Rheumatoid arthritis of late onset occurs in the population over 65 years of age, presenting differences in clinical and laboratory manifestations compared to rheumatoid arthritis in younger people, with a higher risk of presenting with aggressive forms of the disease, and with systemic compromise. The presence of a probable relationship between the interstitial lung disease associated with late-onset rheumatoid arthritis is established in this case. In most cases pulmonary involvement occurs after the joint problems, although they may appear simultaneously, and may even be the first manifestation. Pathological findings in interstitial pneumonias associated with collagen diseases are similar to idiopathic interstitial pneumonias. A case is presented of a woman who had pulmonary involvement due to idiopathic interstitial pneumonia, and subsequently presented with joint pain with rheumatoid arthritis being documented.
La artritis reumatoide de inicio tardío es considerada en la población mayor de 65 años, presentando diferencias a las manifestaciones clínicas y de laboratorio respecto a la artritis reumatoide en población joven, con mayor riesgo de presentar formas agresivas de la enfermedad y de comenzar con un compromiso sistémico. Se establece en este caso clínico la presencia de una probable relación entre la enfermedad pulmonar intersticial asociada con artritis reumatoide de inicio tardío. En la mayoría de casos la afectación pulmonar se presenta posterior al compromiso articular, aunque puede aparecer simultáneamente e incluso ser la primera manifestación. Los hallazgos patológicos de las manifestaciones pulmonares asociadas con enfermedades autoinmunes son similares a las neumonías intersticiales idiopáticas. Se describe el caso de una paciente que presenta compromiso pulmonar por neumonía intersticial idiopática y posteriormente presenta dolor articular, por lo que se documentó artritis reumatoide.
The late onset rheumatoid arthritis (LORA) is a systemic disease of autoimmune origin, characterized by compromising both large and small synovial joints, which starts after 65 years of age. Other authors make reference to LORA after the age of 60 years.1 The reported prevalence of LORA is close to 2%.2 Classically, in rheumatoid arthritis (RA) it has been described a ratio of 2 or 3 women for each man affected by the disease; this ratio has been attributed to the role that sex hormones play in women. In patients older than 60 years, a tendency to 1:1 ratio is observed; however, this probably has to do not only with the estrogenic fall in postmenopausal women, but also with the alteration in the concentration of androgenic hormones in men.2
The clinical presentation of the LORA is more acute and with a greater commitment of the joints; It has been described in the literature that the clinical behavior presents a more systemic commitment.3 Among the extra-articular manifestations, the interstitial lung disease (ILD) associated with RA (RA-ILD) stands out. Because of its difficult clinical evaluation, frequency and severity, it becomes a diagnostic and management challenge. The purpose of this case is to present an elderly female patient presenting lung involvement due to nonspecific interstitial pneumonia (NSIP) and who subsequently has joint pain in which RA was documented.
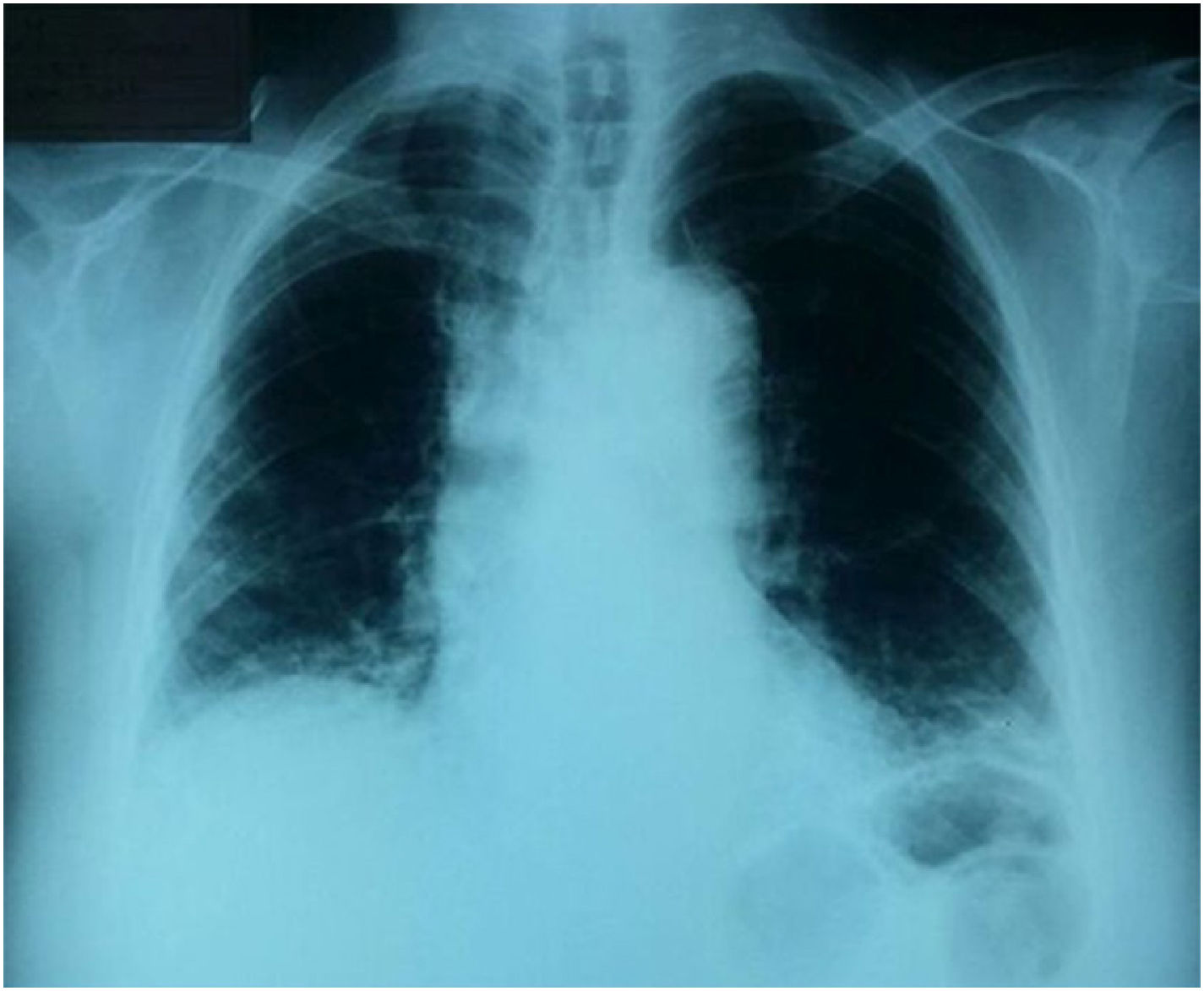
Case presentationA 68-year-old woman with no significant pathological, pharmacological, toxic or occupational antecedents consults for a picture of dyspnea associated with non-productive cough of 9 months of evolution without other related symptomatology. The physical examination showed: blood pressure 120/70, pulse: 80/min, respiratory rate: 24/min, temperature 36.8°C, oxygen saturation at room air of 90%, body mass index of 27kg/m2. At pulmonary auscultation, the patient presented velcro-like crackles at the basal level; the rest of organ systems had no alterations. Due to the presence of dyspnea and the finding on physical examination, it was performed a chest radiograph that showed interstitial reticular opacities distributed in the lower lobes and subpleural (Fig. 1).
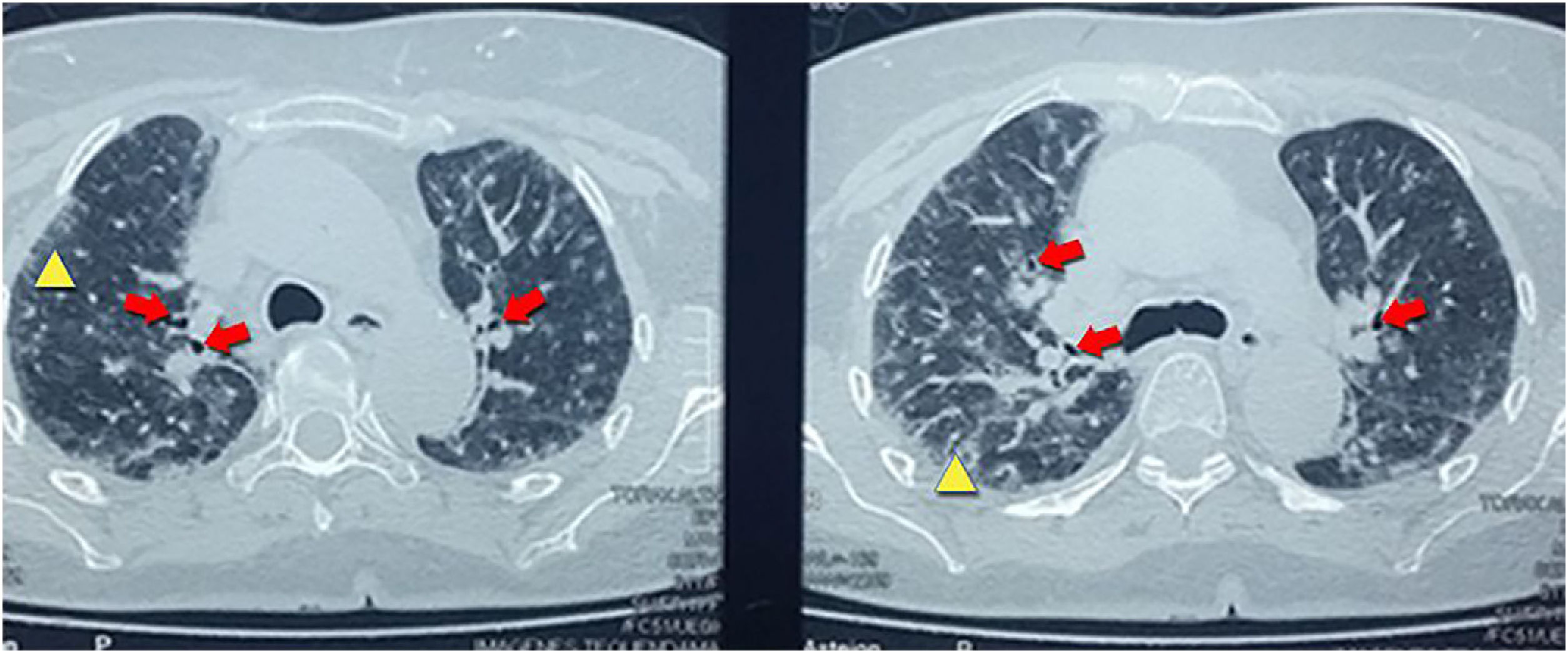
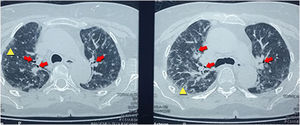
With the finding in the chest X-ray, it was performed a high-resolution computerized tomography that reported centrilobular nodules distributed in the upper lobes, associated with ground-glass areas, interlobular thickening with traction bronchiectasis suggestive of interstitial pneumonia type NSIP (Fig. 2).
With the foregoing, respiratory function tests were requested, which showed in spirometry a forced vital capacity of 2l (53% of the predicted), forced expiratory volume within the first second of 1.76l, compatible with a moderate restrictive pattern. Carbon monoxide diffusing capacity of 13.63ml/min/mmHg (48% of the expected), arterial blood gasometry on room air with PaO2: 61.6mmHg, O2 saturation: 91.8%, HCO3 of 17.7mmHg. An electrocardiogram that ruled out arrhythmias was also performed, as well as an echocardiogram that showed no compromise of systolic function of the left and right ventricles or valvulopathies. The mean pulmonary arterial pressure was 20mmHg.
Because of the findings we proceeded to seek possible etiologic factors that explain the interstitial commitment. When the patient was interrogated, she denied occupational, pharmacological and toxic exposures, as well as other systemic symptoms such as joint pain. Antinuclear antibodies, extractable nuclear antibodies, anti-smooth muscle antibodies and thyroid tests were performed, all of which were negative. In addition, studies were conducted to rule out hepatitis B and C, opportunistic infections with ELISA for HIV, fungi, and culture for mycobacteria, all of them being negative, which ruled out an infectious cause.
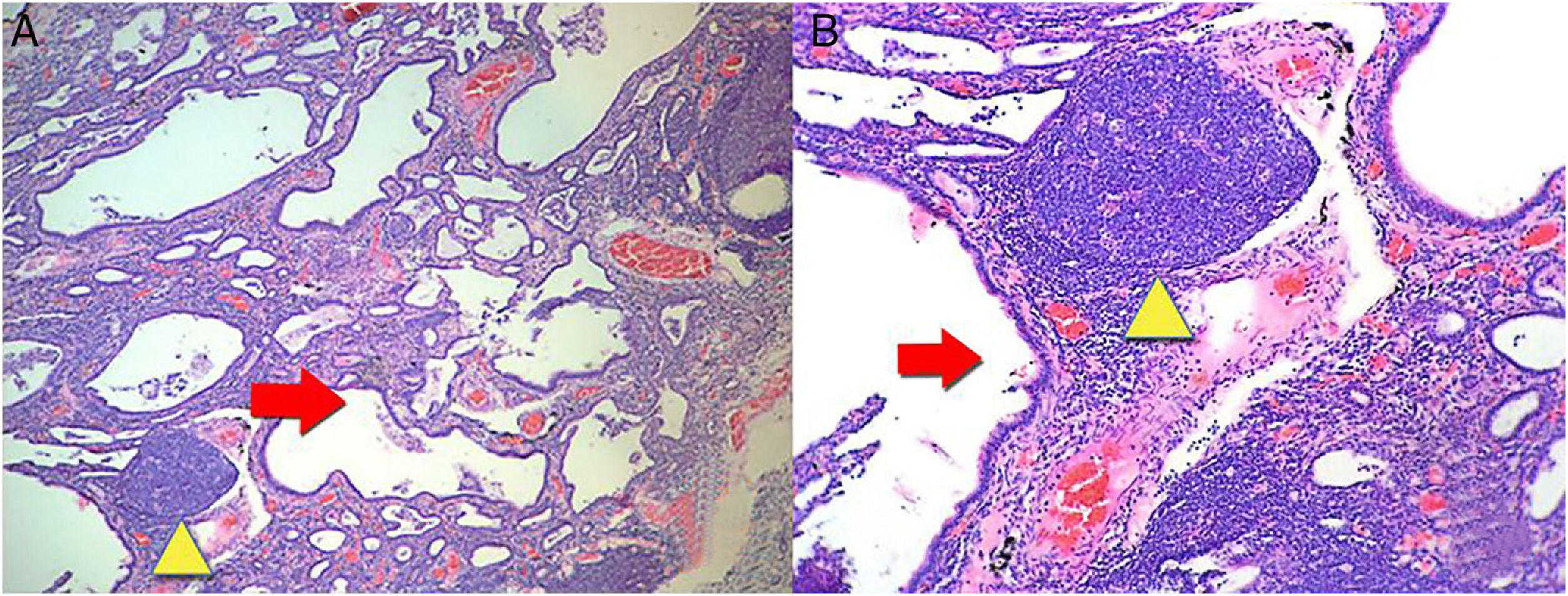
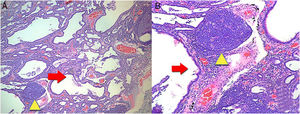
Then we proceeded to perform a lung biopsy that reported interstitial fibrosis accompanied by inflammatory infiltrate in patches, with the presence of a remarkable formation of lymphoid follicles, histological characteristics related to a NSIP in the fibrotic stage (Fig. 3). With the result, management with N-acetylcysteine and pulmonary rehabilitation was started.
(A) A biopsy of the lung where there are lesions with a pattern of usual interstitial pneumonia. Irregular advanced lesions with the presence of a remarkable formation of cysts (red arrow) and lymphoid follicles in patches (yellow triangle). 4× magnification. (B) More details with 10× magnification. Hematoxylin and eosin staining.
The patient remained asymptomatic from the pulmonary point of view during 2 years after diagnosis. Then started a clinical picture of 3 months of evolution consisting in pain and edema at the level of the proximal interphalangeal joints, metacarpophalangeal joints, as well as compromise of the shoulders and knees, in addition to the morning stiffness during 2h, with a count of 6/28 painful joints and 3/28 swollen joints, which allowed to determine an evaluation of clinical activity with a CDAI of 21. A rheumatoid factor (RF) of 50IU/ml (cut-off value 1–14IU/ml), anti-cyclic citrullinated peptide antibodies of 90IU/ml (cut-off value 25IU/ml) and C-reactive protein 55mg/l (cut-off value less than 5mg/l) were determined. These clinical data are correlated with the pulmonary and joint findings and the diagnosis of pulmonary compromise by RA was raised.
Management was started with disease-modifying antirheumatic drugs (DMARDs) based on methotrexate (MTX) at low doses of 12.5mg per week; the stability of pulmonary function and the control of the RA were maintained with this treatment.
DiscussionThe patient had a LORA because it started after 65 years of age. LORA, unlike the early onset RA, is characterized by having the same prevalence in women as in men; while the prevalence of early-onset RA is higher in women than in men, with a 3:1 ratio. In the LORA the clinical picture is much more acute, and its main symptoms are a longer duration of morning stiffness, frequent proximal joint involvement with apparent benign clinical course and systemic clinical manifestations2 that in the patient began with ILD.
The ILD encompasses a spectrum of diffuse fibrotic lesions and inflammatory parenchymal lesions. The idiopathic pulmonary fibrosis (IPF) is the ILD more closely related to aging: the onset before 50 years of age is not frequent and the incidence of IPF increases with age.4 However, beyond the association between IPF and aging, the epidemiology of other forms of ILD in the elderly is not known.
With the aging population, the ILD in the elderly is increasingly found in clinical practice. In addition, considerations related to age have implications for the care of the ILD. Compared with younger patients, the risks of surgical lung biopsy and immunosuppression are higher in elderly patients.5 Age can also have an impact on survival in ILD. However, it is not known whether ILD is more aggressive at the extremes of age, and the epidemiology of other ILD subtypes according to age is not known exactly. In contrast, in a recent study of patients older than 70 years it was found that the incidence of other forms of ILD changes with aging, being the most frequent IPF in 34%, and interstitial lung diseases associated with connective tissue diseases in 11%, being very significant.6
LORA is one of the entities most representative of the connective tissue diseases with lung involvement in aging. Approximately 5% of people with RA have clinical manifestations of lung involvement and 18% of mortality due to RA is attributed to pulmonary causes.7 The lung affectation by RA can occur at the level of the parenchyma, the pleura, the airway, the blood vessels and the respiratory muscles.
The most frequent lung involvement by LORA is the ILD. The presentation of ILD is similar to the idiopathic interstitial pneumonias (IIP). The histological patterns of interstitial disease found in RA are the same as in IIP. The patterns include: usual interstitial pneumonia, NSIP, cryptogenic organizing pneumonia, desquamative interstitial pneumonia, respiratory bronchiolitis associated with ILD, acute interstitial pneumonia and lymphoid interstitial pneumonia.8 An ILD with histologic pattern of NSIP in fibrotic phase was documented by biopsy in the patient (Fig. 3).
The factors that have been associated with RA-ILD are male gender, smoking habit and advanced age at the time of diagnosis of LORA; the latter is characterized by having a more acute onset and with systemic symptoms. The presence of anti-cyclic citrullinated peptide antibodies and RF has been related with the presence of extra-articular manifestations of LORA. High titers of RF with decreased carbon monoxide diffusing capacity have been associated with ILD-LORA as presented by the patient.9
The results of the imaging tests were related to the histological changes. The most frequent imaging findings in patients with a histological pattern of NSIP are ground-glass opacities, patchy or diffuse, bilateral, symmetrical and predominantly peripheral, subpleural and in lower lobes, and traction bronchiectasis, although this pattern predominates in the fibrotic phase (Fig. 2). In the initial stages of NSIP predominates the cellular phase, in which an interstitial infiltrate composed of lymphocytes, plasma cells and histiocytes is observed. As the disease progresses, there is a predominance of the fibrotic phase, where there is less infiltrate and it is replaced by fibrous tissue9,10 (Fig. 3).
Although the lung disease usually appears when RA is already diagnosed, in some individuals ILD may be the first manifestation of autoimmune disease, as it occurred in this patient; consequently, whenever an individual with ILD is studied, it should be interrogated for systemic symptoms, especially for Raynaud's phenomenon, cutaneous symptoms, dry syndrome, and muscular and joint symptoms,1 symptomatology that at the onset of the clinical picture the patient did not manifest. ILD may be the first clinical manifestation of an autoimmune disease, and it has been demonstrated that the lungs can be the initial site of immune deregulation in RA.11
The confirmation of an underlying autoimmune disease in people with ILD can be challenging. It has been documented in different follow-up studies of patients with ILD that over time up to 19% develop a classifiable autoimmune disease.7,9 In a case–control study of individuals initially diagnosed with ILD it was documented later an autoimmune disease in 4%, being RA the most frequent diagnosis. In their evolution, these individuals showed polyarthralgia and rheumatoid factor positivity.12 For this reason, early detection of the underlying RA would lead to opportune application of DMARD therapies that could improve clinical and radiological outcomes.
There is no standardized approach for the identification of an underlying autoimmune disease in people with ILD. The current practice to determine if an underlying autoimmune disease is present often includes a medical history, physical examination and circulating autoantibodies tests.7 A complete clinical evaluation of the characteristics of autoimmune disease, the realization of tests for specific autoantibodies and the consideration of radiographic and histopathological findings, which were carried out for the diagnostic approach of this patient, are important components to document a hidden autoimmune disease.13
The definitive diagnosis was an LORA that started with pulmonary manifestations with parenchymal involvement compatible with ILD-LORA, with a histological pattern of NSIP in the fibrotic phase. Then she presented joint manifestations, which allowed us to clarify the etiology of the underlying disease.
The treatment of ILD-LORA is still empirical and depends on the medical judgment based on the symptomatology of each individual. Due to the heterogeneity of the course of the lung disease, there are no controlled studies. It has been described that MTX, despite being the first line treatment for RA, along with anti-TNF, should be avoided in individuals with ILD because of the risk of progression or aggravation of the lung disease.14 On the other hand, the majority of medicines have been associated with acute pneumonitis, including azathioprine. However, there are studies that call into question the association between DMARD and acute pneumonitis; for example, in a cohort of patients with RA-ILD it was demonstrated that neither MTX nor anti-TNF-α agents were associated with acute complications or higher mortality.15 Something similar occurred in the study conducted by Suissa et al.16 in one of the stratified analyzes; MTX was a protective factor against the development of an acute interstitial complication in patients with previous lung damage: RR: 0.4 (95% CI: 0.2–0.9). Therefore, MTX at low doses was suggested for the management of the patient, because in the context of LORA with pulmonary compromise it is not an absolute contraindication, in addition, the eventual risk of MTX-induced lung disease has occurred in less than 5% of the reported series.15 In this order of ideas, this drug was chosen taking into account that in some studies it has not been observed that treatments with low doses of MTX cause worsening of ILD.17
ConclusionLORA is a systemic inflammatory disease of multifactorial autoimmune etiology, which can affect any structure of the respiratory system. The most frequent is the involvement of the pulmonary parenchyma, but it can also affect the pleura, the blood vessels, the airway and the respiratory muscles. The lung involvement can occur even before the joint manifestations of the disease and it should be addressed towards early diagnosis and treatment. The ILD-LORA is an important cause of morbidity and mortality in patients with LORA, and therefore it is important to classify the type of interstitial involvement. Given the worse prognosis that patients with ILD-LORA have, the diagnosis should be confirmed as soon as possible with the different aids available, both clinical and imaging or histological studies, in order to offer the appropriate and early treatment that allows us to avoid the progression of the disease, improve the quality of life and prevent dependence due to the disease. However, there is no consensus for the management of this disease, and for this reason it is necessary to re-evaluate the current recommendations for treatment through clinical trials with appropriate designs.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Ocampo-Chaparro JM, Hernández H, Reyes-Ortiz CA. Artritis reumatoide de inicio tardío asociada a enfermedad pulmonar intersticial. Rev Colomb Reumatol. 2018;25:287–291.