Alopecia areata (AA) is an autoimmune disease that generates non-scar loss of hair with varying degrees of involvement, including total loss of hair follicles. Despite being a benign entity, it has a great impact on the emotional and psychosocial life of patients. A wide variety of topical and oral treatments are currently available. We present the case of a 24-year-old patient with severe recurrent alopecia areata without response to multiple previous treatments, in which a secondary cause was ruled out and the histological diagnosis was confirmed with biopsy. Treatment with tofacitinib, a JAK inhibitor, was started, showing an excellent clinical response after one month of treatment.
La alopecia areata (AA) es una enfermedad autoinmune que genera pérdida no cicatrizal de cabello con diferentes grados de afectación, incluyendo la pérdida total de tallos pilosos. A pesar de ser una entidad benigna, tiene un gran impacto en el ámbito emocional y psicosocial de los pacientes. En la actualidad, se dispone de una amplia variedad de tratamientos tanto tópicos como orales. Se presenta el caso de una paciente de 24 años, con alopecia areata recurrente severa, sin respuesta a múltiples tratamientos previamente prescritos, en quien se descartó una causa secundaria y se confirmó diagnóstico histológico con biopsia. Se inició tratamiento con tofacitinib, un inhibidor de la JAK, con una excelente respuesta clínica al mes de iniciado el tratamiento.
Alopecia areata (AA) is an autoimmune disease that generates a non-scarring loss of hair, which can range from affecting circumscribed areas of hair (patchy alopecia), the total loss of the scalp (alopecia totalis), up to the total loss of terminal hair shafts (alopecia universalis). It affects 1–2% of the world population, with equal predilection for men or women.1 It is considered to have a multifactorial etiology and it has been reported its association with multiple inflammatory-mediated entities, such as lichen planus, systemic sclerosis, vitiligo, atopic dermatitis, allergic rhinitis, ulcerative colitis, Hashimoto's thyroiditis and systemic lupus erythematosus, among others,2–4 and with psychiatric disorders, mainly mood disorders and anxiety.5
Although it is considered a benign entity, it can have a high emotional impact and in the psychosocial sphere.6 Treatment depends on the extent of the disease; currently there are multiple pharmacological aids such as topical, oral or intralesional corticosteroids, topical minoxidil and, in more severe cases, topical immunotherapy or Janus kinase (JAK) inhibitors such as tofacitinib, ruxolitinib and baricitinib.7 The case of a patient with severe AA treated with tofacitinib, who showed a notable improvement in the first month of treatment is presented.
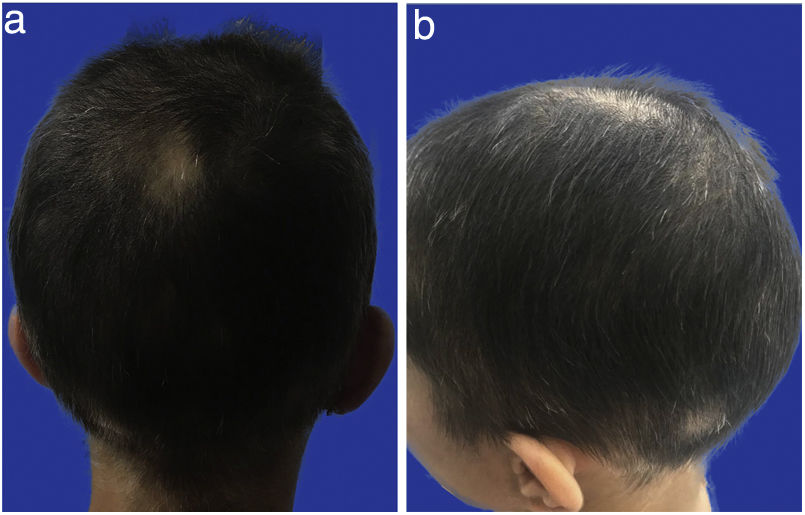
Case presentationA 24-year-old female patient who presents to the rheumatology consultation with a clinical picture of four years of poorly defined alopecic areas that extend throughout the scalp, recurrent, managed with multiple treatments that include minoxidil lotion 5%, clobetasol lotion 0.05%, oral corticosteroids at variable doses between 5 and 20&#¿;mg/day, intralesional corticosteroids for two years, finasteride 1&#¿;mg/day and three sessions of cryotherapy, without improvement and with rapid progression until compromising the entire scalp (Fig. 1a and b). The underlying skin was smooth, with no changes in color or consistency and no scars; there was no involvement of the eyebrows or the hair shafts of the rest of the body. On the review by systems, the patient reported episodes of conjunctival injection with epiphora, polyarhtralgias without synovitis, muscle weakness, paresthesia in the hands and feet, and conciliation insomnia; she denied oral ulcers, Raynaud’s phenomenon, gastrointestinal or urinary symptoms, or skin lesions.
There was no family history of AA or rheumatic diseases. During the physical examination of the consultation, poorly defined alopecic areas that extended throughout the scalp were observed, no nail involvement, muscle weakness, ocular or otorhinolaryngological involvement, neurological deficit, or other findings were documented. The laboratory tests, which included complete blood count, transaminases, blood glucose, urinalysis, C-reactive protein (CRP), vitamin B12, ferrokinetic profile, creatine kinase (CPK), thyroid-stimulating hormone (TSH), serology for hepatitis B, C, HIV, VDRL, extractable nuclear antibodies (ENA), antiphospholipid antibodies, chest and long bones X-ray, skull tomography and scalp fungal culture were normal, while positive antinuclear antibodies (ANA) in 1:160 with a speckle pattern were documented.
AA of primary origin was suspected, a scalp biopsy was taken by dermatology, finding a decrease in the count of terminal follicles, with lymphoid inflammatory infiltrates around the lower segment of the hair follicles (Figs. 2 and 3), which confirms the diagnosis. Due to the severity of the compromise and the failed previous treatments, tofacitinib was prescribed at a dose of 11&#¿;mg per day (presentation of extended release), and a notable improvement was observed after one month of treatment, with repopulation of 80% of the scalp with healthy terminal hair shafts and of normal thickness (Figs. 4–6 Figs. 4–6). After six months of treatment, taking the current medication, the patient persisted without alopecic areas.
AA is an entity that, despite not having a wide prevalence and having a benign course, its importance lies in the associated high emotional and social burden in the patients who present it.6,8 In the majority of the cases its origin is primary, although it has also been documented as a clinical manifestation of other pathological entities mediated by inflammation and endocrine disorders2–4; therefore, for therapeutic purposes, in its presence, and only if the anamnesis or the physical examination suggest it, a possible underlying secondary cause should be studied. In our patient, despite the fact that there were some symptoms reported in the review by systems, it was not possible to document a secondary cause, there were not findings on the physical examination that suggested it, and the extension studies were normal. The only finding was an elevation of ANA, which has been reported in the literature to be elevated in this population, even at titers such as those of our patient, a phenomenon that has been observed especially in women.4,9,10
There are multiple therapeutic options for this entity, several of them used by the patient, without any improvement. Among the current treatments there are topical corticosteroids, especially of high potency; oral at high doses; and intralesional, preferable for those patients with limited patchy alopecia or with involvement of less than 50% of the scalp. For those patients with a greater extension compromised, topical immunotherapy with agents such as dinitrobenzene (DNCB), squaric acid dibutylester (SADBE) or diphenylcyclopropenone (DPCP) is preferred, which have shown good effectiveness in patients with extensive patchy alopecia, although such benefits are less in patients with alopecia totalis.7,11
Thanks to a better understanding of the pathophysiology of this entity, as well as the discovery of the important role played by the activation of cytotoxic T lymphocytes - CD8+, which are necessary and sufficient to trigger the disease through inflammatory pathways that involve a response to interferon gamma and signaling through JAK,12 JAK inhibitors have been studied, among them tofacitinib, ruxolitinib and baricitinib, used especially in patients with severe alopecia.13 The efficacy of these drugs has been reported in different cases and studies to date, with the most recent meta-analysis with a systemic review that included 30 studies and 289 cases of patients treated with JAK inhibitors, in whom a 72.4% of response was observed and 45.7% of the patients had a response greater than 50%, as well as an average of 2.2 months of treatment to obtain this response. In the patients with alopecia totalis o universalis14 a longer duration was required, a data which is consistent with the case of our patient, who presented a response greater than 80% in the first month of treatment.
Regarding tofacitinib, the first successful case of treatment in a patient with psoriasis was reported in 2014.15 In a study of 66 patients treated with 5&#¿;mg de tofacitinib, an improvement of more than 50% was observed in 32% of them.16 In another study of 90 patients with varying degrees of involvement, 77% had a response to treatment with tofacitinib, and of them, 58% had a response greater than 50%.17 In 2018, in an open study of 12 patients with moderate and severe alopecia, tofacitinib 5&#¿;mg was administered and the dose was increased up to 10&#¿;mg in those who did not respond; a response was observed in 11 patients, eight of them with a response higher than 50%.18 In our case, this would be the first report in Colombia on the use of tofacitinib in a patient with AA.
ConclusionAA and its variants are a pathology with a high impact on the quality of life of the patients and resistance and relapses can occur sometimes despite the use of multiple treatments. Today there is important evidence on the use of JAK inhibitors in this entity, especially in cases that are difficult to control. Our case is the first reported in the literature on the successful use of tofacitinib in a patient with AA in Colombia.
Ethical considerationsInformed consent was requested and the research complies with the current bioethical regulations.
FundingNone.
Conflict of interestNone.
Thanks to Dr. Julia Inés Mesa Villegas, MD, specialist in Dermatology and Dermatopathology, for performing and interpreting the histological images.