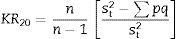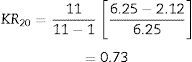Psoriatic arthritis screening tools are useful for the rheumatologist in optimizing referrals to the dermatology clinic. The Toronto Psoriatic Arthritis Screening Questionnaire (ToPAS) is a tool that has been developed and validated in Canada.
ObjectivesTo translate, and perform some validation tests on TOPAS for use in dermatology clinics in Colombia.
MethodsTranslation and validation of scales.
ResultsToPAS was translated using standardized methodology. A pilot test was conducted on 20 patients diagnosed with psoriasis and psoriatic arthritis using the final version of the document translated into Spanish, in order to evaluate items of aspects such as understanding, ambiguity, and response time. The tool was then used on 108 patients, including 65 (60.2%) males, with 36 (33.3%) cases with a diagnosis of psoriatic arthritis, and 72 (64.7%) with psoriasis only. Using a cut-off score >8 points, it showed a sensitivity of 75% and specificity 92%, positive predictive value 82% and negative predictive value of 88%. To assess the reliability test–retest, 25 participants were selected and Pearson correlation coefficient was calculated to evaluate the correlation between the first and second application of ToPAS, which gave a value of p=0.94, which represents a high level of correlation between the first and second application.
ConclusionsA translation, validation and evaluation of operational characteristics of the ToPAS questionnaire was conducted, being a practical tool for use in the dermatology clinic.
Los instrumentos de tamizaje de artritis psoriásica son una herramienta útil en la consulta dermatológica, para optimizar la remisión al reumatólogo. La herramienta Toronto Psoriatic Arthritis Screening Questionnaire (ToPAS) ha sido desarrollada y validada en Canadá.
ObjetivosTraducir y aplicar algunas pruebas de validación de la escala ToPAS para ser utilizada en la consulta dermatológica en Colombia.
MétodosEstudio de traducción y validación de escalas.
ResultadosSe llevó a cabo la traducción y validación del ToPAS con una metodología estandarizada, obteniendo un documento en español con el cual se realizó una prueba piloto del documento final en 20 pacientes con diagnóstico de psoriasis y artritis psoriásica, con el fin de evaluar los ítems en cuanto a aspectos como comprensión, ambigüedad y tiempo de respuesta. Posteriormente, el instrumento se aplicó a 108 pacientes, 65 (60,2%) eran hombres, 36 (33,3%) tenían diagnóstico de artritis psoriásica y 72 (66,6%) solo psoriasis. Se consideró como punto de corte un puntaje>8 puntos, encontrando una sensibilidad del 75% y una especificidad del 92%, valor predictivo positivo del 82% y valor predictivo negativo del 88%. Para evaluar la confiabilidad test-retest se seleccionaron los últimos 25 participantes, calculándose el coeficiente de correlación de Pearson para evaluar la correlación entre la primera y la segunda aplicación del ToPAS; el valor de p fue de 0,94, valor que representa un alto nivel de correlación entre la primera y la segunda aplicación.
ConclusionesSe realizó la traducción, validación y evaluación de características operativas de la encuesta ToPAS para detección de artritis psoriásica, constituyéndose en una herramienta práctica para uso en la consulta dermatológica.
Psoriatic arthritis is an inflammatory arthropathy associated with psoriasis1 that affects between 6 and 39% of patients with psoriasis, with a prevalence in the general population ranging from 0.02 to 0.2%.2 A delay in the diagnosis and treatment can lead to joint damage, deformity and functional sequelae.3 Given that the skin lesions usually precede the joint involvement in up to 10 years,4 the dermatologists are who usually make the initial assessment and handle the majority of cases of psoriasis.5 Taking into account that the manifestations of psoriatic arthritis go beyond the dermatological commitment, it becomes necessary an instrument for de evaluation of the patient, from the point of view of osteoarticular symptoms, which alerts the dermatologist about the possibility of psoriatic arthropathy and in this way improves the referral to the rheumatologist. Worldwide have been designed several psoriatic arthritis screening instruments, all of them in the English language, among which are included the questionnaires Psoriatic Arthritis Screening and Evaluation (PASE),6 Psoriasis Arthritis Questionnaire (PAQ),7 Psoriasis Epidemiology Project (PEST)8 and Toronto Psoriatic Arthritis Screening Questionnaire (ToPAS).9
The PASE6 was developed and validated by Abrar Qureshi in the clinic of skin and musculoskeletal diseases of the Brigham and Women's Hospital in Boston, Massachusetts. The population object of this study included patients between 18 and 85 years of age with a diagnosis of psoriasis or psoriatic arthritis, who were able to read and understand the scale in its original language (English). The PASE is a questionnaire of 15 questions divided into 2 groups; the first (7 questions), makes reference to the symptoms of the disease in relation to the presence of joint swelling or pain, and low back pain. The second group of questions (8 questions), refers to aspects of functionality. Each of the 15 questions is answered using the Likert scale (strongly disagree=1 point, disagree=2 points, neutral=3 points, agree=4 points, completely agree=5 points). The minimum score is 15 points and the maximum is 75. It was determined a cut-off score of 44 points, with which the PASE obtains a sensitivity of 76% and a specificity of 76%.
The PAQ questionnaire was presented in the form of abstract by Peloso and it was later modified by Gerd-Marie Alenius in the University Hospital of Umea in Sweden.10 The original PAQ consists of 10 questions with a minimum score of 1 point and a maximum score of 10 points, while in the PAQ modified by Alenius the minimum score is 1 point and the maximum is 8 points. The cut-off score is 4 points, with which is obtained a sensitivity of 60% and a specificity of 62.2%.
The PEST8 questionnaire was developed by Phillip Helliwell. The study and validation of this scale was carried out on patients with a diagnosis of psoriasis of the section of musculoskeletal diseases of the Chapel Allerton Hospital, attached to the University of Leeds in the United Kingdom. The PEST consists of 5 questions, obtained from the instruments PAQ7 and PAQ modified by Alenius,10 including an additional question that explores the presence of dactylitis in fingers or toes. Each affirmative question adds one point and a total of 3 or more affirmative answers is indicative of psoriatic arthritis, with a sensitivity of 97% and a specificity of 79%, positive predictive value of 65% and negative predictive value of 99%. As a particular feature, the PEST includes the drawing of a mannequin that allows the patient to identify joints or body areas in which he has suffered discomfort (edema, pain or stiffness).
The ToPAS9 questionnaire is a psoriatic arthritis screening tool for patients with psoriasis, but it has also been validated to be applied for the detection of psoriatic arthritis in the general population. The ToPAS was designed by Dafna Gladman from the Western Research Institute in Toronto, Canada. 5 groups were taken into account for the selection of the patients. The first included patients with a diagnosis of psoriatic arthritis, who were being followed-up at the Toronto Western Hospital; the second, patients with a diagnosis of psoriasis, who were followed-up at the Psoriasis Education and Research Center, where they received specific treatment for psoriasis and education about their disease; the third group included patients of the general dermatological clinic of the Toronto Western Hospital; the fourth group included patients under follow-up by rheumatology, excluding individuals with a diagnosis of psoriatic arthritis; and the fifth group included patients under regular follow-up by the clinics of family medicine of the Toronto Western Hospital and the Women's College Hospital. All patients were assessed by a rheumatologist following a standardized protocol, which included a complete medical history and physical examination, routine laboratory tests, rheumatoid factor and antinuclear antibodies; X-rays were performed only when there was a clinical suspicion of arthritis (joint pain or low back pain, limitations in the ranges of motion or joint deformities). The patients were diagnosed as cases of psoriatic arthritis according to the CASPAR criteria.11 The instrument is made up of 3 domains that assess cutaneous, ungueal and joint changes related to psoriatic arthropathy. The ToPAS contains a total of 14 questions (Annex). The first 10 have 2 answer options: affirmative (yes) or negative (no), within which are included questions about cutaneous changes, changes in the nail bed and osteoarticular symptoms. The last 4 questions are related to the personal history of psoriatic arthritis or other rheumatic diseases and a family history of psoriasis or psoriatic arthritis. For the first 3 questions there is a visual aid with photos of typical psoriatic skin lesions and psoriatic nail involvement (pits and onycholysis). With a cut-off point of 8, the ToPAS has a sensitivity of 86.8% and a specificity of 93.1%.
Given the operational characteristics previously described and because it is a practical tool and easy to fill out by the patient, and without a version translated into Spanish and validated, our group decided to translate and validate the ToPAS in Colombia. This work was carried out with the idea of obtaining a questionnaire in Spanish, which will be useful for the screening of psoriatic arthritis in the dermatological consultation in our country.
ObjectiveTo translate into Spanish and validate the ToPAS tool in a group of Colombian patients.
MethodsA study of translation and validation of scales was carried out. In the first phase of the study, the original ToPAS was translated into Spanish. This translation was done by 2 official translators (Spanish native language) and the resulting document was retranslated into English by 2 official translators (English native language), after which the 2 documents in English were compared against the original and the one that was more similar to the original questionnaire in English was chosen. Subsequently, a pilot test of the document chosen in Spanish was carried out, with 12 patients with a diagnosis of psoriasis and 8 patients with a diagnosis of psoriatic arthritis, in order to evaluate the items in terms of understanding, ambiguity and response time. In the second phase of the study, it was conducted the validation of the instrument (appearance validity, construct validity, content validity, usefulness testing), measurement of the operational characteristics and test–retest reliability.
Results108 patients participated in the study, 65 (60.2%) were men. Thirty-six (33.3%) had a diagnosis of psoriatic arthritis according to the CASPAR criteria and 72 (64.7%) had only psoriasis without articular involvement. The average age of the patients was 51.19 years (±18.9 years). The average evolution time of the patients with psoriasis was 31.46 (±17.3 years) and the average time of evolution of the patients with psoriatic arthritis was 5.96 years (±2.6 years).
A pilot test of the translated tool was conducted in order to evaluate the items and it was found an adequate understanding of the questions, no ambiguous terms or questions with affective load were detected, nor items with answers in a given direction in more than 95% of the times; there was not extreme aversion bias. For the evaluation of the appearance validity of the instrument, a group of 5 patients, 2 expert rheumatologists and one expert dermatologist analyzed the scale and determined that the items measured the concepts that were intended to be measured (psoriatic arthritis and psoriasis). For the evaluation of the construct validity, given the non-availability at that time of a scale translated into Spanish and validated that would measure the same concept, it was decided to compare it against the classification criteria of CASPAR, which evaluate the same concept, and the operational characteristics of the scale for the diagnosis of psoriatic arthritis were measured. As defined in the original article that gave rise to the ToPAS tool, a score >8 points was considered as the cut-of point. When evaluating the patients, taking as gold standard the assessment by the expert rheumatologist that coincided with the diagnosis of psoriatic arthritis according to the CASPAR criteria, the Spanish version of the ToPAS tool had a sensitivity of 75% and a specificity of 92%, positive predictive value of 82% and negative predictive value of 88%.
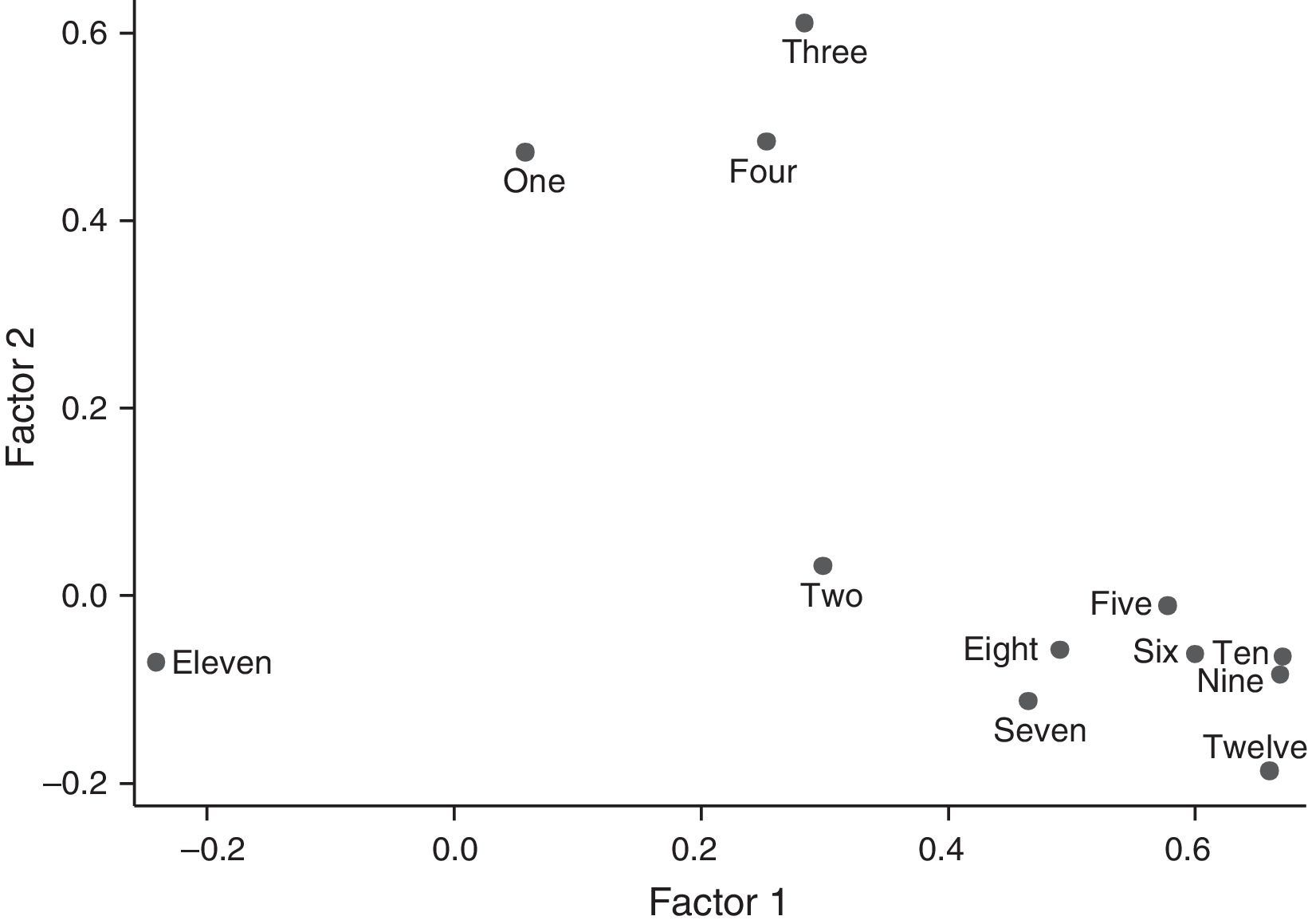
An exploratory factor analysis was performed for the content evaluation. For the factor analysis, the 2 first factors were taken, because they had the highest eigenvalues. From the factor analysis it can be concluded that items 1, 3 and 4 would be forming a domain for the scale, determined by the dermatological variables, since they are best represented in factor 1, and that items 2, 5, 6, 7, 8, 9, 10 and 12, the other domain, because it is best represented in factor 2, which corresponds to the rheumatological characteristics. Although the question 11 is not clearly identified with the foregoing domains because this item does not question on symptoms but about the presence of some type of arthritis (Fig. 1).
In this study it was not possible to evaluate the sensitivity to change, since the patients were not followed-up in the long term, which would allow to detect a modification in their clinical condition, which for the purpose of the instrument would be the development of a clinical picture of psoriatic arthritis, outcome that can take years to appear.
Regarding the reliability tests to evaluate the internal consistency or homogeneity, the Kuder–Richardson formula 20 was applied to assess the reliability of the scale, based on the data obtained in the first application of the test to the 108 patients.
where, n: total number of items; st2: variance of the total scores; p: proportion of subjects who passed one item divided by the total of subjectsq=1−p
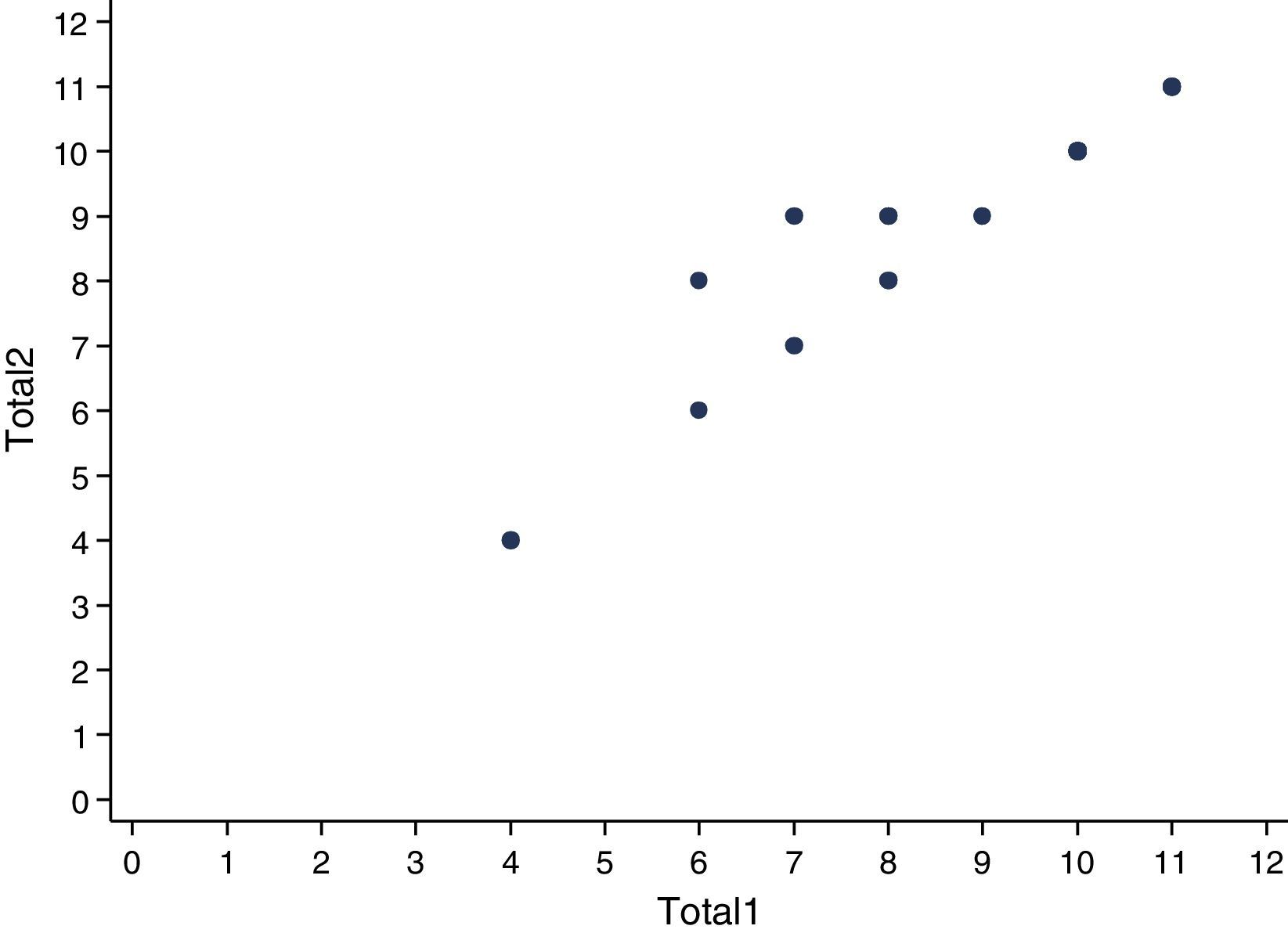
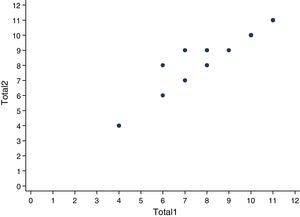
The test–retest reliability was evaluated in order to assess the stability of the scale over time; for this purpose were selected the last 25 participants, calculating the Pearson correlation coefficient to evaluate the correlation between the first and the second application of the ToPAS (with a month of difference between both), obtaining a value that indicates a very high positive correlation (r=0.94) (Fig. 2). Since the ToPAS scale is self-administered, the measurement of the inter-rater reliability is not applicable.
As for the evaluation of the usefulness of the scale, the average response time was 3min, no previous training was required to fill out the scale and its rating is easy and it does not require complex mathematical methods. The application of the tool was quick with an average duration of 3min per patient.
DiscussionThe human resource in different medical specialties in Colombia is limited; specialties such as dermatology, and especially rheumatology, have a small number of specialists in our country. Taking into account that the patients with psoriasis initially consult to dermatology, since in 75% of cases the skin lesions precede the articular manifestations,4 it becomes useful and necessary to have an instrument that allows to optimize the referral to the rheumatologist. Several screening tools for psoriatic arthritis have been designed, such as PASE, PEST, PAQ and ToPAS. Our research group, after evaluating the operational characteristics of each of these screening instruments, decided to choose the ToPAS for its translation and validation in a population of Colombian patients. As in the original work of the ToPAS, despite being a screening tool, it was obtained an instrument with a specificity value higher than its own sensitivity, keeping these operational characteristics with values similar to the original instrument, and with adequate test–retest reliability. Compared with the Spanish version of the PASE (screening and evaluation questionnaire) translated by the group of Dr. Soriano,12 our instrument had a similar sensitivity (PASE: 76%, ToPAS: 75%) with a higher specificity (PASE: 74.4%, ToPAS: 92%). The ToPAS instrument in Spanish does not replace the assessment by the rheumatologist and, on the contrary, seeks to improve the referral from the dermatological consultation, with the idea of identifying early cases of psoriatic arthropathy, and in this way, achieving an early diagnostic and therapeutic intervention, that ultimately results in a better care to the patient with psoriasis and suspicion or diagnosis of psoriatic arthropathy.
ConclusionThe translation, validation and evaluation of operational characteristics of the ToPAS survey for the detection of psoriatic arthritis were carried out, making it a practical tool for use in the dermatology clinic.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they do not have any conflict of interest.
Thanks to the Colombian Association of Rheumatology for its support through a research grant with which the present research work was partially funded.
Please cite this article as: Fernández-Ávila DG, Beltrán A, González C, Castro L, Rincón-Riaño DN, Díaz MC, et al. Traducción y validación de la versión en español del cuestionario ToPAS (Toronto Psoriatic Arthritis Screening Questionnaire), para el tamizaje de pacientes con artritis psoriásica en la consulta dermatológica en Colombia. Rev Colomb Reumatol. 2017;24:79–83.