Multiorgan dysfunction syndrome is the most common cause of mortality in intensive care units. The lungs and kidneys are frequently affected, so up to 60 % of patients require simultaneous respiratory support and renal replacement therapy.
Extracorporeal CO2 elimination systems have now been developed with the aim of reducing the incidence of acute lung injury. These systems can be combined with renal support therapies in patients with dysfunction of both organs.
We present a case of respiratory septic shock with renal failure and respiratory distress syndrome, in which extracorporeal elimination of CO2 therapy facilitated the use of protective ventilation, with a low tidal volume of 4 ml/kg, plateau pressure below 30 cmH2O, and PaCO2 values of less than 60 mmHg.
El síndrome de disfunción multiorgánico es la causa más frecuente de mortalidad en las Unidades de Cuidados Intensivos. Los pulmones y riñones son dos órganos frecuentemente afectados, por lo que hasta el 60% de los pacientes precisan simultáneamente soporte respiratorio y terapia de sustitución renal.
En la actualidad se han desarrollado sistemas de eliminación extracorpórea de CO2 con el objetivo de reducir la incidencia de lesión pulmonar aguda, que pueden ser combinados con terapias de soporte renal en pacientes con disfunción de ambos órganos.
Presentamos un caso de shock séptico de origen respiratorio con fracaso renal y síndrome de distrés respiratorio, en el que se llevó a cabo terapia de eliminación extracorpórea de CO2 que permitió facilitar pautas de ventilación de protección con disminución del volumen corriente hasta 4 ml/kg y reducción de la presión meseta por debajo de 30 cmH20, con valores de PaCO2 inferiores a 60 mmHg.
Multiorgan dysfunction syndrome (MODS) has been defined as "the progressive dysfunction of 2 or more organ systems in acutely ill patients, such that homeostasis cannot be maintained without intervention".1 MODS is the most frequent cause of mortality in intensive care units. The lungs and kidneys are the organs frequently affected, so up to 60 % of patients require simultaneous respiratory support and renal replacement therapy.2 The extracorporeal oxygenation and/or ventilation systems available today can reduce the incidence of acute lung injury in patients under mechanical ventilation.3 They can also be combined with renal support and adsorption therapies in patients with both lung and kidney dysfunction, particularly in the context of sepsis.
Case reportThis was a 69-year-old man, height 177 cm, weight 85 kg, with a history of severe chronic obstructive pulmonary disease, who presented at the emergency department with worsening of his habitual dyspnoea and increased cough with yellowish expectoration, with no fever or chest pain. The patient’s status deteriorated rapidly during his stay in the emergency department, with BP 85/45 mmHg, heart rate 102 bpm, tachypnea 32 rpm, and arterial oxygen saturation 88 % with Ventimask® 40 %. An angio-CT scan was performed to rule out pulmonary thromboembolism. The images showed pulmonary infiltrate in the left lower lobe, probably associated with pneumonia, so the sepsis code was activated, meropenem 2 g was administered, and resuscitation with fluid therapy and vasopressors was initiated. Given the patient’s clinical deterioration, he was admitted to the critical care unit with a diagnosis of septic shock of respiratory origin. He was monitored with the VolumeView® system, intubated, and connected to mechanical ventilation. He presented cardiac arrest that required advanced cardiac life support manoeuvres for 8 min until haemodynamics were stabilised. He was diagnosed with refractory shock and required norepinephrine at 0.9 μg/kg/min and adrenaline at 0.3 μg/kg/min for cardiac index values of 2 l/min/m2, left ventricular end-diastolic volume index of 800 ml/m2, extravascular lung water index of 10 ml/kg, and systemic vascular resistance index of 500 dyn*s*cm−5*m2. Laboratory findings showed lactate 0.46 mmol/l, pro-BNP 25,000 pg/ml and procalcitonin 65 ng/ml.
Transoesophageal echocardiogram showed severe left ventricular dysfunction with an ejection fraction of 15 %, so levosimendan perfusion was started.
Respiratory parameters were PaO2/FiO2 ratio 180 and PaCO2 40 mmHg, with the following mechanical ventilation settings: tidal volume 500 ml, positive end expiratory pressure (PEEP) 12 cmH2O, plateau pressure 29 cmH2O, and pulmonary compliance 25 ml/cmH2O.
In the first 24 h, he presented anuria with elevated creatinine, establishing the diagnosis of stage 3 acute renal failure accroding to the Kidney Disease Improving Global Outcomes classification, so polymyxin haemoadsorption was started, followed by continuous veno-venous haemodiafiltration with an oXiris® filter. This system provides both renal replacement therapy and adsorption of endotoxins and cytokines in septic patients.
Antigens for Streptococcus pneumoniae were detected in urine, so levofloxacin was added to the treatment. The final diagnosis was severe community-acquired pneumococcal pneumonia that caused septic shock with MODS, acute respiratory distress syndrome (ARDS), and kidney failure.
Although the patient’s haemodynamic status had improved within 24 h, allowing us to withdraw adrenaline, his respiratory status worsened in the following days, presenting PaO2/FiO2 ratio of 100 on the third day of admission, despite recruitment manoeuvres. On mechanical ventilation with a tidal volume of 500 ml, PEEP of 12 cmH2O and respiratory rate of 15 rpm, the patient’s PaCO2 value was 84 mmHg, with a pH of 7.15.
We decided to connect a Prismalung® system (Gambro-Baxter) (Fig. 1) to eliminate CO2 and provide lung-protective ventilation. The Shaldon 12 F catheter in the right femoral vein was replaced using guidewire exchange with a 13.5 F Shaldon catheter to allow a blood flow of up to 450 ml/h. Renal replacement therapy was maintained to achieve an ultrafiltration rate of 25 ml/kg and a removal rate of 250 ml/h, adding a fresh gas flow of 10 l oxygen to the Prismalung® gas exchanger.
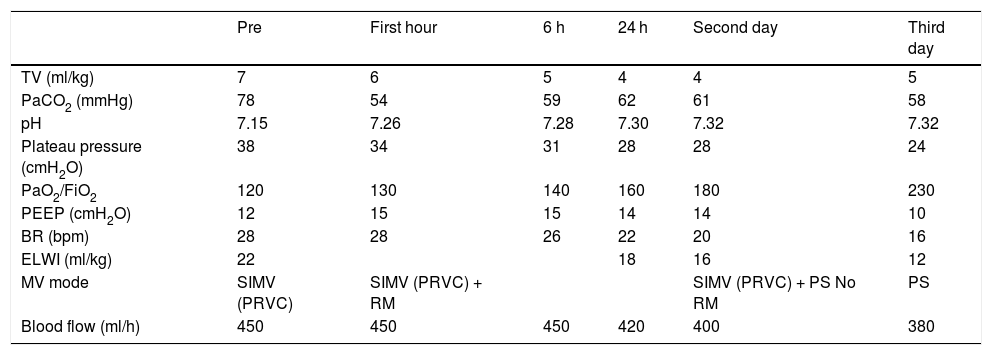
Table 1 shows the evolution of the main respiratory variables during therapy. The use of the Prismalung® membrane allowed us to reduce tidal volume to 4 ml/kg and plateau pressure to under 30 cmH2O, with PaCO2 values of under 60 mmHg.
Evolution of mechanical, respiratory and haemodynamic ventilation parameters during the first 3 days of therapy with Prismalung®.
| Pre | First hour | 6 h | 24 h | Second day | Third day | |
|---|---|---|---|---|---|---|
| TV (ml/kg) | 7 | 6 | 5 | 4 | 4 | 5 |
| PaCO2 (mmHg) | 78 | 54 | 59 | 62 | 61 | 58 |
| pH | 7.15 | 7.26 | 7.28 | 7.30 | 7.32 | 7.32 |
| Plateau pressure (cmH2O) | 38 | 34 | 31 | 28 | 28 | 24 |
| PaO2/FiO2 | 120 | 130 | 140 | 160 | 180 | 230 |
| PEEP (cmH2O) | 12 | 15 | 15 | 14 | 14 | 10 |
| BR (bpm) | 28 | 28 | 26 | 22 | 20 | 16 |
| ELWI (ml/kg) | 22 | 18 | 16 | 12 | ||
| MV mode | SIMV (PRVC) | SIMV (PRVC) + RM | SIMV (PRVC) + PS No RM | PS | ||
| Blood flow (ml/h) | 450 | 450 | 450 | 420 | 400 | 380 |
BR: breathing rate; ELWI: extravascular lung water index; FiO2: fraction inspired oxygen; MV: mechanical ventilation; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; PEEP: positive end-expiratory pressure; RM: recruitment manoeuvres; PS: pressure support; SIMV: synchronized intermittent mechanical ventilation; VRPC: volume regulated pressure control; VT: tidal volume according to ideal weight.
The Prismalung® system was removed after 72 h (sixth day after admission), but renal replacement therapy was maintained. Percutaneous tracheotomy was performed 24 h later, and amines were withdrawn, leaving sedation with dexmedetomidine 0.5 μg/kg/h, and pressure-support ventilation at 14 cmH2O and PEEP 8 cmH2O. At this stage, the patient presented isocoric, normorreactive pupils, with blink and cough reflexes, but no response to stimuli. Evoked potentials performed on the tenth day of admission showed bilateral absence of cortical responses, and the electroencephalogram obtained on the eleventh day of admission showed signs of grade IV encephalopathy, with a dismal prognosis. We decided to limit therapeutic effort, and the patient died within 24 h.
DiscussionThe Acute Respiratory Distress Syndrome Network4 clinical trial showed that reducing tidal volume to 6 ml/kg ideal weight was associated with a relative 22 % decrease in mortality. This, coupled with plateau pressures of less than 30 cmH2O, has been defined as "protective ventilation",5 and is one of the main goals of mechanical ventilation in this sepsis patients. Other authors subsequently suggested that reducing the mechanical stress caused by mechanical ventilation can further protect the lung, thus creating the concept of "ultra-protective ventilation", in which a tidal volume of 3−4 ml/kg appears to reduce pulmonary oedema and preserve, at least in part, alveolar and endothelial epithelial integrity. This strategy reduces the inflammatory response both at the pulmonary and plasma level.6 However, ultra-protective ventilation reduces alveolar ventilation, increases PaCO2, and promotes respiratory acidosis,7 which may limit its use in patients with chronic obstructive pulmonary disease, as in our case.8
The extracorporeal CO2 removal system used in this case consists of a polymethylpentene membrane with a 0.32 m2 gas exchange surface connected in parallel with a haemodiafiltration circuit, in our case the oXiris filter®, and connected to a rotameter to administered fresh gas. Blood is drawn passively from the arterial side or actively by a pump from the venous side to a gas exchange membrane, where fresh, CO2-free gas sweeps the hollow fibres and removes CO2 from the patient’s blood by diffusion. The device requires a blood flow of between 350 and 600 ml/h through 13–15.5 F cannulas to achieve the desired effect.
Review articles9 and clinical studies10 have been published on the combined use of both devices, particularly in patients with chronic obstructive pulmonary disease and in patients with ARDS under protective ventilation with secondary hypercapnia. Although the results of these studies should be evaluated with caution, the clinical evolution of most patients was favourable, and the number of days on mechanical ventilation was reduced; however, there is no evidence of a reduction in mortality.
After starting this therapy in our patient, we achieved PaCO2 values of between 54 and 62 mmHg by reducing the tidal volume to 4 ml/kg and plateau pressure to less than 30 cmH2O for the 72 h the treatment lasted.
Combining renal replacement therapy and ventilatory support in a single device can be beneficial in the management of ARDS patients, and the system can also be connected to endotoxin and cytokine adsorption systems in the case of sepsis, such as those used in this case.
More, high quality studies with a large sample size are needed to evaluate both the impact of this strategy on survival and the type of patient it would most benefit. Based the available literature, we recommend considering CO2 removal systems in patients with ARDS and PaCO2 above 65 mmHg that require renal replacement therapy. If there is no kidney failure, the system should be individualized, and we do not recommend it in patients with ARDS without hypercapnia or kidney failure.
ConclusionThe extracorporeal CO2 removal system facilitates protective ventilation that has been proven effective in patients with respiratory distress. Furthermore, the addition of an endotoxin and cytokine adsorption system in a single device is particularly useful in patients with sepsis
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Méndez Hernández R, Ramasco Rueda F, Planas Roca A. Eliminación extracorpórea de CO2 en un caso de síndrome de distrés respiratorio por sepsis. Rev Esp Anestesiol Reanim. 2020;67:35–38.