Vitamin D deficiency is considered a worldwide epidemic, with an estimated one billion people suffering from this condition. According to different studies, 100% of adult population of the United States and Europe have this condition, and has been considered as a causal factor in many diseases, such as osteoporosis. In Colombia, there are few studies that have estimated the prevalence in people with osteoporosis.
OutcomeTo determine the prevalence of vitamin D deficiency in a population of patients with osteoporosis treated in a high complexity clinic in Colombia.
Materials and methodsRetrospective descriptive study of the diagnosis of osteoporosis and vitamin D deficiency in an outpatient clinic of different Internal Medicine specialities in a high complexity hospital in Cali, Colombia in the year 2013–2014. Sociodemographic characteristics, medical history, vitamin D with parathormone levels, and bone densitometry results were collected. The prevalence of vitamin D deficiency in patients with osteoporosis was determined.
ResultsA total of 206 patients with osteoporosis were included, of which 114 had a low 25 hydroxycholecalciferol level. The prevalence of vitamin D deficiency was 55.3%. The mean vitamin D level was 22ng/ml (p=0.00), with bone densitometry reports that showed a mean T score of −2.1 in the spine (p=0.55), and −1.7 at the femoral neck (p=0.00).
ConclusionLow vitamin D levels have been identified as one of the factors associated with osteoporosis. In a south-western region of Colombia a population with osteoporosis showed a prevalence of 53% of low vitamin D levels, lower than other results reported in studies made in other regions of Colombia.
La insuficiencia de vitamina D se considera una epidemia mundial. Se estima que un billón de personas a escala mundial padecen de insuficiencia de vitamina D. Según diferentes estudios el 100% de la población adulta mayor de Estados Unidos y Europa presentan esta condición, la cual se ha tratado de atribuir a la hipovitaminosis D, como factor causal de muchas patologías, entre ellas a la osteoporosis, por su rol esencial en el metabolismo del calcio y en la prevención de fracturas. Se ha tratado de establecer su frecuencia a escala mundial y en Colombia son pocos los estudios que han estimado la prevalencia en población con osteoporosis.
ObjetivoDeterminar la prevalencia de la insuficiencia de vitamina D en una población de pacientes con osteoporosis, atendidos en una clínica de alta complejidad en Colombia.
Materiales y métodosEstudio retrospectivo y descriptivo que incluía pacientes atendidos en la consulta externa, en las diferentes especialidades de medicina interna, en un hospital de alta complejidad en Cali, Colombia, entre los años 2013 a 2014, con diagnóstico de osteoporosis e insuficiencia de vitamina D. Se describieron las características sociodemográficas, antecedentes médicos y resultado de niveles de vitamina D, parathormona, densitometría ósea. Se determinó la prevalencia de déficit de vitamina D en pacientes con osteoporosis.
ResultadosSe incluyeron 206 pacientes con diagnóstico de osteoporosis, de los cuales 114 presentaron insuficiencia de 25-hidroxicolecalciferol, para una prevalencia de 55.3%. El promedio de niveles de vitamina D fue 22ng/ml (p=0.00), con reportes de densitometría ósea que para el momento del estudio presentaban un promedio de T score de –2.1 en columna vertebral (p=0.55) y T score de –1.7 en cuello femoral (p=0.00).
ConclusionesLa insuficiencia de vitamina D se ha determinado como uno de los factores asociados con osteoporosis. En una población con osteoporosis del suroccidente colombiano, la prevalencia de la insuficiencia de vitamina D fue de 53%, más baja que la reportada en otras ciudades de Colombia.
Osteoporosis is a progressive systemic bone disease, characterized by a low bone mass that causes increased bone fragility and fracture susceptibility.1 Among the risk factors, vitamin D plays an essential role since it takes part in the bone mineralization; low levels of 25-hydroxyvitamin D (25-OH vitamin D) have been associated with fractures,1,2 and supplementation with vitamin D (at least 800 units per day) prevents hip and non-vertebral fractures in individuals aged 65 years or older.3
The global prevalence of vitamin D deficiency is high, up to 86% in patients with osteoporosis.1,4 Vitamin D deficiency is considered a worldwide epidemic. It is estimated that, in the world, one billion people suffer from vitamin D deficiency. According to different studies, 100% of the older adult population of the United States and Europe has this condition.5 In the NHANES III study which included 18,883 patients, of whom 9491 were older than 40 years, vitamin D deficiency was observed in 50% of men and in 40% of women.6,7
It has been considered that people residing in regions close to the equator, exposed to the sun without protection, have sufficient levels of vitamin D. However, studies conducted in Turkey and Australia show the opposite with vitamin D levels of 17ng/ml and <20ng/ml, respectively.8,9 The reference values of 25-OH cholecalciferol for different populations are not standardized; it has been proposed a spectrum of serum levels of vitamin D, where individuals with levels below 10ng/ml are considered deficient, while those who have levels lower than 30ng/ml are considered insufficient or inadequate.10,11 The purpose of this study is to determine the prevalence of vitamin D deficiency in a population of patients with osteoporosis treated in a high complexity clinic in Colombia.
Materials and methodsIt was conducted a retrospective descriptive study in patients with current or previous diagnosis of osteoporosis, treated in the Foundation Valle del Lili, a high complexity institution located in the city of Cali, Colombia, at 1000m above sea level. In this institution are treated patients coming from the Southwest of Colombia. For the study were eligible the patients with new or previously confirmed diagnosis of osteoporosis by the M80, M81, M82 codes of the ICD-10, obtained from the records of medical histories of the hospital and outpatient units of medical subspecialties, within the period from June 2013 to June 2014; and were included those individuals who had a report of vitamin D levels, measured in the institutional laboratory as 25-hydroxyvitamin D (D2 and D3 cholecalciferol) by electrochemiluminescence, history of osteoporosis and report of bone densitometry in the clinical history, within the 6 months before obtaining the vitamin D levels.
A calculation of the sample size was made according to the population treated in this year, with a prevalence of vitamin D deficit reported in the literature of 64%, confidence level 95%, accuracy 5%, for a total of 250 patients. A simple randomized sampling was conducted to select the patients and it was built a database for the electronic registration in the Microsoft® Excel® 2013 program. Demographic variables such as age, sex, type of affiliation to a health care system, medical antecedents such as arterial hypertension, diabetes menopause, presence of rheumatologic, respiratory, or neurological diseases, use of medications related to osteoporosis, and previous fractures were included. Likewise, physical examination variables such as weight, height, body mass index, blood pressure readings, laboratory studies including levels of creatinine, TSH (thyroid stimulating hormone), calcium, phosphorus, parathyroid hormone levels, levels of vitamin D and findings in the bone densitometry.
Osteoporosis was defined according to the World Health Organization criteria as a bone mineral density (BMD) lower than −2.5 standard deviations in the T scale, and severe or established osteoporosis when fragility fractures were present. For individuals younger than 50 years, osteoporosis was defined as a bone densitometry with a Z score less than −2.0 standard deviations or the presence of fracture. The presence of vitamin D deficiency was determined at serum levels of 25-0H D lower than 30ng/ml. For the statistical analysis, the quantitative variables were reported as means or medians, measures of dispersion, standard deviation and interquartile range in accordance with the compliance of assumptions of normality. The categorical variables were described as the absolute value and the percentage. Frequency tables were created according with the presence or absence of vitamin D deficiency. Each variable was compared using the Student's t test or the Wilcoxon Mann–Whitney for the quantitative variables and with the Chi-square or the Fisher's exact tests for the categorical variables according to the compliance with assumptions. This study was approved by the Ethics Committee for Institutional Biomedical Research.
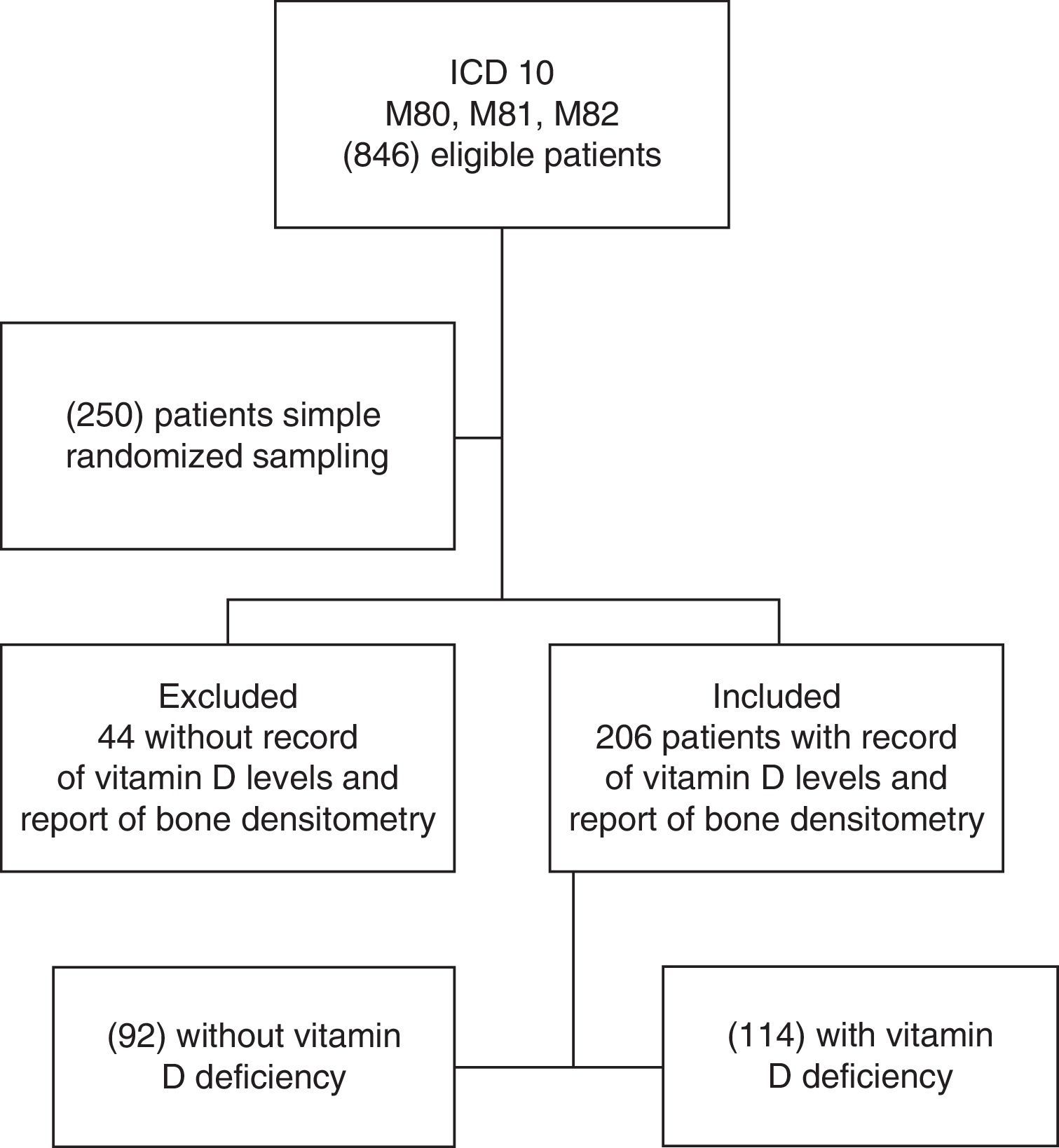
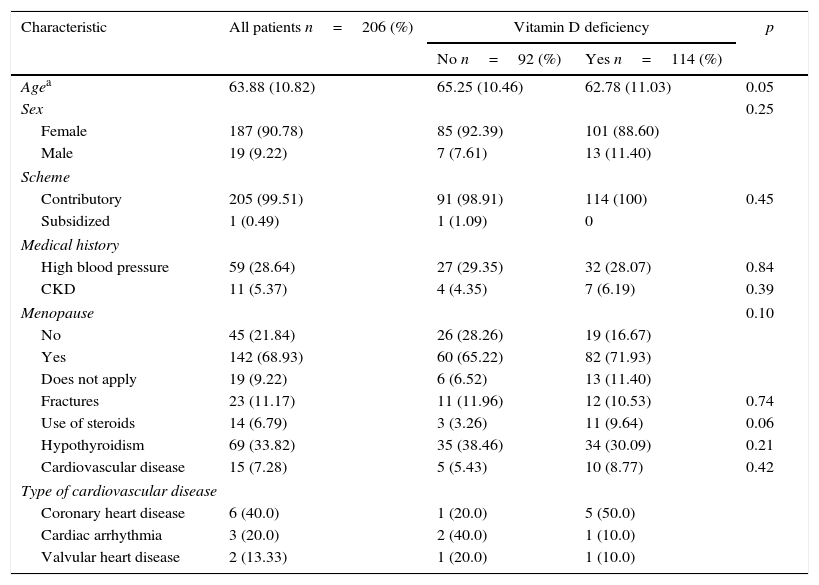
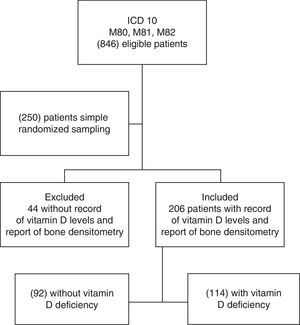
Results846 individuals with previous diagnosis of osteoporosis were found in the hospital records within the period from June 2013 to June 2014. Then, 250 records were selected by simple randomized sampling, of which 206 patients had a confirmed diagnosis of osteoporosis and a report of serum levels of 25-OH vitamin D (Fig. 1). The prevalence of vitamin D deficiency in patients with osteoporosis was 55.3% (114 of 206 patients), of which 39.4% were already receiving supplementation of vitamin D orally. The characteristics of the population are represented in Table 1. The average age was 63 years, with predominance of the female gender (90.78%), and 99% of individuals belonged to the contributory scheme. Among women, the prevalence of vitamin D deficiency was 54.30%, and 65% in men.
General characteristics and medical history of the patients with osteoporosis.
| Characteristic | All patients n=206 (%) | Vitamin D deficiency | p | |
|---|---|---|---|---|
| No n=92 (%) | Yes n=114 (%) | |||
| Agea | 63.88 (10.82) | 65.25 (10.46) | 62.78 (11.03) | 0.05 |
| Sex | 0.25 | |||
| Female | 187 (90.78) | 85 (92.39) | 101 (88.60) | |
| Male | 19 (9.22) | 7 (7.61) | 13 (11.40) | |
| Scheme | ||||
| Contributory | 205 (99.51) | 91 (98.91) | 114 (100) | 0.45 |
| Subsidized | 1 (0.49) | 1 (1.09) | 0 | |
| Medical history | ||||
| High blood pressure | 59 (28.64) | 27 (29.35) | 32 (28.07) | 0.84 |
| CKD | 11 (5.37) | 4 (4.35) | 7 (6.19) | 0.39 |
| Menopause | 0.10 | |||
| No | 45 (21.84) | 26 (28.26) | 19 (16.67) | |
| Yes | 142 (68.93) | 60 (65.22) | 82 (71.93) | |
| Does not apply | 19 (9.22) | 6 (6.52) | 13 (11.40) | |
| Fractures | 23 (11.17) | 11 (11.96) | 12 (10.53) | 0.74 |
| Use of steroids | 14 (6.79) | 3 (3.26) | 11 (9.64) | 0.06 |
| Hypothyroidism | 69 (33.82) | 35 (38.46) | 34 (30.09) | 0.21 |
| Cardiovascular disease | 15 (7.28) | 5 (5.43) | 10 (8.77) | 0.42 |
| Type of cardiovascular disease | ||||
| Coronary heart disease | 6 (40.0) | 1 (20.0) | 5 (50.0) | |
| Cardiac arrhythmia | 3 (20.0) | 2 (40.0) | 1 (10.0) | |
| Valvular heart disease | 2 (13.33) | 1 (20.0) | 1 (10.0) | |
Values reported as absolute number (percentage). OAD: occlusive arterial disease; CKD: chronic kidney disease.
The frequency of menopause was 76.34% with no differences between those who had or not a deficit of vitamin D. Less than 10% of patients with osteoporosis had chronic kidney disease, chronic steroid use, cerebrovascular disease, occlusive arterial disease, heart failure, congenital malformations, syncope, and pulmonary, neurologic or psychiatric disease (Table 2). It was observed a frequency of 40% of coronary heart disease, 28% of arterial hypertension, and 33% of hypothyroidism. There were no differences in the frequency of these antecedents and osteoporosis between those who had vitamin D deficit and those with did not.
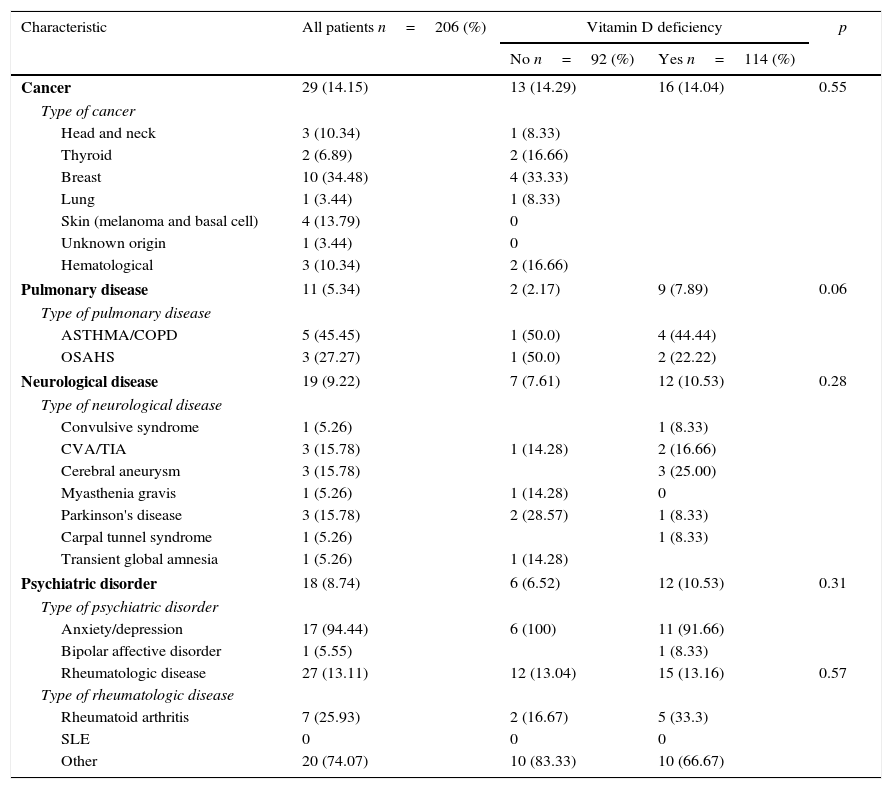
History of neoplastic, rheumatologic, pulmonary and neurological diseases of the patients with osteoporosis.
| Characteristic | All patients n=206 (%) | Vitamin D deficiency | p | |
|---|---|---|---|---|
| No n=92 (%) | Yes n=114 (%) | |||
| Cancer | 29 (14.15) | 13 (14.29) | 16 (14.04) | 0.55 |
| Type of cancer | ||||
| Head and neck | 3 (10.34) | 1 (8.33) | ||
| Thyroid | 2 (6.89) | 2 (16.66) | ||
| Breast | 10 (34.48) | 4 (33.33) | ||
| Lung | 1 (3.44) | 1 (8.33) | ||
| Skin (melanoma and basal cell) | 4 (13.79) | 0 | ||
| Unknown origin | 1 (3.44) | 0 | ||
| Hematological | 3 (10.34) | 2 (16.66) | ||
| Pulmonary disease | 11 (5.34) | 2 (2.17) | 9 (7.89) | 0.06 |
| Type of pulmonary disease | ||||
| ASTHMA/COPD | 5 (45.45) | 1 (50.0) | 4 (44.44) | |
| OSAHS | 3 (27.27) | 1 (50.0) | 2 (22.22) | |
| Neurological disease | 19 (9.22) | 7 (7.61) | 12 (10.53) | 0.28 |
| Type of neurological disease | ||||
| Convulsive syndrome | 1 (5.26) | 1 (8.33) | ||
| CVA/TIA | 3 (15.78) | 1 (14.28) | 2 (16.66) | |
| Cerebral aneurysm | 3 (15.78) | 3 (25.00) | ||
| Myasthenia gravis | 1 (5.26) | 1 (14.28) | 0 | |
| Parkinson's disease | 3 (15.78) | 2 (28.57) | 1 (8.33) | |
| Carpal tunnel syndrome | 1 (5.26) | 1 (8.33) | ||
| Transient global amnesia | 1 (5.26) | 1 (14.28) | ||
| Psychiatric disorder | 18 (8.74) | 6 (6.52) | 12 (10.53) | 0.31 |
| Type of psychiatric disorder | ||||
| Anxiety/depression | 17 (94.44) | 6 (100) | 11 (91.66) | |
| Bipolar affective disorder | 1 (5.55) | 1 (8.33) | ||
| Rheumatologic disease | 27 (13.11) | 12 (13.04) | 15 (13.16) | 0.57 |
| Type of rheumatologic disease | ||||
| Rheumatoid arthritis | 7 (25.93) | 2 (16.67) | 5 (33.3) | |
| SLE | 0 | 0 | 0 | |
| Other | 20 (74.07) | 10 (83.33) | 10 (66.67) | |
Values expressed as absolute value (percentage). CVA: cerebrovascular accident; TIA: transient ischemic attack; COPD: chronic obstructive pulmonary disease; SLE: systemic lupus erythematosus; OSAHS: obstructive sleep apnea–hypopnea syndrome.
Regarding the rheumatologic conditions it was seen a higher frequency of rheumatoid arthritis and to a lesser extent other pathologies such as Sjögren syndrome, fibromyalgia and osteoarthritis. A higher frequency of rheumatologic, neoplastic, pulmonary, neurologic or psychiatric diseases was not observed among patients who had vitamin D deficiency.
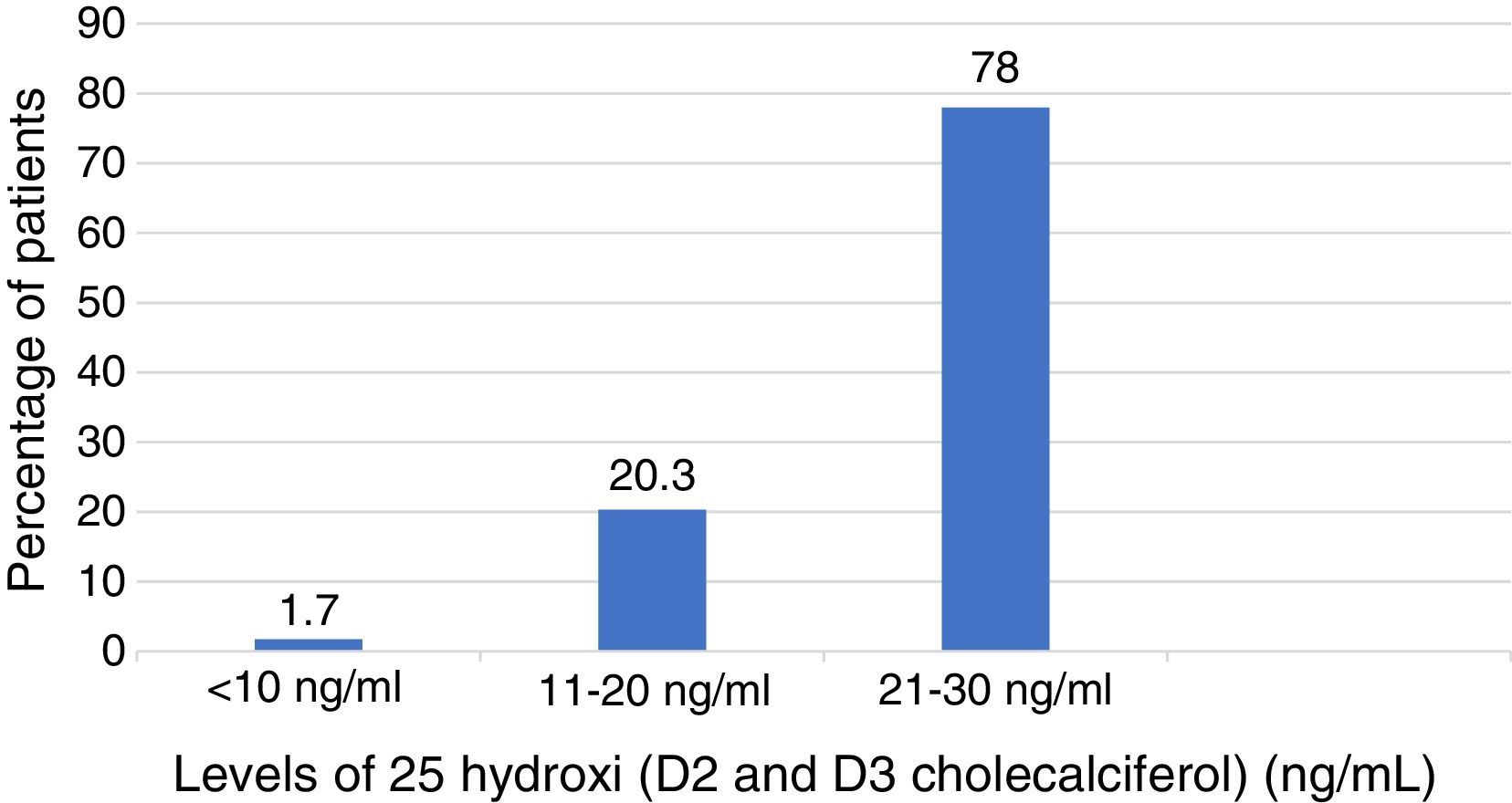
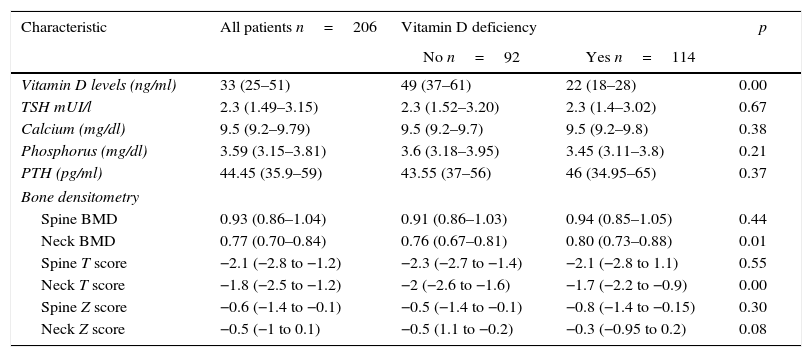
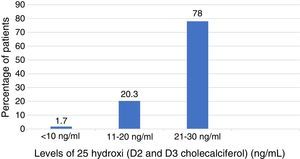
In Table 3 are described the medians of the levels of vitamin D among those who had vitamin D deficiency and those with did not. The median of the levels of vitamin D among those who presented deficiency was 22ng/ml (interquartile range 18–28ng/ml) of which 78% had levels lower than 30ng/ml; 20.3% levels lower than 20ng/ml and 1.7% had levels below 10ng/ml (Fig. 2). There were no deficits of calcium or phosphorus disorders. 34% of patients had levels of parathyroid hormone higher than 55pg/ml. The BMD among patients with previous diagnosis of osteoporosis was found in the osteopenic range with a lower value in the femoral neck BMD, among those who did not have vitamin D deficit compared with those who presented it (femoral neck BMD 0.76 vs. 0.88; p=0.00) as well as the T score of the femoral neck which was worse in patients without vitamin D deficiency (femoral neck T score −2 vs. −1.7; p=0.00). The prevalence of severe osteoporosis was 11% with no differences in relation to the presence or absence of vitamin D deficiency.
Paraclinical exams of the patients with osteoporosis.
| Characteristic | All patients n=206 | Vitamin D deficiency | p | |
|---|---|---|---|---|
| No n=92 | Yes n=114 | |||
| Vitamin D levels (ng/ml) | 33 (25–51) | 49 (37–61) | 22 (18–28) | 0.00 |
| TSH mUI/l | 2.3 (1.49–3.15) | 2.3 (1.52–3.20) | 2.3 (1.4–3.02) | 0.67 |
| Calcium (mg/dl) | 9.5 (9.2–9.79) | 9.5 (9.2–9.7) | 9.5 (9.2–9.8) | 0.38 |
| Phosphorus (mg/dl) | 3.59 (3.15–3.81) | 3.6 (3.18–3.95) | 3.45 (3.11–3.8) | 0.21 |
| PTH (pg/ml) | 44.45 (35.9–59) | 43.55 (37–56) | 46 (34.95–65) | 0.37 |
| Bone densitometry | ||||
| Spine BMD | 0.93 (0.86–1.04) | 0.91 (0.86–1.03) | 0.94 (0.85–1.05) | 0.44 |
| Neck BMD | 0.77 (0.70–0.84) | 0.76 (0.67–0.81) | 0.80 (0.73–0.88) | 0.01 |
| Spine T score | −2.1 (−2.8 to −1.2) | −2.3 (−2.7 to −1.4) | −2.1 (−2.8 to 1.1) | 0.55 |
| Neck T score | −1.8 (−2.5 to −1.2) | −2 (−2.6 to −1.6) | −1.7 (−2.2 to −0.9) | 0.00 |
| Spine Z score | −0.6 (−1.4 to −0.1) | −0.5 (−1.4 to −0.1) | −0.8 (−1.4 to −0.15) | 0.30 |
| Neck Z score | −0.5 (−1 to 0.1) | −0.5 (1.1 to −0.2) | −0.3 (−0.95 to 0.2) | 0.08 |
Values reported in medians (interquartile range). BMD: bone mineral density; PTH: parathyroid hormone; TSH: thyroid stimulating hormone.
Vitamin D deficiency is a common condition, and therefore it has been considered a global epidemics.5 This pathology becomes important due to its association with a low BMD, increased risk of osteoporosis and fractures.12,13 In Colombia, studies have been conducted in different altitudes such as those of Díaz et al., who found a prevalence of vitamin D deficiency in postmenopausal population in Bogota, defined by a level lower than 25ng/ml in 81%, and in 89% of individuals with low sun exposure. González et al. reported a prevalence of the deficit of vitamin D associated with osteoporosis with a value lower than 69.5% in another population from Bogota. But in the latter case they defined as deficit, vitamin D levels lower than 32ng/ml.14 Another study conducted in Medellin found levels lower than 30ng/ml in 77.1%.15
In our study, the prevalence of vitamin D deficiency in patients with osteoporosis was 55%, lower than the reported in other studies conducted in Colombia, but with important differences in the evaluated population, since Cali is not only located in a tropical zone, but it is also situated about 1000m above sea level, with a climate favorable for increased sun exposure, an important substrate for the synthesis of vitamin D compared with Bogota and Medellin which are located at 2625m and at 1538 above sea level, respectively. There are also differences in the racial distribution, where even though previous studies have shown that afro-descendant patients have lower bone density, there is no correlation with the vitamin D levels in this population, and they are attributed to other causes that have not been identified yet.16
However, it never ceases to amaze such a high prevalence in a tropical zone, attributable to the life styles that affect the degree of exposure to the sun, such as the type of clothing, the use of sunscreen, variables not evaluated in our study, which could increase the prevalence.17,18 The main limitation has been that the individuals included come from a high complexity center, although it is a reference center for the South-west of Colombia, the prevalence could have been underestimated due to early diagnoses and treatments of a population covered by specialized outpatient units, which implies that patients with a longstanding diagnosis of osteoporosis and vitamin D deficit might, at the time of the study, be corrected, findings which are consistent with the bone densitometry reports where only 34.6% of individuals remained in a range of osteoporosis within the 6 months prior to obtaining the vitamin D levels. Likewise, 99% of the individuals included belonged to the contributory scheme, which indicates at least a minimum income, and greater access to basic measures of food intake that include sea products containing vitamin D. Therefore, these results cannot be extrapolated to the subsidized population.
However, it should be taken into account that the city of Cali, located in Southwestern Colombia, is situated in the equatorial zone, where it would be expected that the exposure to the ultraviolet rays would favor a lower frequency of vitamin D deficiency, which would be consistent with our results, although there are other factors that may be related with inadequate vitamin levels. It is necessary to conduct further studies that allow to evaluate other variables not included and to extend the study to the population without osteoporosis.
In recent years, attempts have been made to associate the vitamin D deficiency with the development of high blood pressure, cardiovascular disease, cancer and even diabetes mellitus.19–21 Even though the objectives of our study are not intended to search an association with these and other pathologies, when performing the analysis of the different risk variables included in the study, there were no significant differences between the patients who had low vitamin D levels and those who had normal levels. Finally, supplementation of vitamin D is important in the primary and secondary prevention of osteoporosis, with a potential, today clear, in diabetes and cardiovascular risk. Therefore, The American Society of Endocrinology recommends supplementary doses of vitamin D of 1000–2000IU, although there is still no clarity on the optimal dose of supplementation.22,23
ConclusionThe prevalence of vitamin D deficiency in patients with osteoporosis treated in a high complexity institution of the Colombian Southwest was 55.3%, although it is lower in relation to the global statistics and other studies conducted in other geographic areas of the country. However, further studies with a larger population size are needed in order to demonstrate these findings and thus implement public health measures that will contribute to reduce the risk of developing osteoporosis.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were performed on human beings or animals for this research.
Data confidentialityThe authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consentThe authors state that patient data do not appear in this article.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Mendoza E-PN, Marín J-WT, Carrillo DC, Guzmán GE, Arango LG. Prevalencia de la insuficiencia de vitamina D en pacientes con osteoporosis. Rev Colomb Reumatol. 2016;23:17–23.