The mesenchymal stromal cells (MSCs) are hematopoietic stem cells with high capacity of differentiation to other cellular lineages, depending on the microenvironment in which they live as well as on the interaction and signaling pathways they establish with the extracellular matrix. Several properties have been described in these cells: proangiogenic, antifibrotic and immunomodulatory. These properties are being studied as a therapeutic approach for autoimmune diseases such as cutaneous systemic sclerosis (SSc). SSc is a systemic chronic disease, with an approximate prevalence of 35.6 cases per 100,000 inhabitants in North America and of 0.02% in Colombia in 2018. There are two different clinical variants, diffuse and localized. In both variants an important skin involvement and a rapidly deterioration of organs is present, which can overshadow the clinical prognosis and increase the mortality. Options for the treatment of advanced diffuse SSc are scarce mainly targeting symptomatic control with little impact on the progression and mortality. Therefore, there is an increasing interest in new therapies like advanced cellular therapy with hematopoietic stem cells and stromal mesenchymal cells. This article reviews the information related to the use of stromal mesenchymal cells in patients with this disease.
Las células mesenquimales estromales (MSC) son células madre no hematopoyéticas pluripotenciales con alta capacidad de derivación a diferentes linajes celulares, dependiendo tanto del microambiente en el que se encuentren, como de la interacción y señalización que establezcan con la matriz extracelular del entorno, esto ha permitido describir un potencial proangiogénico, antifibrótico e inmunomodulador, que ha sido blanco de investigación en enfermedades autoinmunes como la esclerosis sistémica cutánea (SSc). Considerando que la SSc es una enfermedad inflamatoria crónica, con una prevalencia estimada de 35,6 casos por cada 100.000 habitantes en Norte América y de 0,02% en nuestro país para el 2018, se caracteriza por presentar dos variables clínicas principalmente; una variante limitada y una variante difusa, presentando en ambas un compromiso extenso de piel y órganos que puede ser rápidamente progresivo y deteriorar el pronóstico de los pacientes que la padecen, aumentando su mortalidad. Debido a que las opciones terapéuticas en esta entidad son limitadas y buscan únicamente el control de síntomas, pero con poco impacto en progresión y mortalidad, terapias celulares avanzadas han surgido como nuevas opciones terapéuticas incluyendo el trasplante de células madre hematopoyéticas y las células mesenquimales estromales. A continuación, se revisará acerca de la utilidad y evidencia de células mesenquimales estromales en pacientes con esta enfermedad.
Cutaneous systemic sclerosis (SSc) is a chronic autoimmune disease affecting connective tissue; it has unknown etiology and is characterized by having vasculopathic phenomena, fibrotic compromise with increased deposits of extracellular matrix and inflammation of the skin and organs such as the lungs, digestive tract, heart, blood vessels and kidneys. This disease mainly affects women (8:1 ratio), having a prevalence estimated as 39.9 cases per 100,000 adults in the USA,1 and 0.02% of the population over 18 years-old in Colombia.2 The average age at presentation ranges from 35 to 55 years old.3
Two variations of its clinical presentation have traditionally been described: limited SSc, having skin involvement in the limbs’ distal regions, accompanied by sclerodactylia, calcinosis, Raynaud's phenomenon and involvement of the internal organs, i.e., the lungs and kidneys. The anti-centromere antibody in up to 70% of cases is its characteristic serological marker4; diffuse SSc is the second variant where skin involvement is proximal in the limbs and includes the trunk and the back. Renal, cardiac and pulmonary involvement is frequent and can quickly become progressive. Anti-topoisomerase I or anti-SCL70 in up 30% and anti-RNA polymerase III in 20% of cases are the serological markers for diffuse SSc, another biomarker are the anti-nuclear antibodies, that are positive in up 90% of cases with different nuclear and centromeric patterns. The disease significantly affects the quality of life (QoL) and the functional class of patients suffering from it.5
Its cause remains unknown; however, it is known that genetic, hormonal and environmental factors are associated with its presentation. The disease's consequences can be fatal, having a 33% 10-year survival rate in patients suffering pulmonary involvement and a 45% 3-year survival rate in pulmonary hypertension (PH) patients.6
Given its multifactorial etiology, it is considered a heterogeneous disease; it is currently classified as an orphan disease by the Colombian Ministry of Health, meaning that there are few effective therapeutic options regarding the severe forms of presentation.4 Around 20% of patients become refractory to conventional therapy thereby forming part of the already described poor prognosis. Treatment of the disease currently focuses on early detection, follow-up and complication management depending on the organ involved: renal crisis, interstitial lung disease (ILD) and PH. Furthermore, treatment involves controlling symptoms regarding the other organs involved, such as gastroesophageal reflux disease (GERD) and Raynaud's phenomenon.6,7
Few therapeutic options focus on controlling the physiopathological mechanisms favoring the disease's progression.8,9 Some therapies have been studied which target the triggering physiopathological mechanisms regarding this disease, such as some biological and antifibrotic therapies and stem cell transplants, in order to modify the pathogenesis and identify which options can modulate its progression. Regarding cellular therapy, experience concerning stem cell transplantation (HSCT) has been extremely promising, leading to a significant improvement of the disease's symptoms and progression.10–13
Another experience has involved the use of mesenchymal stromal cells (MSCs) which have an anti-inflammatory, antifibrotic and anti-proliferative effect which seems to have had a great impact on managing cutaneous manifestations, acral ulcers and reducing the progression of pulmonary involvement.14,15 MSC have been used in different scenarios; clinical trials have supported their use regarding autoimmune diseases such as Crohn's disease,16 systemic lupus erythematous (SLE) and rheumatoid arthritis (RA), leading to frankly positive results related to controlling disease activity and remission, having a significant safety margin and a low adverse events (AE) rate associated with their use.17,18 However, no controlled clinical trials support and/or formally recommend their use in therapy of SSc due to the regulations regarding their use and costs.
This review thus discuses MSC use as part of SSc patients’ treatment.
MethodologyOriginal articles were selected by reading every report identified in the search using terms such as: “diffuse scleroderma”, “systemic scleroderma”, “refractory scleroderma”, “stem cell”, “Wharton's jelly stem cell” and “diffuse and limited scleroderma treatment”. Articles which were reports and/or case series dealing with adult patients over 18 years-old, randomized trials, topic reviews and narrative reviews investigating the origin/source, immunological mechanisms and the therapeutic effects of using Wharton's jelly-derived mesenchymal stem cells for SSc were included.
The search using the selected terms led to accumulate around 55 articles, 7 of which were reports and case series and 48 dealt with systematic and narrative reviews and experimental trials involving animal models. Evidence from experience regarding adults aged 18 years-old suffering from progressive systemic sclerosis or diffuse scleroderma was selected, plus 43 articles for a literature review (i.e., 49 articles). Further 8 articles were added to the search for information about systemic sclerosis patients’ conventional therapy which included international guidelines (European and American) and controlled clinical trials concerning SSc-related immunomodulators and biological therapy (giving 56 articles as the final selection).
Mesenchymal stem cellsMSC are non-hematopoietic stem cells which can be derived from different cell lines, depending on the microenvironment in which they are found and their signaling and interaction with the environment's extracellular matrix,19 They have common characteristics regarding phenotype and potential but may differ in terms of some receptors stimulating their activity and mechanism of action (MOA), thereby creating MSC subgroups.20
The International Society for Cellular Therapy (ISCT) has provided criteria for verifying these cells’ characteristics observed in in vitro culture. The ISCT has described characteristics such as their plastic binding ability during culture, having a specific immunophenotype by surface molecule expression of CD105, CD73 and CD90 and negativity for CD45, CD34, CD14 (or CD11b), CD79α (or CD19) or human leukocyte antigen (HLA)-DR isotype molecules. They must be differentiated in vitro into osteoblasts, adipocytes and chondroblasts.21,22
Other MSC subpopulations are produced in response to an increase in cytokine levels in the environment; for example, MSC1 (pro-inflammatory profile) can be produced when MSC have low IFN-γ and TNF-α levels, which would not lead to stimulating an increase in IL6 levels and trigger a monocyte type-1 response. It has been suggested that activating TLR4 would be the key to a proinflammatory MSC-mediated response.23,24 MSC2 (anti-inflammatory profile) would be the other subpopulation, being produced when MSC identify a local inflammatory environment having high IFN-γ and TNF-α levels. This would lead to stimulating IL-6, IDO, PGE2 and TGF-β secretion, thereby producing a monocyte type-2 response (IL-10 secreting, anti-inflammatory). A study has shown that TLR3 activation has led to an immunosuppressive anti-inflammatory response.25,26
MSC can be derived from many sources (i.e., amniotic liquid), fetal or neonatal annexes (i.e., placenta and/or amniotic membrane (AM)), umbilical cord Wharton's jelly (WJ), umbilical cord blood (UCB), numerous adult tissues (i.e., bone marrow (BM)), adipose tissue (AT), periosteum, perichondrium, synovia, dental pulp (DP), lymphoid tissues and/or menstruation.23,27
Regardless of the source, basic, preclinical and clinical research regarding MSC must be carried out from different sources so that they can be compared in terms of their therapeutic potential. MSC derived from different sources have advantages and disadvantages regarding the appropriation of knowledge, ethical implications, the method for obtaining them, accessibility, population duplication, pluripotent markers, senescence activity, immunomodulation potential and differentiation potential.28
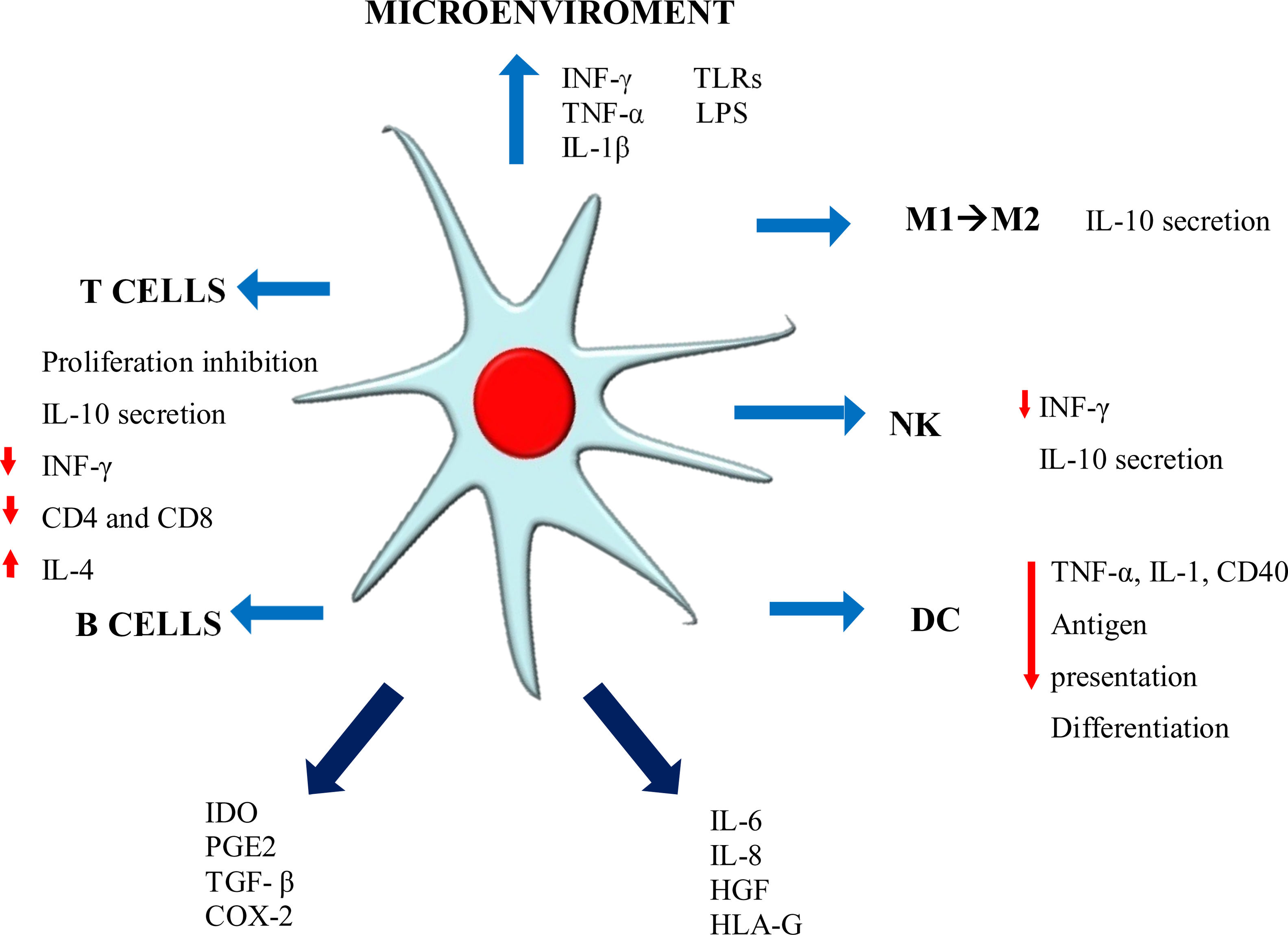
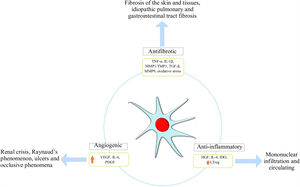
Immunomodulation mechanismsThe MSC immunomodulator MOA functions in a paracrine manner, inducing the secretion of growth factors, cytokines and adhesion molecules which can reduce immune cell proliferation in culture, thereby affecting both innate and adaptive immunity. Such properties enable MSC to escape recognition by the immune system, modulating/mediating T-cell, B-cell, NK, dendritic cell (DC) and macrophage functions21,26,28 (Fig. 1).
Such modulation is mediated by mechanisms depending on cell contact and the exosome secreted in response to the environment. Cell contact-dependent mechanisms work through proteins such as programmed death ligand-1 (PDL-1) TSG-6, ICAM-1, VCAM-1, COX-1, and soluble factors such as IL-10, IL-6, TGF-β1, nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), PGE2 and HLA-G5, EGF, FGF, PDGF, TGF-β, VEGF, HGF and angiopoietin-1.20,23,29
Activated MSC acquire immunomodulatory properties that can vary, depending on the proinflammatory cytokine stimulant; for example, IFN-γ confers antigen-presenting cell (APC) properties, IDO secretion and PD-L1 expression.30 TNF-α or IL-1β induce HLA class I expression and increase ICAM-1 and VCAM-1 expression. If activation is simultaneously promoted by IFN-γ, TNF-α produces chemokines and chemokines receptors such as CCR5, CCR10, CXCR3, CXCL9 and CXCL10 and there is an increase in IL-6, IL-8, HGF, PGE-2 and COX-2 expression.31 It has been demonstrated that WJ-MSC have the potential for improving lymphocyte recruitment using chemokines such as CCL2, CCL5, CCL20, CXCL2 and CXCL10.25
MSC and T-lymphocyte responseMSC inhibit stimulated T-lymphocyte proliferation, arresting cell division by halting it during G0/G1 phase, thereby impeding their entry during cell cycle S (synthesis) phase.31 T-lymphocytes accumulate during pG0/G1 phase after 24h exposure to MSC, an increase in p16, p21, p27 expression being observed and a reduction in A, D2, E and B cyclins, leading to the arrest of the cell cycle.21 Interestingly, it has been demonstrated that T-lymphocytes do not form part of the IL-6-associated apoptosis phenomenon.32 It is worth stressing that MSC do not just suppress CD4 T-lymphocyte activation but also escape cytotoxic CD8 T-lymphocyte and natural killer (NK) lysis. MSC have been seen to induce the arrest of T-cell division in in vitro assays involving mixed lymphocyte reaction (MCR) in culture and such T-cell inhibition has seemed to be antigen-specific.32 It is quite likely that MSC can inhibit T-cell proliferation in allogeneic and xenogeneic environments.33
Activated T-lymphocytes with MSC in coculture have been shown to increase FOXP3 positivity, suggesting an increase in regulatory T-cell (Tregs) immunomodulator population at the expense of the other T-lymphocyte populations.21 MSC-mediated immunomodulation relies heavily on the local inflammatory medium for activating IDO expression, thereby inducing T-cell differentiation into CD4+ CD25+ and FOXP3+.22 MSC exposed to interleukin-1β have significantly induced Tregs VIA regulation for increasing ICOSL; ICOSL-ICOS signaling has played an important role in contact-dependent regulation of Tregs differentiation by activating the phosphoinositide 3-kinase-Akt pathway. An in vitro study has shown that ICOSL expression and overexpression coincided with Tregs induction in MSC coculture with CD4+ T-cells. The neutralization or knockdown of ICOSL has been seen to significantly reduce Tregs and IL-10 secretion.31 MSC lead to effector cell energy, accompanied by a reduction in IFN-γ proinflammatory cytokines, tumor necrosis factor (TNF)-α, IL-17 and an increase in IL-10 and IL-4 (change to Th2 and/or regulatory phenotype).26,34
MSC and B-lymphocyte responseDetaining the cell cycle affects B-cells in the same way, causing MSC to reduce chemokine receptor expression and immunoglobulin production. However, this does not alter TNF-α, IFN-γ, IL-4 and IL-10 expression or the expression of molecule stimulators such as HLA-DR, CD40 and the B7 family.35
IFN-γ- or TNF-α-activated MSC inhibit B-cell proliferation and differentiation since they can regulate B7-H1 and PD-1 and IgG- and IgM-secreting B-lymphocytes.36 CCL2, B7-H1, VEGF, C3, GAL-9 and IDO membrane vesicles (MV) are B-cell immunoregulation-related soluble factors and are anti-inflammatory, whilst BAFF, PGE2 and APRIL are proinflammatory.34 It has been stated that MSC secrete CCL2, which is a chemoattractant for monocytes and macrophages in inflamed areas, and that it is involved in B-cell inhibition. Its overexpression suppresses circulating autoantibody levels.35
MSC and monocyte line responseRecruited macrophages and monocytes migrate to the site of a lesion in response to proinflammatory environmental signals; unstimulated MSC constitutively secrete IL6 and express HLA-G5 which is fundamental in MSC-mediated immune regulation; MSC-mediated immunomodulation secretes IL-6 and PGE2 directing the monocyte line toward the formation of IL-10-expressing monocyte-derived cell population (MDC). IL-10 activates MSC for positively regulating HLA-G (soluble and membrane bound) expression which is related to immunomodulatory effects concerning NK and adaptive immune cells. MSC become activated via cell–cell contact, IL-10, IL-6 or other secreted factors following monocyte–MSC interaction for positively regulating their PGE2 expression, thereby further reinforcing monocyte bias toward MDC.25
Trophic potentialMSCs’ trophic role can currently be highlighted for their multiple applications covering a wide range of disorders. This is due to MSC secreting bioactive molecules being able to mediate immunomodulatory and trophic activities22 since they express growth factors such as brain derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF), anti-apoptotic factors and proliferative and angiogenic factors. Other reports have highlighted GW-MSCs’ trophic properties.20 It has been shown that GW-MSC endogenously express mRNA for neurotrophins and increase remyelination following transplants in experimental autoimmune encephalomyelitis (EAE) mice, thereby indicating their potential for providing trophic support for the central nervous system (CNS). Cell-based therapies can thus enhance remyelination as well as their anti-inflammatory properties which, together, can promote endogenous tissue regeneration, this being crucial for suppressing multiple sclerosis progression.37
The WJ-MSC tropic effect has induced better microvasculature formation and cell migration in co-cultured endothelial cells.29 WJ-MSC thus have proangiogenic, anti-apoptotic and antioxidant characteristics. Serval studies have sought to standardize methodologies so that whilst morbidity is low,29,38 at the same time CD34 cells can be presented and disappear ex vivo when the cells proliferate.31
Microvesicles are released in response to increases in intracellular calcium. The integrins, extracellular matrix (ECM) proteins, lectins, proteoglycans or glycolipids in extracellular vesicles (EV) enable coupling to cells expressing appropriate receptors on their surfaces,33,34 resulting in plasmatic membrane remodeling and vesicle detachment.39 They can also act as transcription modulators and influence cell phenotypes since they enable genetic information to be exchanged between cells as the vesicles encapsulate and protect degrading enzymes’ DNA, messenger RNA (mRNA), long non-coding RNA (ncRNA) and micro RNA (miRNA).40 Ratajczak et al.,41 demonstrated that the microvesicles obtained from murine embryonic stem cells improved negative-lineage Sca-1-positive progenitor cells’ survival and expansion by improving Oct-4, Rex-1, HoxB4, Scl and GATA 2 expression.
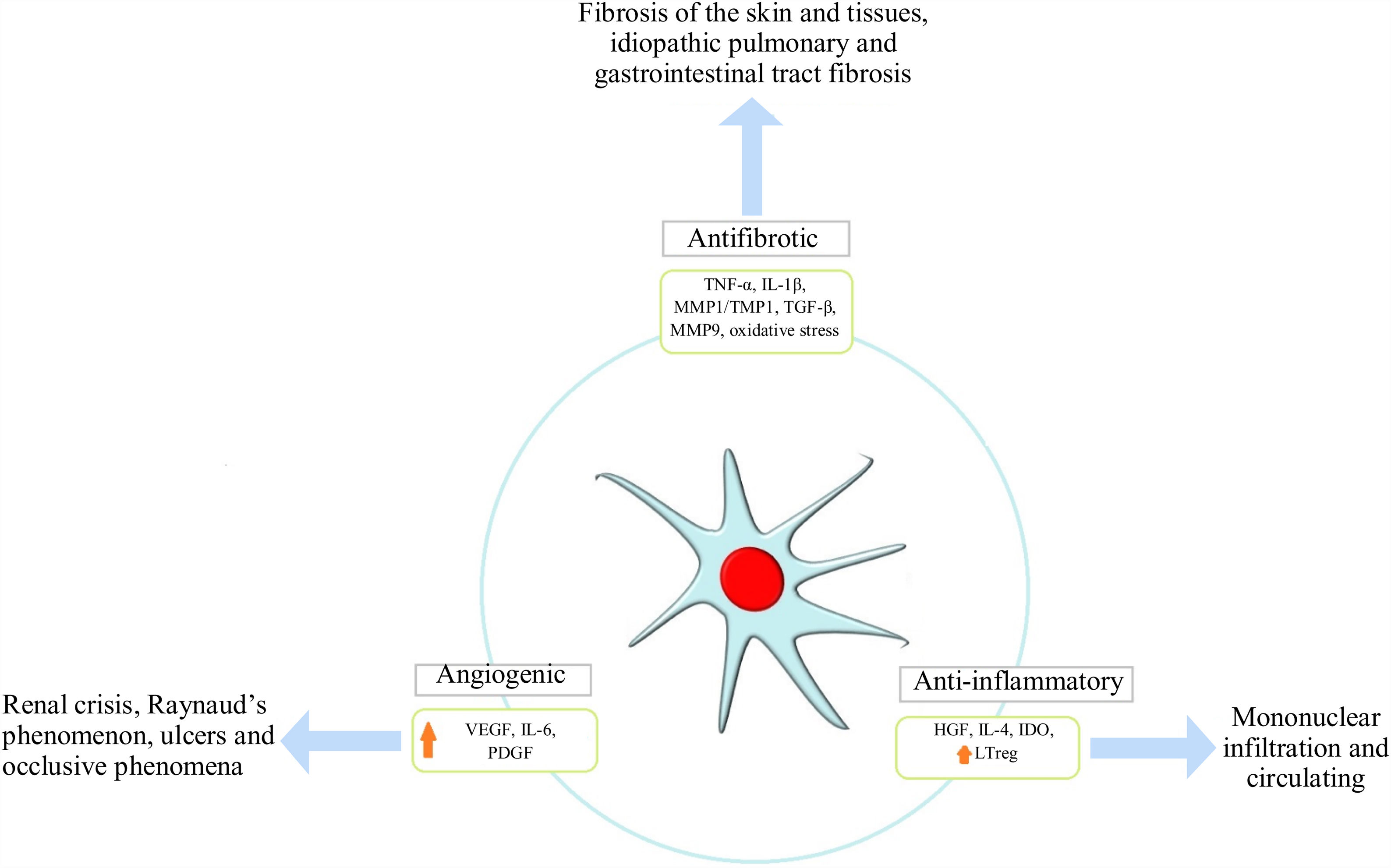
Pro-angiogenic effectSSc is characterized by vasculopathy secondary to endothelial dysfunction thereby unleashing tissue hypoxia which is not compensated for by the physiological response enabling endothelial progenitor cell activation and the development of neovascularization. MSCs’ role in this mechanism is related to the release of growth factors, such as hepatic growth factor (HGF), vascular endothelial growth factor receptor type 1 and 2 (VEGFR1,2), and adhesion molecules, such as VCAM-1 which promotes angiogenesis20; they can also reverse contractile protein expression, i.e., smooth muscle-actin proteins SMAα and SM22α.42 Using MSC by IV infusion has been reported to be effective regarding SSc patients suffering critical limb ischemia due to greater VEGF secretion accompanied by reduced ischemia and angiography which revealed lower limb revascularization, whilst skin biopsy revealed an increase in angiogenic factors.43
WJ-MSC seem to be more angiogenic than AD-MSC. For example, IL-8 and PDGF-AA expression was much greater in WJ-MSC according to the pertinent literature. IL-8 acts directly on the endothelial cells participating in cell proliferation, migration and survival whilst PDGF-AA is a mesenchymal angiogenic factor which is not expressed in epithelial cell lineages, acting as an important migratory factor in angiogenesis and wound healing. PDFG-AA can stimulate VEGF-A expression; this has been the most studied factor regarding angiogenesis.42
Antifibrotic effectFibrosis is a main characteristic of this disease's physiopathology; it is produced by excessive collagen production and/or thickening of the skin and internal organs thereby affecting their architecture and function, being an irreversible and difficult to control mechanism in SSc patients. It is unleashed in response to tissue hypoxia and oxidative stress where fibroblasts and TH2 lymphocytes produce an exaggerated amount of transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF). MSC exercise an antifibrotic effect via two mechanisms initially described in animal models; one of them results from MSCs’ ability to reduce TGF-β1 levels and increase VEGF levels.44 The second mechanism enables them to stimulate overexpression of the thioredoxin (Trx) system, inhibit hypoxia-induced apoptosis and thus fibrosis accompanied by reduced expression of collagen 1α, collagen 3α and other extracellular matrix components.45,46 Furthermore, a reduction in cytokine levels (i.e. tumor necrosis factor α and IL-1β) has been seen in human models treated with adipose tissue-derived MSC47 (Fig. 2).
ImmunogenicityMSC are considered to be hypoimmunogenic due to their low MHC class I molecule expression; however, they lack MHC class II expression, the same as HLA-DR.32,47 Nevertheless, human MSC positively regulate costimulatory molecules (CD80, CD86) in response to IFN-γ. Low MHC I expression is important for protecting MSC from natural killer cell-mediated cytotoxicity and detaining T-cells in cell cycle G0 phase, which could also help MSC escape immune recognition.23 Allogeneic cells are thus not rejected by a receptor's immune system in the absence of immune suppression, meaning that they can be used for therapeutic purposes regarding different diseases.48
MSC as SSc-related therapyPhysiopathological principleSSc is characterized by a classic triad consisting of fibrosis, vascular damage and uncontrolled inflammatory response,3 thereby altering tissue architecture and function due to increased myofibroblast activity resulting in excessive production of extracellular matrix components such as collagen and other glycoproteins. This increases tissue contraction and the irreversible infiltration of organs which is associated with microvascular phenomena promoting hypoxia, the release of reactive oxygen species (ROS) and cytokines stimulating a TH2 response with IL4 and IL13 stimulating IgE synthesis. In turn, this increases fibroblast differentiation and fibrogenesis thereby creating a vicious circle regarding this mechanism, contributing to fibrosis, dysfunction and vital organ failure (i.e., the lungs, the kidneys and the heart).44
Different immunosuppressive therapies have been studied based on the above and being aware of the entity's proinflammatory model. One such is autologous hematopoietic (stem) cell transplantation (AHCT) which requires an immunosuppressive, myeloablative regime for depleting the immunological system (T-cells and B-cells), thereby re-establishing immunological tolerance32,35; significant AHCT-related clinical improvement has been demonstrated in randomized clinical trials. However, the pertinent literature states that a high rate of complications could arise from the required cytotoxic regimes involving exposure to infection during the first months’ follow-up, on some occasions leading to fatal outcomes and relapse rates after 5 years’ intervention.12,13 MSC could thus be extremely useful because they do not need to be myeloablative, thereby reducing the risk of infection and associated toxicity (Table 1).30,31
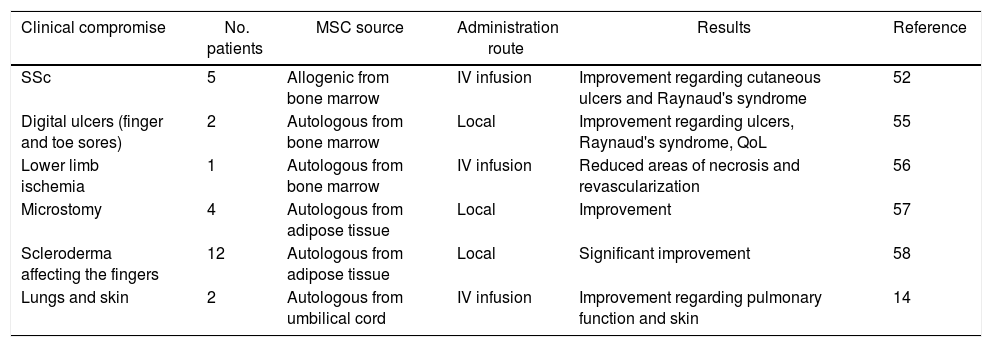
Reports in the pertinent literature regarding the use of MSCs in SSc (50).
| Clinical compromise | No. patients | MSC source | Administration route | Results | Reference |
|---|---|---|---|---|---|
| SSc | 5 | Allogenic from bone marrow | IV infusion | Improvement regarding cutaneous ulcers and Raynaud's syndrome | 52 |
| Digital ulcers (finger and toe sores) | 2 | Autologous from bone marrow | Local | Improvement regarding ulcers, Raynaud's syndrome, QoL | 55 |
| Lower limb ischemia | 1 | Autologous from bone marrow | IV infusion | Reduced areas of necrosis and revascularization | 56 |
| Microstomy | 4 | Autologous from adipose tissue | Local | Improvement | 57 |
| Scleroderma affecting the fingers | 12 | Autologous from adipose tissue | Local | Significant improvement | 58 |
| Lungs and skin | 2 | Autologous from umbilical cord | IV infusion | Improvement regarding pulmonary function and skin | 14 |
SSc: cutaneous systemic sclerosis; MSCs: stromal stem cells; IV: intravenous administration.
Source: Coopman et al. 50
MSCs’ therapeutic usefulness was first related to bone marrow transplant patients for reducing transplant rejections; this was related to their ability to secrete endogenous factors regulating cell activity.21,27 MSC properties were then described as being anti-proliferative, anti-inflammatory and immunomodulatory. This was due to their ability to adapt to the microenvironment in which they were found, their interaction with cells in their immediate setting and their response to cytokine influx for maintaining homeostasis.48 Due to such properties MSC begun to be used during the last 10 years in different scenarios; they were initially used in murine skin models and systemic lupus erythematosus (SLE)17 due to the favorable and safe outcomes resulting from starting therapy on humans in cases of autoimmune disease,49,50 graft-transplant rejection,51 sepsis, occlusive vascular disease (OVD)44 and degenerative diseases such as osteoarthrosis,49 considering the ease of administering them and the low adverse events (AE) rate in the patients studied.
The immunological benefits of using MSC with SSc patients are currently being studied. Several case reports recording promising results have been published; the first of these, published in 2008, was aimed at assessing the response of a patient having acral ulcer-derived cutaneous involvement. A 1×106MSC cell/kg weight dose was used with this patient; a significant improvement regarding changes in the skin and ulcerous lesions was observed.19 These results led to case series being published regarding patients having skin involvement (thickening and fibrosis), vasculopathy and Raynaud's phenomenon.
One publication (2011) dealt with 5 diffuse SSc patients suffering severe cutaneous involvement due to acral ulcers, myositis and mainly interstitial lung involvement who had received prior therapy involving cyclophosphamide (CPM), methotrexate (MTX) and azathioprine (AZA). The patients received a dose of MSC equal to that involved in the first case, administered by intravenous (IV) route; there was a significant improvement of the skin, ulcers disappeared and some patients experienced an improvement regarding their functional class indexes, lung function tests and QoL and no AE were reported in any of these 5 cases.52 A case series involving 2 patients suffering from PH and pulmonary failure refractory to conventional treatment was published in 2016; the patients were treated with rituximab-associated stem cell therapy, administering the dose described in the previous cases by IV route. This involved administering the dose on two occasions, 30 days apart, with no complications or AE associated with its application. Significant improvement was documented during the 12-month follow-up regarding activity and QoL indexes, accompanied by changes in the skin and normalization of lung pressure in echocardiographic follow-up.14
MSC dose/therapy safety and adverse eventsMSC must be subjected to cell-surface marker detection, differentiation potential, microbiological culture tests, karyotype analysis and tumorigenicity tests to guarantee the safety of patients undergoing this type of therapy. Such identification guarantees the low risk involved in using MSC as therapy according to ISCT recommendations and cell stability, thereby avoiding a loss of immunomodulatory or multipotential capability due to alterations during cell expansion and culture.53,54
MSC have been administered by local injection and IV route regarding autoimmune diseases, particularly SSc; these facts are relevant as they could influence MSC migration and elimination ability. Local injection has been used with greater frequency on patients having skin or vascular involvement, unlike the IV route which has been chosen for patients having multisystemic or organ involvement. However, this has not affected MSC safety or the results regarding this therapy in trials and case reports published to date.53
Regarding AE, stem cells have been characterized by having a good safety profile and low AE rate. This has been described in an analysis of safety regarding patients suffering from autoimmune diseases (i.e., SLE, SSc and rheumatoid arthritis) who had received an infusion of MSC from different sources (umbilical cord and bone marrow). The study sought to establish MSC safety profile and long-term follow-up49,53 by analyzing different cases published to date regarding patients suffering from autoimmune diseases, including SLE, RA, SSc. The suggested dose was 1×106 cells per kg of weight administered by IV route during an estimated time of 30min and an average 12–24h follow-up. The most commonly occurring AE related to hyperacute manifestations (those occurring during the first 48h following infusion) were fever, headache, palpitations, facial flushing/redness and insomnia in up to 11.9% of the applications.
Fever has been considered the most frequently reported AE in the clinical series and trials published so far (occurring in up to 5.9% of the cases); fever was still the main event reported as not being associated with infectious events in follow-up during the first 30 days. Regarding infection, cases have been described involving the respiratory tract, urinary tract and herpes zoster (shingles); however, no associations with applying MSC-related therapy have been established since it is known that stem cells have immunogenic/antimicrobial potential due to their capability for promoting phagocytosis, as well as releasing some antimicrobial peptides which they naturally produce.
Furthermore, no cases of neoplasia associated with MSC therapy have been described and it is known that 5-year survival rates are around 90.35%, making it a safer therapy than treatments involving stem cell transplants where survival rate has been recorded as around 70%.53
ConclusionsInterest in stromal stem cells has been increasing for more than 15 years now regarding the autoimmune disease scenario due to their immunomodulatory and precursor properties, having been used in pathologies such as SSc since 2008. Experience since then in different centers worldwide regarding patients having limited therapeutic alternatives and those suffering from autoimmune disease refractory to conventional treatment has led to successful results in terms of QoL and functionality, representing hope for those suffering from this disease and a clinical challenge for rheumatologists.
However, controlled clinical trials are required for supporting such therapy's use regarding these types of disease, thereby enabling this type of therapy to be recommended as part of the approach to managing SSc patients.
Conflict of interestThe authors have no conflict of interest.
Please cite this article as: Reyes-Martínez V, Londoño J, Ávila-Portillo LM, Rueda JC, Padilla-Ortiz DM, Salgado D, Muñoz N, Santos AM. Células estromales mesenquimales representan una opción terapéutica en pacientes con esclerosis sistémica. Rev Colomb Reumatol. 2020;27:126–134.