In patients with autoimmune diseases, the simultaneous occurrence of lupus anticoagulant and blood coagulation factors inhibitors is infrequent and is associated with hemorrhagic events. In these cases, the initial approach requires a thorough interpretation of coagulation laboratory tests and mixing studies to reach a definitive diagnosis. We report the case of a patient with systemic lupus erythematosus and Sjögren’s syndrome who presented with hemorrhagic diathesis caused by circulating inhibitors against factors VIII and XI coexisting with lupus anticoagulant. The inhibitors eradication was made with rituximab, achieving good results.
La ocurrencia simultánea de anticoagulante lúpico e inhibidores circulantes contra los factores de la coagulación es infrecuente en los pacientes con enfermedad autoinmune, y está relacionada con eventos hemorrágicos. El abordaje inicial requiere una adecuada interpretación de los tiempos de coagulación y prueba de mezcla con plasma para alcanzar el diagnóstico definitivo. Se reporta el caso de una paciente con lupus eritematoso sistémico y síndrome de Sjögren, quien se presentó con trastorno hemorrágico amenazante de la vida ocasionado por inhibidores circulantes contra los factores VIII y XI de la coagulación en coexistencia con anticoagulante lúpico. El tratamiento de erradicación de los inhibidores se realizó con rituximab, con buenos resultados.
Systemic lupus erythematosus (SLE) is the prototype of chronic inflammatory disease with immune system deregulation and production of autoantibodies.1 The presence of antiphospholipid antibodies in SLE is frequent (15–34 % for lupus anticoagulant [LA]) and is associated with the development of thrombotic events.2 To a lesser extent, antibodies directed against coagulation factors (II, VIII, IX, XI, XII and XIII) have been reported.3 These antibodies alter the function or promote the rapid clearance of coagulation factors and therefore, they manifest themselves by bleeding disorders.4 In patients with autoimmune disease, the simultaneous occurrence of LA and blood coagulation factor inhibitors is infrequent.
Clinical caseA 65-year-old woman diagnosed 6 months before with late-onset SLE, according to the 2012 SLICC criteria, supported by: alopecia, oral sores, photosensitivity, consumption of C3 complement, thrombocytopenia < 100,000 µl, leukopenia < 4000 µl, positive direct Coombs test ++, ANA (1/160 homogeneous pattern), anti-Sm 14.74 (positive); with negative LA at the time of diagnosis. In addition, secondary Sjögren’s syndrome given by the presence of xerostomia, xerophtalmia, xerodermia, anti-Ro 26.89 (positive), anti-La 51.2 (positive), under management with prednisolone 5 mg/day, azathioprine 50 mg/day and hydroxychloroquine 200 mg/day.
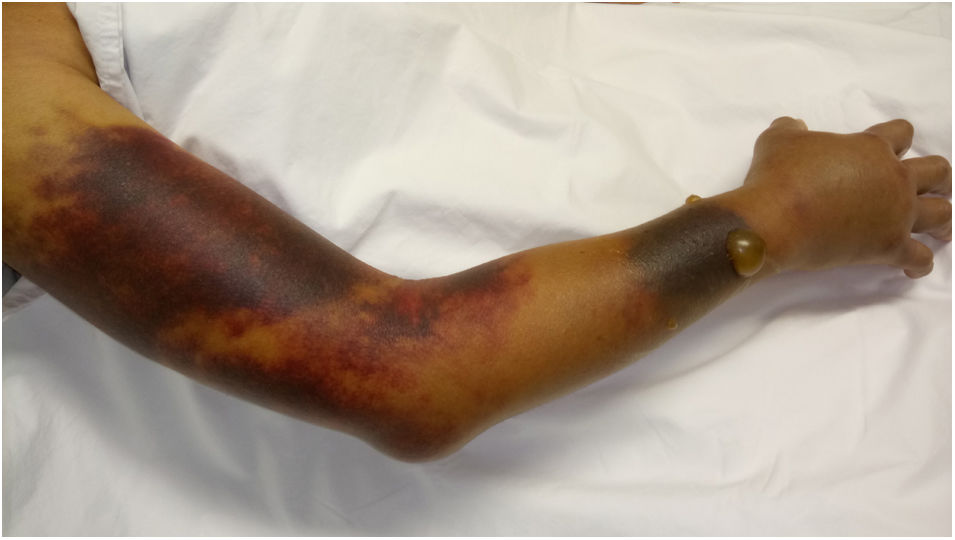
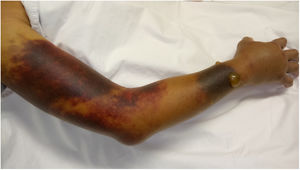
The patient was admitted to the hospital due to abdominal pain and hematomas in the extremities. No family or personal antecedents of hemorrhagic diathesis; there was no history of trauma and the consumption of medications or herbal products with anticoagulant action was denied. At physical examination she was found in fair general condition, with mucocutaneous paleness, arterial hypotension and tachycardia. An ecchymotic lesion was observed in the posterior region of the pharynx, as well as hematomas in the right arm, the left forearm, the right inguinal region and the ipsilateral thigh (Fig. 1). The laboratory studies revealed leukopenia, severe anemia, moderate thrombocytopenia and lymphopenia. The activated partial thromboplastin time (aPTT) was prolonged at 190 s (normal: 20−35 s), with prothrombin time (PT) of 10.3 s (normal: 9−13 s) and mixing test with normal plasma that did not correct. The ultrasound examination showed hemarthrosis in the right shoulder, hematomas in the vastus lateralis and right biceps femoris muscles, retroperitoneal hematoma adjacent to the right psoas muscle, and venous thrombosis of the cephalic, cervical and brachial veins. There were no signs of critical limb ischemia that would have required an urgent surgical intervention.
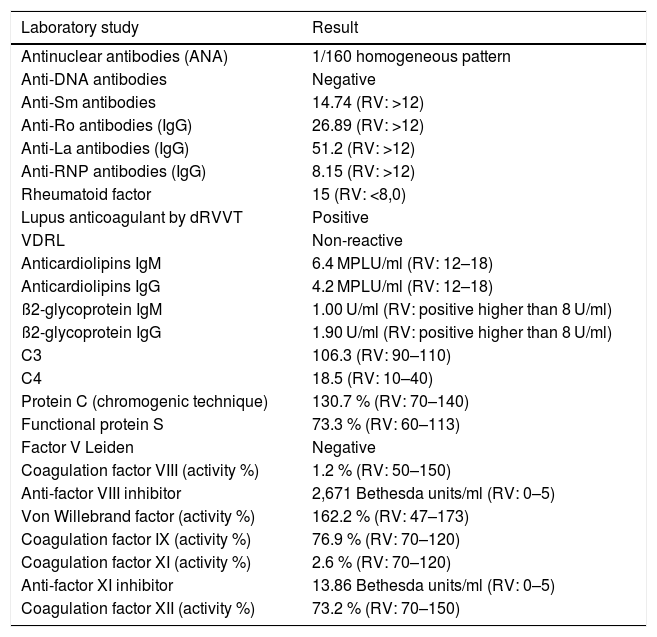
Additional studies revealed the presence of positive LA and circulating inhibitors against coagulation factors VIII and XI, as well as a quantitative deficiency of their levels (Table 1).
Laboratory studies during hospitalization.
| Laboratory study | Result |
|---|---|
| Antinuclear antibodies (ANA) | 1/160 homogeneous pattern |
| Anti-DNA antibodies | Negative |
| Anti-Sm antibodies | 14.74 (RV: >12) |
| Anti-Ro antibodies (IgG) | 26.89 (RV: >12) |
| Anti-La antibodies (IgG) | 51.2 (RV: >12) |
| Anti-RNP antibodies (IgG) | 8.15 (RV: >12) |
| Rheumatoid factor | 15 (RV: <8,0) |
| Lupus anticoagulant by dRVVT | Positive |
| VDRL | Non-reactive |
| Anticardiolipins IgM | 6.4 MPLU/ml (RV: 12–18) |
| Anticardiolipins IgG | 4.2 MPLU/ml (RV: 12–18) |
| ß2-glycoprotein IgM | 1.00 U/ml (RV: positive higher than 8 U/ml) |
| ß2-glycoprotein IgG | 1.90 U/ml (RV: positive higher than 8 U/ml) |
| C3 | 106.3 (RV: 90–110) |
| C4 | 18.5 (RV: 10–40) |
| Protein C (chromogenic technique) | 130.7 % (RV: 70–140) |
| Functional protein S | 73.3 % (RV: 60–113) |
| Factor V Leiden | Negative |
| Coagulation factor VIII (activity %) | 1.2 % (RV: 50–150) |
| Anti-factor VIII inhibitor | 2,671 Bethesda units/ml (RV: 0–5) |
| Von Willebrand factor (activity %) | 162.2 % (RV: 47–173) |
| Coagulation factor IX (activity %) | 76.9 % (RV: 70–120) |
| Coagulation factor XI (activity %) | 2.6 % (RV: 70–120) |
| Anti-factor XI inhibitor | 13.86 Bethesda units/ml (RV: 0–5) |
| Coagulation factor XII (activity %) | 73.2 % (RV: 70–150) |
The initial management was carried out with transfusional support of globular concentrate and fresh frozen plasma in combination with pulses of methylprednisolone for 3 consecutive days, followed by oral prednisolone at doses of 1 mg/kg/day and tranexamic acid without obtaining a response, the emergency therapeutic option of recombinant activated factor VII was not available; given the severe leukopenia and lymphopenia, for safety profile it was decided not to apply cyclophosphamide, for this reason, management with rituximab was indicated at a dose of 1 g intravenous in a scheme of week 0 and 15 days later, during hospitalization, for the eradication of the inhibitors, obtaining a good clinical evolution and correction of the coagulation times, after the first application, the patient continued under quarterly controls by hematology and the levels of coagulation factors increased, with reapplication of rituximab at 6 months and studies negative for hidden neoplasm, without the need for hospital readmission due to infection or bleeding after 12 months.
DiscussionWe describe the case of a patient with SLE and Sjögren’s syndrome who was admitted to the hospital due to an hemorrhagic disorder in whom the initial laboratories revealed an elevated aPTT with normal PT and mixing test with normal plasma that did not correct, suggesting the presence of acquired coagulation inhibitors, either LA or circulating inhibitors against the coagulation factors.4,5 To help clarify the etiology, the clinical presentation is useful because, although LA in vitro increases the aPTT, in vivo is related to venous and arterial thrombotic events6; while the circulating inhibitors against coagulation factors are associated with hemorrhagic events.7 In addition to the clinic, definitive confirmation by the laboratory with measurement of LA, coagulation factor levels and circulating inhibitors is required.4,5 In this case, the confirmatory result of the laboratory tests demonstrated the presence of circulating inhibitors against factors VIII and XI in very high titers, as well as a quantitative decrease in the levels of the respective factors.
The appearance of inhibitors against coagulation factors in nonhemophilic patients is uncommon, with an approximate incidence of 2 people per million,8 with an average age of onset of 64 years and a similar male to female incidence ratio.9 Unlike the congenital form in which articular hemorrhages (hemarthrosis) are the main characteristic, these are not common in acquired hemophilia, manifesting mainly with cutaneous and soft tissue hemorrhages.8 About 50 % of cases are related to an underlying clinical condition (autoimmune disorders, lymphoproliferative diseases, solid tumors, drugs, pregnancy and postpartum),9 being SLE the most frequent of the rheumatological causes.10 Given the immune deregulation that occurs in the patient with SLE, polyclonal autoantibodies mainly of type IgG 1 and 4 are directed against factor VIII A2 or C2 domains neutralizing its function or promoting its rapid clearance in the circulation.4,11 Even in the presence of high titers of inhibitors such as those seen in this case, it can be found a quantifiable residual activity of coagulation factors, evidencing a complex interaction in the kinetics between inhibitor and factor, which is different from what occurs in congenital hemophilia. Therefore, the severity of bleeding poorly correlates with the factor levels.8,11
An aggravating factor in this patient was the deficiency of factor XI, which has been rarely described in patients with SLE causing life-threatening bleeding.12 The combined deficiency of factors VIII and XI in an acquired form has not been previously reported, although presumably they share the same pathogenesis.
Simultaneous occurrence of circulating coagulation inhibitors with LA is unusual. Since 1993, there have been reports in association with connective tissue diseases and myeloproliferative disorders,13 mainly causing hemorrhagic disorders, but also thrombotic events as seen in this patient. The fact that the hematological manifestation in this patient occurred in the absence of other clinical manifestations of disease activity stands out.
The therapeutic objective was based on controlling bleeding and eradicating the inhibitor. Timely bleeding control decreases morbidity and mortality. Bridging agents such as recombinant factor VIIa and activated prothrombin complex concentrate are first-line agents,5 being fresh frozen plasma less useful because of its low content of the factor; however, the latter was used as the only resource due to the lack of availability of bridging agents in our hospital. The risk of thrombosis with the use of bridging agents should be taken into account, although this does not contraindicate their use since the benefit of bleeding control outweighs the risks. Given the life-threatening bleeding and the absence of use of coagulation bridging agents, it was considered not to use anticoagulant therapy for the management of the thrombosis of the upper limb.
For the eradication of circulating inhibitors against coagulation factors, immunosuppressive medications (cyclophosphamide, azathioprine and rituximab), glucocorticoids, intravenous gammaglobulin and plasmapheresis, have been used in addition to the control of the underlying disease, with satisfactory results. The discussion has focused on the most effective regime. Although different studies show that patients treated in the first line with a combination of steroids and cyclophosphamide were more likely to ensure complete remission than those treated with steroids alone, the end result in terms of survival and sustained remission is the same.5 Rituximab is an increasingly used therapeutic option with results similar to those of cyclophosphamide in terms of efficacy, but with a better safety profile. In this case the use of rituximab resulted in complete remission of the disease with normalization of coagulation factors, eradication of the inhibitor and reduction of immunosuppression.
ConclusionSLE is associated with the production of circulating inhibitors against coagulation factors, among them, factors VIII and XI, causing hemorrhagic disorders. Although this is a not frequent association, it should be studied in patients with SLE with abnormal coagulation tests, even in the absence of other manifestations of the disease, in order to improve the quality of life and decrease the morbidity and mortality rates of these diseases.
FundingThe authors declare that they have not received funding for the development of this work.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: López-Villegas VJ, Hincapié Rubio LM, Saldarriaga Rivera LM. Hemorragia severa secundaria a deficiencia adquirida de factores VIII y XI de la coagulación relacionada con lupus eritematoso sistémico: reporte de caso. Rev Colomb Reumatol. 2019;26:292–295.