Gastrointestinal involvement in patients with systemic lupus erythematosus (SLE) is very diverse, and the frequency of occurrence and location along the digestive tract varies widely. Inflammatory processes secondary to immune complex deposits or vascular events may cause this involvement. One of the most characteristic gastrointestinal manifestations in these patients is the intestinal pseudo-obstruction, which is defined as the ineffective intestinal propulsion that occurs in the absence of mechanical or obstructive factors. This is, however, a rare and poorly understood complication of SLE. The case is presented of a male SLE patient presenting with intestinal pseudo-obstruction, and was successfully treated with steroids and intravenous immunoglobulin. A complete review of the literature and a proposal for the pathophysiology of intestinal pseudo-obstruction are presented.
El compromiso gastrointestinal en pacientes con lupus eritematoso sistémico (LES) es muy diverso. Su frecuencia y ubicación a lo largo del tracto digestivo varían ampliamente. Los procesos inflamatorios secundarios a los depósitos de complejos inmunes o eventos vasculares pueden ser los causantes de este compromiso. Una de las manifestaciones gastrointestinales características en los pacientes con LES es la pseudoobstrucción intestinal, que se define como la propulsión intestinal ineficaz que se produce en ausencia de factores mecánicos u obstructivos. Esta es, sin embargo, una complicación rara y poco entendida del LES. En este artículo, reportamos el caso de un paciente masculino con diagnóstico de LES y pseudoobstrucción intestinal, que fue tratado exitosamente con esteroides e inmunoglobulinas intravenosas. Se presenta una revisión completa de la literatura y una propuesta de la fisiopatología de la manifestación.
Gastrointestinal involvement in patients with systemic lupus erythematosus (SLE) is very diverse. Its frequency and location along the digestive tract vary widely. Inflammatory processes secondary to deposits of immune complexes or vascular events may be the causes of the commitment.1 Oral lesions, esophageal dysmotility, mesenteric vasculitis and protein-losing enteropathy are the most common manifestations.2
Intestinal pseudo-obstruction (IPO) is defined as the ineffective intestinal propulsion resulting from impaired functioning of the visceral smooth muscle, the enteric nerves or the visceral autonomic nervous system.3 In many cases, the origin is primary, but secondary causes may occur, including certain neurological, endocrine and connective tissue diseases. The causes included in the latter group had been reported, mainly, in patients with systemic sclerosis.4 In contrast, the association with SLE has been reported in few cases in the literature in English language around the world. This entity has been recently recognized as a rare and poorly understood complication of SLE.1,5 In this article is presented the case of a male patient with SLE who had IPO and was successfully treated with steroids and intravenous immunoglobulin.
Case reportWe report the case of a 28-year-old man with a history of SLE, diagnosed 3 months prior to admission because a systemic clinical picture characterized by acute abdomen secondary to appendicitis treated with an emergency laparotomy, with development of postoperative ascites and edematous syndrome with evidence of type iv lupus nephritis confirmed by renal biopsy, with arthritis and neurological involvement manifested as a convulsive episode without any other etiology. The immunological tests confirmed the diagnosis of SLE (positive antinuclear antibodies, positive anti-Sm and anti-double stranded DNA antibodies and hypocomplementemia). At this time the patient was treated extra-institutionally with 500mg of intravenous cyclophosphamide every 2 weeks and, subsequently, with 2g of mycophenolate mofetil every 24h, 50mg of losartan every 12h, 30mg of prednisolone every 24h and 250mg of chloroquine every 24h. The patient was referred to our institution because of persistence of the abdominal pain in the lower hemiabdomen, diarrhea without dysentery and dysuria.
Upon admission to our institution, the patient was stable, with the following positive findings on physical examination: hypoventilation on the pulmonary auscultation of both bases and diffuse pain with abdominal palpation, with no evidence of signs of peritoneal irritation. The admission laboratory tests are shown in Table 1.
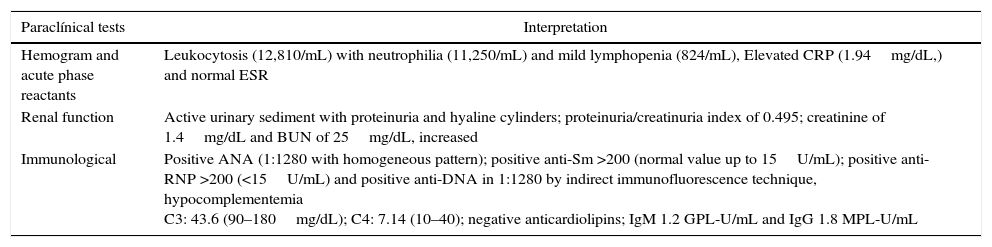
Admission laboratory tests of the patient.
| Paraclínical tests | Interpretation |
|---|---|
| Hemogram and acute phase reactants | Leukocytosis (12,810/mL) with neutrophilia (11,250/mL) and mild lymphopenia (824/mL), Elevated CRP (1.94mg/dL,) and normal ESR |
| Renal function | Active urinary sediment with proteinuria and hyaline cylinders; proteinuria/creatinuria index of 0.495; creatinine of 1.4mg/dL and BUN of 25mg/dL, increased |
| Immunological | Positive ANA (1:1280 with homogeneous pattern); positive anti-Sm >200 (normal value up to 15U/mL); positive anti-RNP >200 (<15U/mL) and positive anti-DNA in 1:1280 by indirect immunofluorescence technique, hypocomplementemia C3: 43.6 (90–180mg/dL); C4: 7.14 (10–40); negative anticardiolipins; IgM 1.2 GPL-U/mL and IgG 1.8 MPL-U/mL |
It was requested a renal ultrasound which showed diffuse bilateral enlargement, with bilateral pyelocaliceal dilatation and diffuse urothelial thickening. A magnetic resonance imaging of the brain was performed, which evidenced 2 hyperintense lesions located in the left frontal lobe in the FLAIR and T2 sequences, with diffusion restriction.
The transthoracic echocardiogram showed concentric hypertrophy of the left ventricle with an ejection fraction of 50–55% and a global pericardial effusion without hemodynamic repercussion. The colonoscopy evidenced mucosal edema in the descending colon with negative histopathological findings. The Systemic lupus erythematosus disease activity index (SLEDAI) at admission was high (value: 16). Due to the high activity of the SLE and the multisystemic involvement, the patient required management with pulses of methylprednisolone (1000mg every 24h) for 3 days, with initial clinical improvement. The evolution of the patient became torpid due to the presence of lower urinary tract symptoms, with a cystoscopy which evidenced trabeculation of the posterior wall of the bladder, with a bladder neck posterior lesion and a diverticulum in the left ureteral meatus.
Since the patient presented recurrent emesis, abdominal distention and, subsequently, constipation and severe abdominal pain, an endoscopy of the upper gastrointestinal tract was carried out, which showed gastric contents with retention of bile (800cc). These findings were interpreted as adynamic ileus.
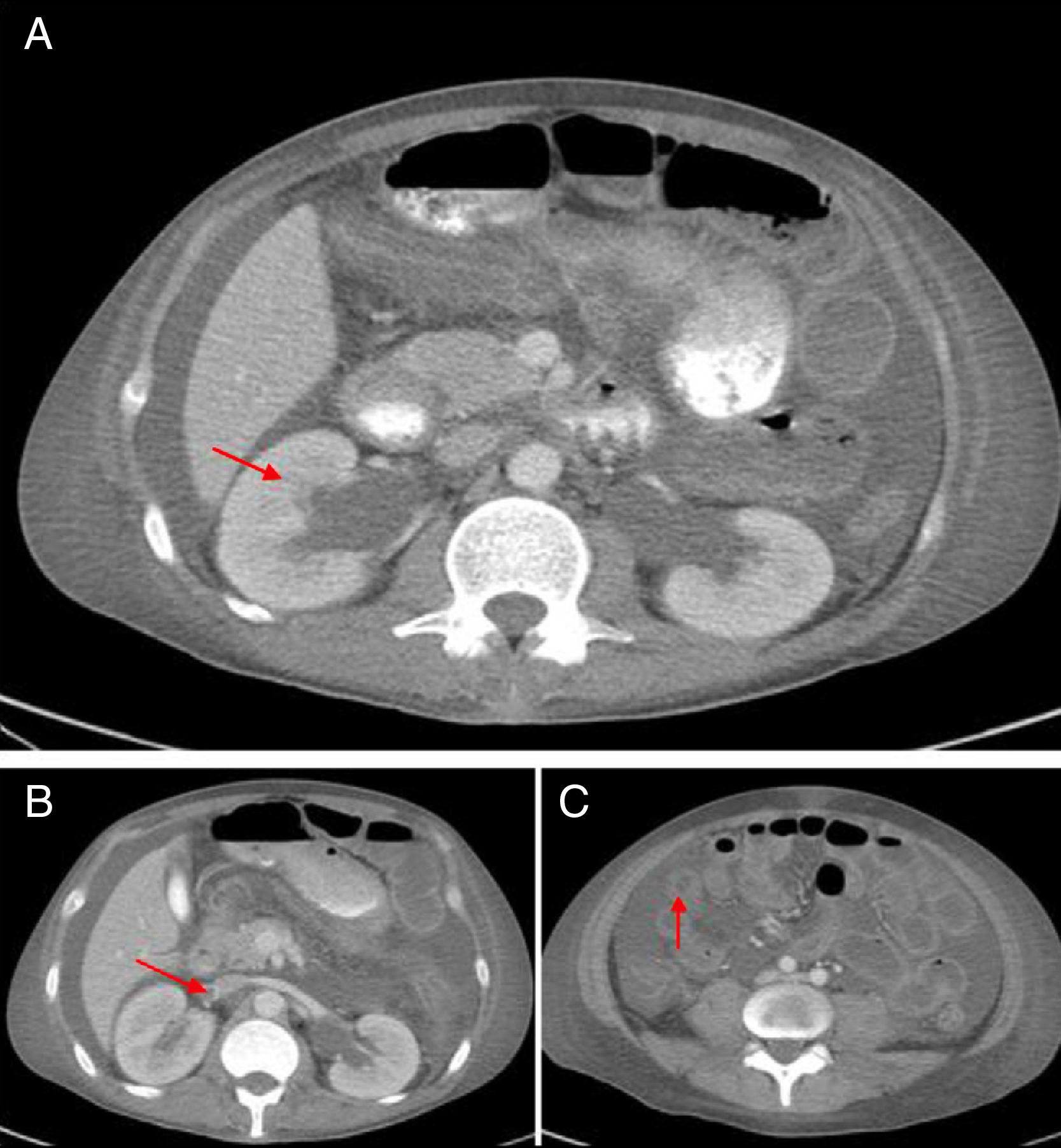
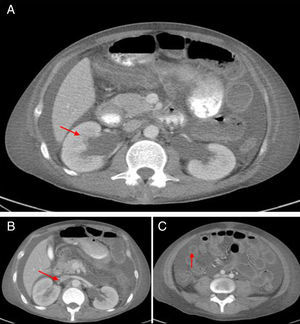
The abdominal CT scan with contrast (Fig. 1) showed hepatomegaly and splenomegaly, ascites, signs of intestinal vasculitis, mild bilateral hydronephrosis and an image suggestive of thrombosis of the right renal vein, which was verified by Doppler echography. Because of this, anticoagulation was started and it was administered a fourth dose of cyclophosphamide. Despite these treatments, the patient continued with poor clinical response, with increased abdominal pain, constipation and the need of high doses of opioids to control the pain. Given the possibility of opioid-induced ileus, an ischemic complication due to intra-abdominal thrombosis or bleeding due to an over-anticoagulation with warfarin, the patient was transferred to the Intensive Care Unit. A new abdominal CT scan revealed a stenosis of the proximal segment of the ileum with a pattern of partial obstruction, without signs of perforation. Due to the evidence of disease activity, it was established the diagnosis of IPO secondary to SLE, refractory to immunosuppressive management (steroids and cyclophosphamide), and for this reason it was decided to start management with intravenous immunoglobulins, total dose of 2g/kg. The patient showed an excellent clinical response with resolution of the gastrointestinal involvement in the course of one week. In the subsequent clinical follow-up the patient has not presented relapse of the intestinal disease, with reduction of doses of steroids up to 5mg of prednisolone daily.
Discussion and literature reviewA systematic literature search was conducted, using the database Medline PubMed and secondary references of articles published until July 2016, with the key terms “lupus erythematosus, systemic”, AND “intestinal pseudo-obstruction” OR “idiopathic intestinal pseudo-obstruction” OR “pseudoobstructive syndrome” OR “paralytic ileus” OR “enteric neuropathy” OR “visceral myopathies” AND “hydronephrosis” without restriction of language. Case reports, case series and case-control studies which met the diagnostic criteria of the American College of Rheumatology of 1997 or 2012 and which included acute or chronic IPO were selected.6
The following review and discussion is based on our case and the comparison with the patients presented in the review of cases of Jin et al.,7 and in the case-control studies of Xu et al.8 and Zhang et al.9
Gastrointestinal involvement in SLE has a frequency that can reach 50% of the patients. These manifestations are usually described as symptomatic commitment, rather than as defined clinical entities. Traditionally, the most common symptoms are nausea and vomiting (53%), anorexia 49% and abdominal pain (19%).10 Sometimes these symptoms are attributed to adverse effects of the medications.2
There are also gastrointestinal conditions that can be life threatening, such as acute abdomen, which is produced by mesenteric vasculitis, hepatobiliary disease, gastroenteritis and appendicitis with a mortality rate ranging between 9.4 and 11% of cases. Other manifestations to be considered include the alteration of esophageal motility, dyspepsia, peptic ulcer, pernicious anemia due to the presence of antibodies against intrinsic factor, intestinal cystic pneumatosis, protein-losing enteropathy, primary lupus peritonitis and ascites.10
IPO is a rare clinical syndrome characterized by ineffective intestinal propulsion with clinical and radiological evidence of intestinal obstruction, but in the absence of an identifiable mechanical lesion.11 The pathogenesis of this syndrome is unknown, although there is histopathological evidence of damage of leimyocytes indicating a systemic autoimmune process targeted to the smooth muscle cells. Another postulated mechanism is the vasculitis, which triggers a chronic ischemia of the intestinal smooth muscle with the consequent muscle damage and hypomotility. Other authors postulate an intrinsic muscular dysmotility (caused by myopathic or neurogenic damage) that affects the muscularis propria, this based on the existing high association between IPO and ureterohydronephrosis.1,4,10,12,13
As more cases are reported in the literature there are more data available about the epidemiology of this manifestation. To date there are 173 cases reported in the literature in the English language. Derived from these cases, it is known that this manifestation has a female predominance (n=163; 94.21%).7–9 In about 50% of cases, the IPO occurs as an early manifestation of SLE (which is the initial manifestation in a large number of cases up to one year before the diagnosis of the SLE).1,8–10 The symptoms of IPO include the acute or subacute onset of abdominal pain, nausea, vomiting, abdominal distension, constipation, diarrhea and weight loss. The small intestine is more frequently involved than the large intestine.12,14
IPO can be classified as acute or chronic according to its duration. The acute form is defined as the onset of one or more symptoms of IPO<6 months before the diagnosis of the IPO, which is the most common form in patients with SLE (n=99; 57.23%). The chronic IPO is defined as the onset of one or more symptoms at least 6 months before the diagnosis (n=74; 42.77%).1,3,8,9,12,15
Likewise, there is an apparent association between IPO related with SLE and urological manifestations. For example, ureterohydronephrosis was present in 103 cases (59.53%) and it has been previously reported in other series in up to 66.7% of the cases of patients with IPO associated with SLE.12 This urological commitment is the initial symptom in 32.5% of patients or it may complicate the evolution of the patient with IPO, which may occur in a time range between one month and 10 years, with a mean onset time of 2.6 years.8 Another urologic manifestation is the chronic interstitial cystitis, a condition that is pathophysiologically associated with deposition of immune complexes. This last manifestation occurred in only 7 cases (4.05% of the patients). Its low frequency is explained by the asymptomatic nature of this presentation, which reaches up to 35% of cases, and also by the low rate of cystoscopies performed.8
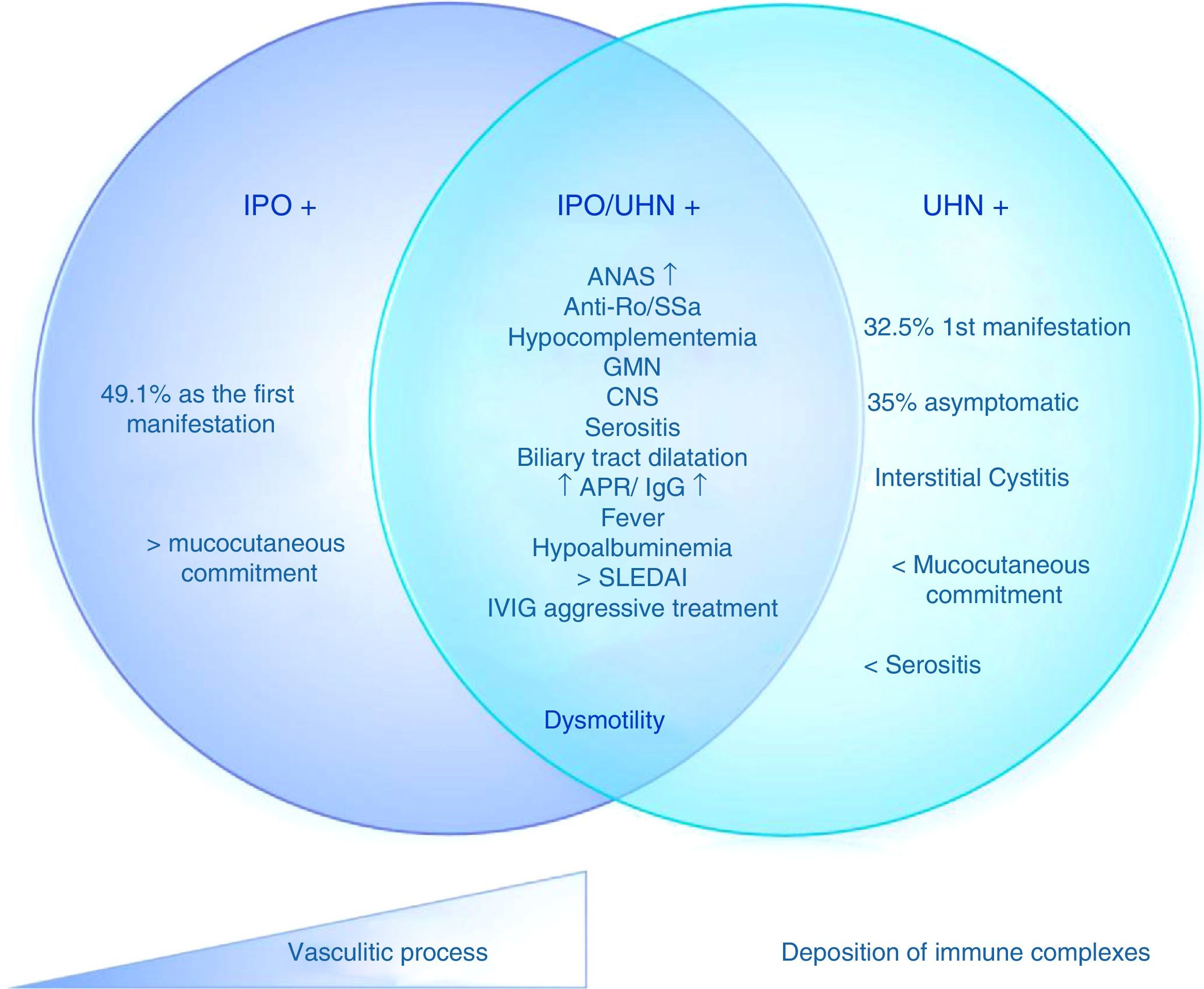
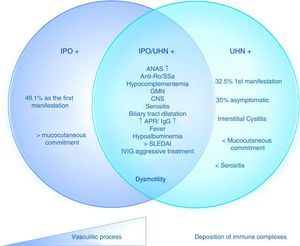
Based on these data, it is possible to determine 3 different subgroups of patients with SLE and IPO, with the same pathogenic mechanism and different degrees of commitment. 1) Isolated IPO; 2) IPO with ureterohydronephrosis; 3) ureterohydronephrosis (Fig. 2). These manifestations may be due, then, to a vasculitis with progressive smooth muscle involvement that leads to dysmotility or to a different spectrum of the syndrome, in which one pathophysiological process predominates over another (for example, the deposition of immune complexes in interstitial cystitis or the presence of progressive vasculitis in the IPO with or without hydronephrosis).
Proposed pathophysiology of the mechanisms of intestinal pseudo-obstruction in patients with systemic lupus erythematosus.
ANA: antinuclear antibodies; GMN: glomerulonephritis; IVIG: intravenous immunoglobulins; IPO: intestinal pseudo-obstruction; APR: acute phase reactants; SLEDAI: systemic lupus erythematosus disease activity index; CNS: central nervous system; UHN: ureterohydronephrosis.
Other organs that can be compromised in IPO are the esophagus3 and the biliary tract.1 The commitment of the biliary tract can trigger multiple complications such as cholecystitis, cholangitis and sepsis. The most severe compromise of the dysmotility syndrome is the so-called generalized megaviscera of lupus, which corresponds to the presence of visceral dilatation and dysfunction, which coexists in more than one organ in patients with SLE, with IPO as the most common manifestation17; there are 9 cases reported in the literature.8,16–18
The definitive diagnosis of IPO is based on the signs and symptoms of intestinal obstruction, evidence of intestinal obstruction in the abdominal X-ray and CT images, without any evidence of anatomical or structural abnormalities.2,10,13 The functional studies, such as antroduodenal manometry, show intestinal hypomotility and esophageal aperistalsis.13
The laboratory findings are nonspecific and there are not specific antibodies in patients with SLE and IPO with or without ureterohydronephrosis. Even the antibodies profile reported data is incomplete or not provided in some patients, but, when they are reported, there is a higher prevalence of positive anti-Ro/SS-A antibodies (n=110; 63.58%) compared with other autoantibodies such as anti-La/SS-B (n=62; 35.83%), RNP (n=29; 16.76%) (only reported in 87 cases) and anti-Sm (n=26; 15.03%) (only reported in 87 cases).
The prevalence of Ro/SS-A in IPO is higher than the prevalence of SS-A (30%) and SS-B (10%) in SLE,8 indicating that the anti-Ro/SS-A antibodies could play a pathological role in muscular dysmotility.6 Interestingly, the presence of anti-Ro antibodies does not result in a higher rate of secondary Sjögren's syndrome, which has been reported in only 13 cases (7.5% of the patients).8
Regarding the commitment of other organs, renal involvement has been reported, manifested as glomerulonephritis in 52 patients (30.05%), nephrotic syndrome in 14 (8.09%) and hematological involvement in 102 (58.95%), which has shown the highest prevalence. Mucocutaneous commitment is not frequently reported, but in case-control studies it has been described one of 68.9%.8
Other relevant systemic commitments with frequent reports in the literature include: ascites/serositis in n=117 (67.63%); arthritis in n=66 (38.15%) and involvement of the central nervous system in n=28 (16.18%). The SLEDAI was reported in the article of Xu et al.,8 and it had an average of 12.4±5.3 with a p-value of 0.012, related with the manifestation of IPO. In the study of Zhang et al.,9 the SLEDAI had an average of 12.1±0.8 with a p-value of 0.017.9 Despite the association with statistical significance, IPO and ureterohydronephrosis can occur with a low disease activity index, with a SLEDAI score ranging from 5 to 10 (22.9%) and <5 (6.6%), even in a stable state of commitment of another organ.8
In terms of treatment, the common denominator is the use of high doses of intravenous steroids in pulses as the basis of the therapy (n=45; 52.33%) (the management was described only in 86 patients). Almost all patients in the revised series responded rapidly to the therapy with steroids. Intravenous immunoglobulins were used in cases refractory to management with steroids, with a good clinical response (n=9; 10.46%).
Along with the steroids, other immunosuppressive agents have been used in 66 cases (76.74%). Among the immunossuppressive drugs most frequently used we found azathioprine, oral cyclosporine and mycophenolate mofetil, which are used with good results. The choice between one or the other is subject to other types of systemic affectation such as nephritis or brain involvement.
At the same time, supportive measures such as parenteral nutrition, oral broad-spectrum antibiotics to decrease bacterial overgrowth and pharmacological stimulation of intestinal motility with cisapride, octreotide and erythromycin (antibiotic with prokinetic properties) should be used.10,13 The doses of the therapy timely and early are essential to avoid relapses and the development of adhesions which complicate the clinical course of the SLE. This is also essential to rehabilitate the visceral peristaltic activity of the gastrointestinal and genitourinary tracts.
The long-term outcomes of SLE-related IPO are variable. Some patients may have recurrent attacks of IPO without commitment of other organs despite the maintenance of the therapy with steroids and other immunossuppressive agents. The mortality rate is 23.52%, due to the development of fungal peritonitis and gastrointestinal bleeding.8,12,19–22
In conclusion, the IPO is an uncommon manifestation of SLE that should be recognized by the physicians because of its potential complications. It can also be the first manifestation of SLE. It represents a real challenge for doctors, since the clinical picture of the patient can be confused with other etiologies of intestinal obstruction and the patient can be taken to unnecessary surgical treatments. A proper diagnosis and treatment will determine the prognosis of the patient.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Echeverry AA, Hormaza AA, Betancur JF, Cañas CA, Posso-Osorio I, Tobón GJ. Tratamiento exitoso con inmunoglobulinas intravenosas en un paciente con pseudoobstrucción intestinal asociada a lupus eritematoso sistémico. Rev Colomb Reumatol. 2017;24:123–128.