning of the operating room after each intervention, including the anaesthesiology and nursing work areas (paying particular attention to computer keyboards, screens and mice), must be extremely thorough in order to reduce the viral and bacterial load.
There is no evidence for the efficacy and safety of eliminating SARS-CoV-2 with ultraviolet lamps or ozone generators. The available evidence suggests that both devices can reduce the surface viral load; however, it is unclear whether this is sufficient to achieve disinfection. Both these devices present a health risk, as they can cause eye damage, skin damage or respiratory tract irritation, and their use must always be regulated.28
Finally, it is essential to promote the use of infection prevention and control programmes and surveillance systems for healthcare-associated infections in order to control their evolution and compare results.29
ConclusionsThe appearance of COVID-19 has already changed our practice, particularly in interventions that carry a high risk of contamination (manual ventilation, orotracheal intubation, etc.). Many of the measures that are currently being taken in most hospitals to prevent the spread of COVID-19 are here to stay. The emergence of SARS-CoV-2 has highlighted the shortcomings inherent to the epidemiological measures hitherto used to prevent bacterial or viral transmission in supposedly clean environments. Hospitals themselves have been a major source of transmission, so the implementation and maintenance of these measures will prevent the spread of pathogens that could emerge as a new clinical challenge in the future.
Most of the measures proposed are standard procedures that should be used all contact isolation protocols or as barrier measures to prevent the transmission of pathogens in a perioperative setting. The truth is that the powerful antibiotics developed in recent years have lulled hospitals into a false sense of security and general relaxation of preventive measures. This pandemic must teach us to keep our guard up, improve control of perioperative infections, and systematically apply protocols for controlling the spread of communicable pathogens.
Please cite this article as: Mata Estévez J. Medidas de control de la transmisión de infecciones en el entorno quirúrgico: cambio de paradigma tras la COVID-19. Rev Esp Anestesiol Reanim. 2021;68:56–61.