Tuberculin is the globally accepted delayed cutaneous hypersensitivity test for the diagnosis of latent tuberculosis. The alteration of cellular immunity induced by disease-modifying drugs used in rheumatoid arthritis may give a false negative result, also known as cutaneous anergy. There are no studies that determine the frequency of anergy in patients with rheumatoid arthritis and on immunosuppressive therapy.
ObjectiveTo determine the frequency and possible factors associated with cutaneous anergy in a group of patients with rheumatoid arthritis and on immunosuppressive therapy.
MethodsCross-sectional analytical observational study including 100 patients with rheumatoid arthritis on immunosuppressive therapy. They were tested for delayed cutaneous hypersensitivity with tuberculin, and a control test with tetanus toxoid. The non-reactivity of both tests was defined as anergy.
ResultsThe overall frequency of cutaneous anergy was 9% (n = 11). It occurred in 33% of men versus 6% of women. The mean age was 57 years, and 89% were over 50 years-old. Being female behaved as a protective variable for the generation of anergy, OR 0.795 [95% CI, 0.658 - 0.959, P<.05]. All patients with anergy were being treated with corticosteroids, 44% with methotrexate, and 33% with biological therapy. Treatment with moderate to high dose prednisone and biological therapy were independently associated as risk factors for presenting with anergy, OR 1.044 [95% CI, 1.008-1.080 P<.05] and OR 1.096 [95% CI, 1.016-1.182, P<.05], respectively. The overall positivity for tuberculin was 13%. Symptoms associated with disease activation were present in 38% of these. All cases (n= 1) of confirmed active tuberculosis were excluded.
ConclusionsThe high prevalence of cutaneous anergy in patients with RA in the present study, and the evidence presented here, supports the recommendation of a second diagnostic test (tuberculin booster or Interferon-Gamma Release Assays) for the diagnosis of latent TB in patients with RA on immunosuppressive therapy.
La tuberculina es la prueba de hipersensibilidad cutánea tardía mundialmente aceptada para el diagnóstico de tuberculosis latente. La alteración de la inmunidad celular inducida por los fármacos modificadores de la enfermedad utilizados en la artritis reumatoide puede dar un resultado falso negativo, también conocido como anergia cutánea. No hay estudios que determinen la frecuencia de anergia en pacientes con artritis reumatoide y terapia inmunosupresora.
ObjetivoDeterminar la frecuencia y los posibles factores asociados con la anergia cutánea en un grupo de pacientes con artritis reumatoide y terapia inmunosupresora.
MétodosEstudio observacional analítico transversal que incluyó a 100 pacientes con artritis reumatoide con terapia inmunosupresora. Se les realizó una prueba de hipersensibilidad cutánea tardía con tuberculina y una prueba de control con toxoide tetánico. La no reactividad de ambas pruebas se definió como anergia.
ResultadosLa frecuencia general de anergia cutánea fue del 9% (n = 11). Ocurrió en el 33% de los hombres versus el 6% de las mujeres, la edad promedio fue de 57 años y el 89% tenía más de 50 años. El sexo femenino se comportó como una variable protectora para la generación de anergia (OR 0,795; IC 95%: 0,658-0,959; p < 0,05). Todos los pacientes con anergia usaron corticosteroides, el 44% fue tratado con metotrexato y el 33% con terapia biológica. El tratamiento con dosis de moderadas a altas de prednisona y terapia biológica se asoció de manera independiente como factor de riesgo para la presentación de anergia: OR 1,044 (IC 95%: 1,008-1,080; p < 0,05) y OR 1,096 (IC 95%: 1,016-1,182; p < 0,05), respectivamente. La positividad general para la tuberculina fue del 13%. Los síntomas asociados con la activación de la enfermedad estaban presentes en el 38% de ellos. Se excluyeron todos los casos de tuberculosis activa confirmada (n = 1).
ConclusionesLa alta prevalencia de anergia cutánea en pacientes con artritis reumatoide en el presente estudio y la evidencia presentada respaldan la recomendación de una segunda prueba de diagnóstico (refuerzo de tuberculina o IGRA) para el diagnóstico de tuberculosis latente en pacientes con artritis reumatoide y terapia inmunosupresora.
In rheumatoid arthritis (RA), synthetic and biological immunosuppressive therapy are the cornerstone of treatment. This type of therapy might carry a greater risk of reactivation of latent chronic infections such as tuberculosis (TB) up to four times above the population without these treatments.1,2 The majority of people control the infectious process after exposure, infection and subsequent dissemination with persistent latent viable germs contained in granulomas without presenting clinical manifestations.3,4
According to the latest global report, Colombia is a country with an intermediate incidence of 25 to 49 TB cases per 100,000 inhabitants and it is estimated that one third of the population may have latent TB.5,6 Between 5 to 15% of people with latent TB develop active infection during life.7 This depends on the virulence of the bacteria and immunity of the host.4,8,9 A relationship has been found with the use of synthetic, biological and corticosteroid immunosuppressive therapy with the transformation of a latent phase to an active phase of TB.4,8,9 Specifically in RA, there is evidence of TB reactivation with disease-modifying medications, in particular tumor necrosis factor alpha (iTNF-a) inhibitors.10–12
The World Health Organization (WHO) and the Center for Disease Control (CDC) recommend the detection of latent disease in high-risk patients with the intention of treating any positive test.4,13 Tests should be practiced systematically in people infected with HIV, in contact with those infected with pulmonary TB, prior to initiating biological therapies, people on dialysis, solid organ recipients or hematological transplantation, among others.13–19 Indirect tests are used for diagnosis by measuring the immune response in vivo such as tuberculin, or in vitro, with the interferon gamma release assay (IGRAs). There is no quality evidence that one is superior to the other.20,21
The tuberculin skin test is based on the principle that Mycobacterium Tuberculosis infection produces a delayed hypersensitivity reaction mediated by T lymphocytes in response to certain antigens present in extracts from filtered cultures called tuberculins. These purified derived proteins (PPD) produce skin induration after vasodilation and chemotaxis of inflammatory cells.4,22–24 The standard dose is 0.1ml intradermally (concentration of 5 tuberculin units) per single puncture. It is done in the forearm area, which should be free of lesions and preferably away from venous vascular areas.23,25,26 The reading should be carried out between 48 and 72hours after the puncture for visual evaluation and / or palpation. The use of the Sokal method is recommended to mark the indurated area.23,25,26 It is a safe test that rarely generates allergic reactions.
The sensitivity of the test is variable and the frequency of false negatives can reach 50% according to some reports in patients with different types of immunosuppression.27–32 On the other hand, there are conditions that may occur with induration in the absence of tuberculous infection such as non-tuberculous mycobacterial exposure.33,34
The tuberculin test depends on the integrity of the cellular immune system that can be altered by noxas such as the use of immunosuppressive therapy. The absence of reaction in a previously exposed patient is known as anergy and is objectified by the inability to express delayed hypersensitivity to skin tests of common antigens.35–38
Anergy has been characterized in people with HIV infection, dialysis, diabetes, malnourished population, among others.22,27–32,39–41 It is also found in healthy people where IL-10 producing T cells are constitutively activated.40–43
It is plausible that pharmacological immunosuppression in RA causes anergy by altering cellular immunity. This has encouraged different scientific societies to recommend conducting a tuberculin booster two weeks later to improve sensitivity or another sequential alternative test (IGRAs).4,13–16,19,44 Thus, boosting the hypersensitivity response and reducing false negatives.14,19 Several studies have assessed these tests in groups of patients on dialysis, increasing the number of positive tuberculins by 12% 17,45 and also in RA where the reinforcement increased positivity by 15%.17,18
However, this recommendation has no scientific support as there are no studies that establish the prevalence of anergy in patients with RA with synthetic or biological immunosuppressive therapy. In our environment with difficulties in accessing health services, the requirement of an additional test may delay the start of therapy for months.
In order to objectify anergy, ubiquitous and frequent antigens in the general population to which a healthy person should always react are used as skin tests. The negativity of these could be interpreted as anergy. The most frequently used antigens are those derived from candida, trichophyton, mumps virus, tetanus toxoid and streptokinase-streptodornase.36,38,46–57 The use of antigens such as tetanus toxoid and candidines have proven to be the diagnostic standard, other candidates historically used such as mumps virus, can lead to confusion and have been relegated from this type of study.58 The interpretation of these tests is also due to the skin induration area such as tuberculin.59–61
The frequency of late cutaneous hypersensitivity to tetanus toxoid in healthy subjects is 79% to 90% and has a good correlation with the leukocyte migration inhibition test, which makes it a valuable antigen in the evaluation of late hypersensitivity.62 The cutaneous reactivity to tetanus toxoid is independent of antibody titers for tetanus and no relationship has been demonstrated between immune response measures and the interval since the last booster immunization.62,63 Tetanus toxoid has proven useful in the evaluation of cellular immunity and as a marker of cutaneous anergy.40,42 It is a sensitive marker, has a low incidence of side effects and produces a slight but beneficial reinforcement of the serum antibody against tetanus toxoid.40,42,43 Additionally, vaccination does not elicit positivity in non-responders, which implies that the non-response is secondary to host factors rather than the absence of antigenic stimulation.64
Induration diameter has varied through numerous studies that have used values greater than 2mm,28,29,32,65–67 3mm 27 and 5mm 39,65,67 to be considered positive. In the present study we use a 5mm cut as the diagnostic standard.
In general terms, the concomitant failure of the response of at least one skin control in patients who have tuberculin applied to them is defined as anergy.27,32,66,67 Whether there is a single negative result 68 or a negative result to all controls (if there are several) as in the case of the CMI Multitest manufactured by Merieux Institute that uses 7 antigens that it's no longer available.69,70 Currently, tests of one or two controls are used, such as the one used in this study, in which the tetanus toxoid was chosen as the most suitable.40,42
The objective of this article is to estimate the frequency of anergy and latent TB in a group of patients with RA and immunosuppressive therapy in Bogotá during 2019. As well as describe the epidemiological, clinical and pharmacological characteristics that are related to this condition.
Materials and methodsMulticenter cross-sectional analytical observational study in which 102 adult volunteers with RA diagnosis were included by 2010 ACR / EULAR criteria with current use of immunosuppressive treatment for more than three months defined as: Methotrexate in doses greater than or equal to 7.5mg / week and / or leflunomide at a dose greater than or equal to 10mg / day and / or sulfasalazine at a dose greater than or equal to 500mg / day and / or prednisone (or its equivalent) at a dose greater than or equal to 5mg per day and / or biological therapy. These subjects were captured in the rheumatology outpatient clinic based at the National University Hospital of Colombia and the integrated north subnet of health services of Bogotá during 2019. The ages of the participants ranged between 23 and 85 years and are stratified in 7 groups (20-30, 31-40, 41-50, 51-60, 61-70, 71-80 and> 80 years). Data on their age, weight, comorbidities, time of diagnosis of RA, immunosuppressive therapy and general symptoms were collected. Treatments such as azathioprine, cyclosporine, cyclophosphamide or mycophenolate mofetil were excluded. Patients with contraindications for skin tests, patients with active malignancy or other rheumatic disease were excluded. Patients with positive tuberculin tests were followed in the outpatient setting with chest x rays to rule out active disease. Patients with active or extrapulmonary pulmonary TB or in contact with confirmed or suspected TB and pregnant patients were also excluded.
SamplingPatients were asked to participate in the study during the consultation or through a telephone call. Informed consent was signed explaining the procedures, advantages and risks of the intervention. A questionnaire was applied and background checks were done. After asepsis, two intradermal reagents were applied. The tuberculin is performed in the forearm by Mantoux technique with 5 UT in 0.1ml of the solution, in the same way 0.1ml of tetanus toxoid is administered 5cm from the first. Induration reading was done 48 to 72hours after application by certified personnel. Anergy is defined if the result of the induration is negative for both tests with a cut-off point less than 5mm.27,28,32,39,65–68
Latent TB is defined as a positive reaction in the tuberculin skin test in the absence of symptoms suggestive of active pulmonary infection (Cough or persistent dyspnea greater than 2 weeks, weight loss over 10% in the last 6 months, recurrent night sweating in the last 6 months or febrile peaks over 38 degrees in the last 6 months without identified infectious focus).71
Statistical analysisFor the descriptive analysis of the sociodemographic aspects and the findings of the skin tests of the patients, absolute distributions, relative distributions and summary indicators such as quartiles, interquartile range, maximum values and minimum values were used.
A non-probabilistic sampling was performed for the selection of the subjects to be included in the study (n = 102) to determine statistical estimates of association, with a power of 80%, a chance risk of 5% and a OR of 1.75 at minus based on an esimated anergy prevalence of 20%.72 The study groups were evaluated as nominal, continuous and percentile variables.
To determine the association of variables with the generation of anergy, Fisher's exact test or Chi square were used for categorical variables, as applicable. A confidence interval of 95% was taken into account. A p less than or equal to 0.05 was considered significant. The risk factors associated with the development of anergy were evaluated through the logistic and Cox regression analysis for the dependent variables of anergy and non-anergy.
Ethical considerationsThe present study meets the requirements for research in humans according to resolution 8430 of 1993 of the Ministry of Health. According to article 11 of the same resolution, the present study is classified as with minimal risk. It was approved by the ethics committee of the institutions involved.
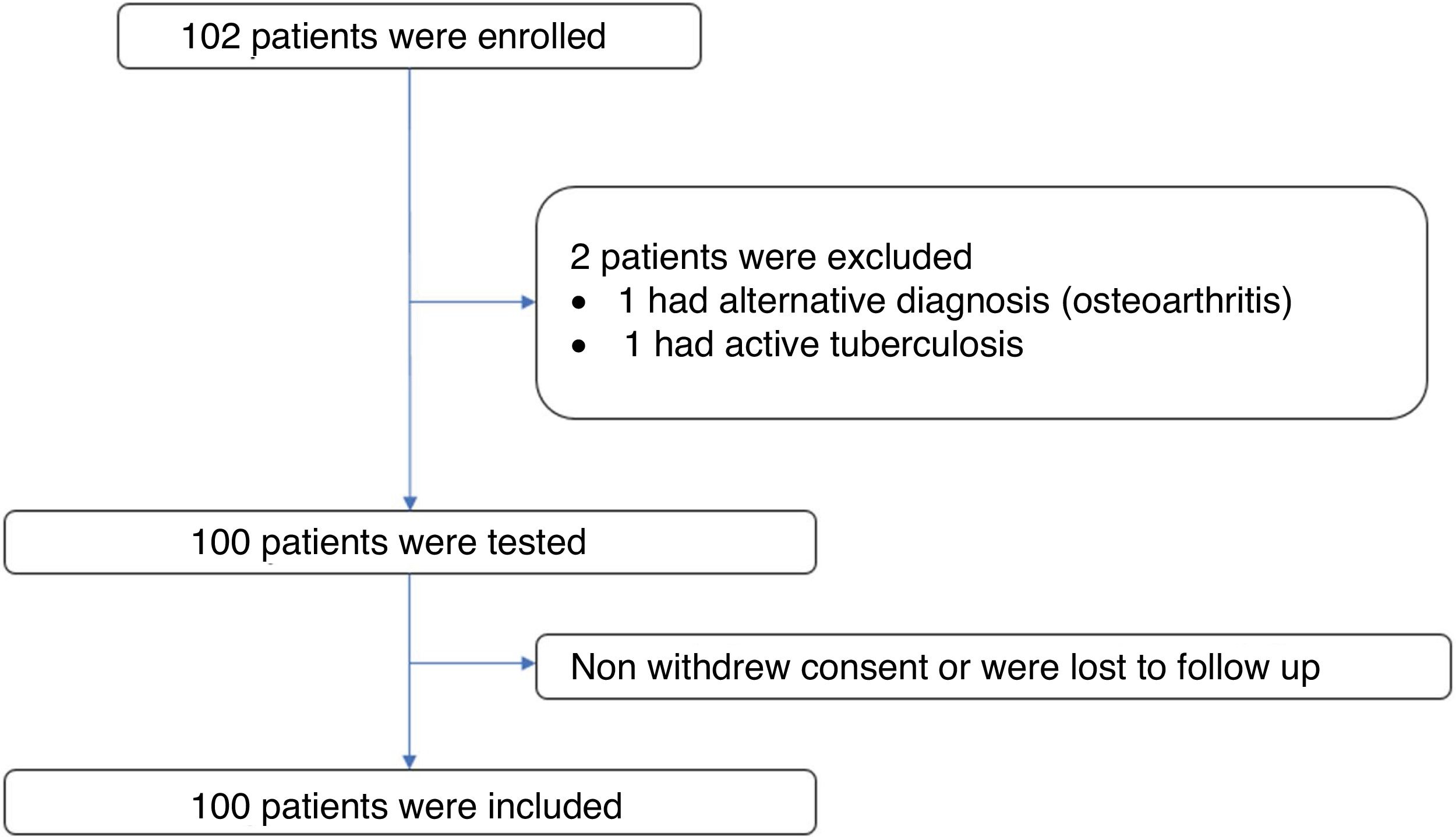
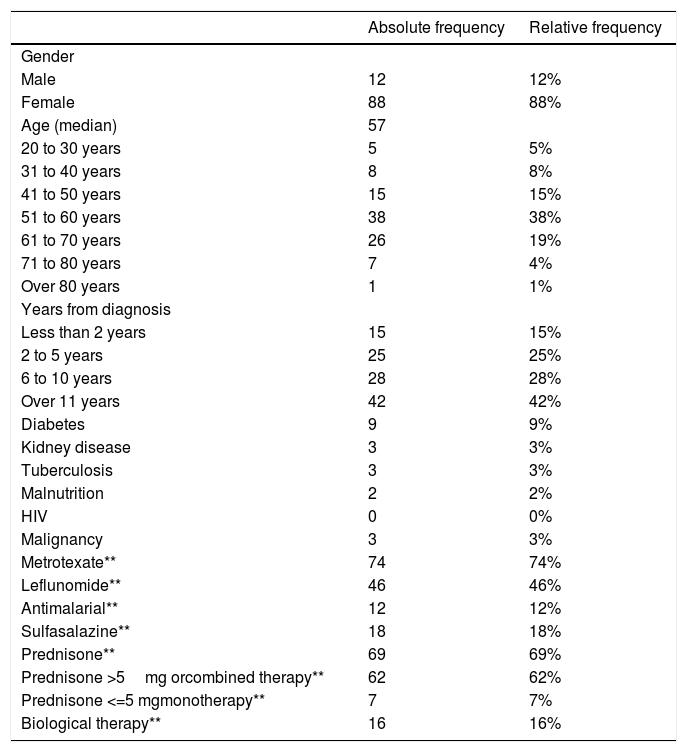
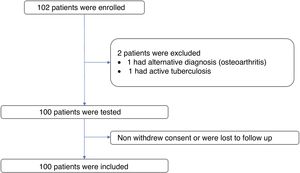
ResultsPatient characteristicsA total of 102 subjects were evaluated and 101 volunteers entered. One subject was excluded from the study because of an alternative diagnosis of osteoarthritis. Of the 101 volunteers, one was excluded due to confirmation of active tuberculosis in the follow up. There was no loss of information. Figure 1 The median age of the participants was 57 years (interquartile range = 12), 88% of these were women (n = 88). The time since diagnosis of RA was over 10 years in 42% of the patients (n = 42) and under 2 years in 15% (n= 15). The most frequent age group was 51 to 60 years old with 38% of the participants (n = 38). The most frequent comorbidity was diabetes mellitus in 9%. The most frequent immunosuppressive treatment was methotrexate followed by prednisone or equivalent and leflunomide with 74%, 69% and 46% of patients respectively. Table 1 shows the summary of the sociodemographic characteristics of the volunteers.
Distribution of the sociodemographic aspects of the participants (n = 100).
| Absolute frequency | Relative frequency | |
|---|---|---|
| Gender | ||
| Male | 12 | 12% |
| Female | 88 | 88% |
| Age (median) | 57 | |
| 20 to 30 years | 5 | 5% |
| 31 to 40 years | 8 | 8% |
| 41 to 50 years | 15 | 15% |
| 51 to 60 years | 38 | 38% |
| 61 to 70 years | 26 | 19% |
| 71 to 80 years | 7 | 4% |
| Over 80 years | 1 | 1% |
| Years from diagnosis | ||
| Less than 2 years | 15 | 15% |
| 2 to 5 years | 25 | 25% |
| 6 to 10 years | 28 | 28% |
| Over 11 years | 42 | 42% |
| Diabetes | 9 | 9% |
| Kidney disease | 3 | 3% |
| Tuberculosis | 3 | 3% |
| Malnutrition | 2 | 2% |
| HIV | 0 | 0% |
| Malignancy | 3 | 3% |
| Metrotexate** | 74 | 74% |
| Leflunomide** | 46 | 46% |
| Antimalarial** | 12 | 12% |
| Sulfasalazine** | 18 | 18% |
| Prednisone** | 69 | 69% |
| Prednisone >5mg orcombined therapy** | 62 | 62% |
| Prednisone <=5 mgmonotherapy** | 7 | 7% |
| Biological therapy** | 16 | 16% |
* Data are presented in median (interquartile range).
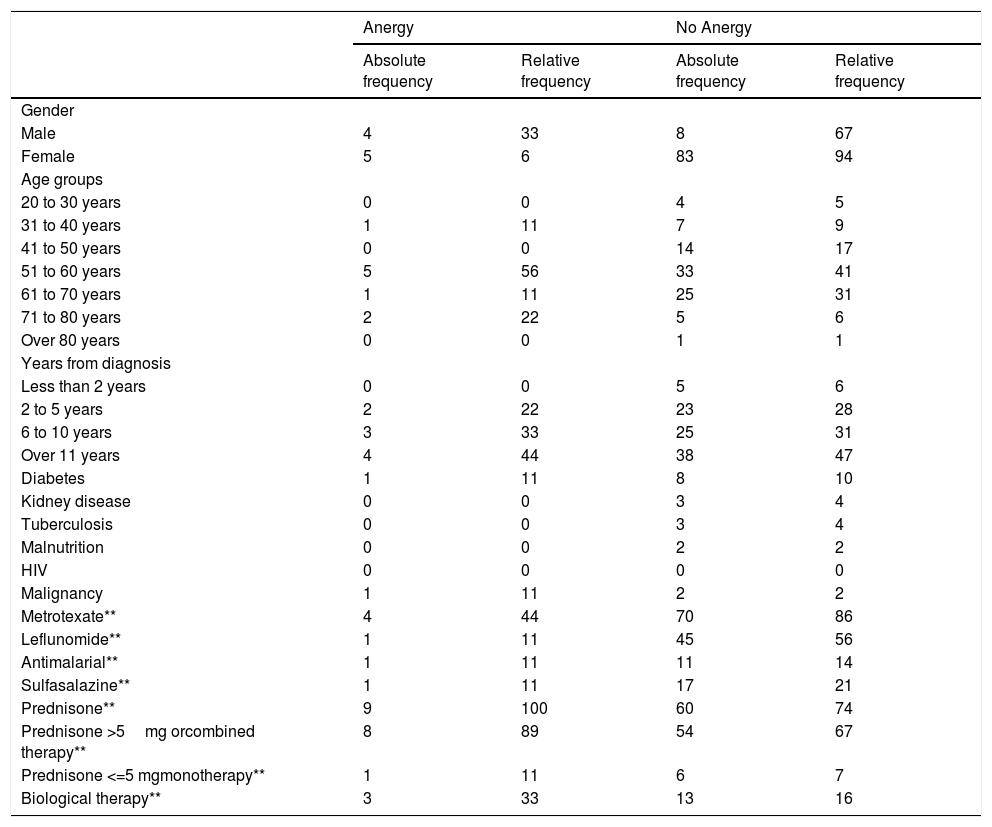
The frequency of cutaneous anergy was 9% (n = 9). It occurred in 33% of men versus 6% of women. Most cases presented the age group of 51 to 60 years with 56% (n = 5) and 89% were older than 50 years (n = 8). More than 10 years of illness was frequent with 44% of patients with anergy (n = 4). Only one of the patients with anergy presented associated comorbidity and it was due to diabetes. Table 2. All patients with anergy were treated with corticosteroids (n = 9) and only one of these used low doses of prednisone or equivalent in monotherapy (less than or equal to 5mg), methotrexate was used in 44% (n = 4) and biological therapy in 33% (n = 3).
Distribution of the Anergy findings (n = 9) an No Anergy (n=81).
| Anergy | No Anergy | |||
|---|---|---|---|---|
| Absolute frequency | Relative frequency | Absolute frequency | Relative frequency | |
| Gender | ||||
| Male | 4 | 33 | 8 | 67 |
| Female | 5 | 6 | 83 | 94 |
| Age groups | ||||
| 20 to 30 years | 0 | 0 | 4 | 5 |
| 31 to 40 years | 1 | 11 | 7 | 9 |
| 41 to 50 years | 0 | 0 | 14 | 17 |
| 51 to 60 years | 5 | 56 | 33 | 41 |
| 61 to 70 years | 1 | 11 | 25 | 31 |
| 71 to 80 years | 2 | 22 | 5 | 6 |
| Over 80 years | 0 | 0 | 1 | 1 |
| Years from diagnosis | ||||
| Less than 2 years | 0 | 0 | 5 | 6 |
| 2 to 5 years | 2 | 22 | 23 | 28 |
| 6 to 10 years | 3 | 33 | 25 | 31 |
| Over 11 years | 4 | 44 | 38 | 47 |
| Diabetes | 1 | 11 | 8 | 10 |
| Kidney disease | 0 | 0 | 3 | 4 |
| Tuberculosis | 0 | 0 | 3 | 4 |
| Malnutrition | 0 | 0 | 2 | 2 |
| HIV | 0 | 0 | 0 | 0 |
| Malignancy | 1 | 11 | 2 | 2 |
| Metrotexate** | 4 | 44 | 70 | 86 |
| Leflunomide** | 1 | 11 | 45 | 56 |
| Antimalarial** | 1 | 11 | 11 | 14 |
| Sulfasalazine** | 1 | 11 | 17 | 21 |
| Prednisone** | 9 | 100 | 60 | 74 |
| Prednisone >5mg orcombined therapy** | 8 | 89 | 54 | 67 |
| Prednisone <=5 mgmonotherapy** | 1 | 11 | 6 | 7 |
| Biological therapy** | 3 | 33 | 13 | 16 |
The overall positivity for tuberculin was 13%. Of these, 62% were asymptomatic (n = 8) and 38% were symptomatic (n = 5). The most frequent symptom was unexplained weight loss in 31% (n = 4) followed by cough and sweating with 23% each (n = 3). Only 15% of patients experienced fever (n = 2). None of the patients experienced dyspnea or hemoptysis.
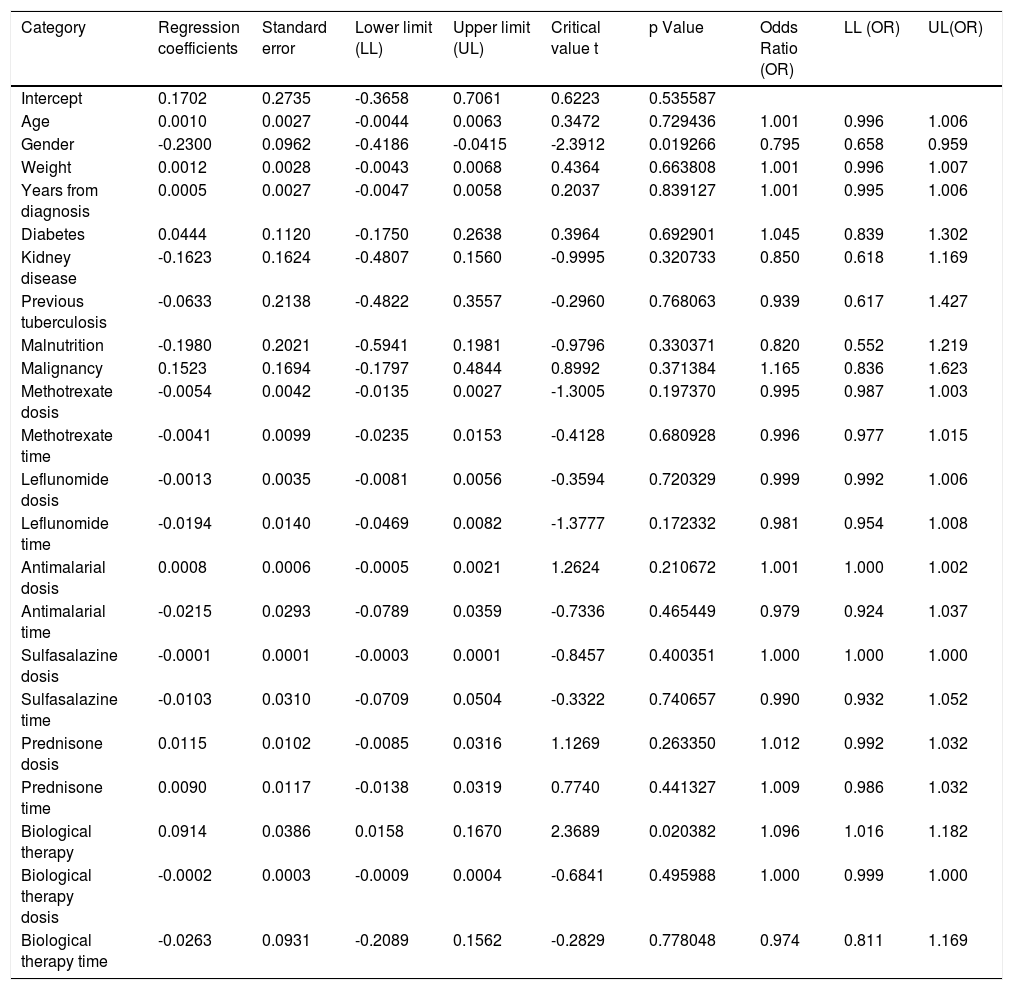
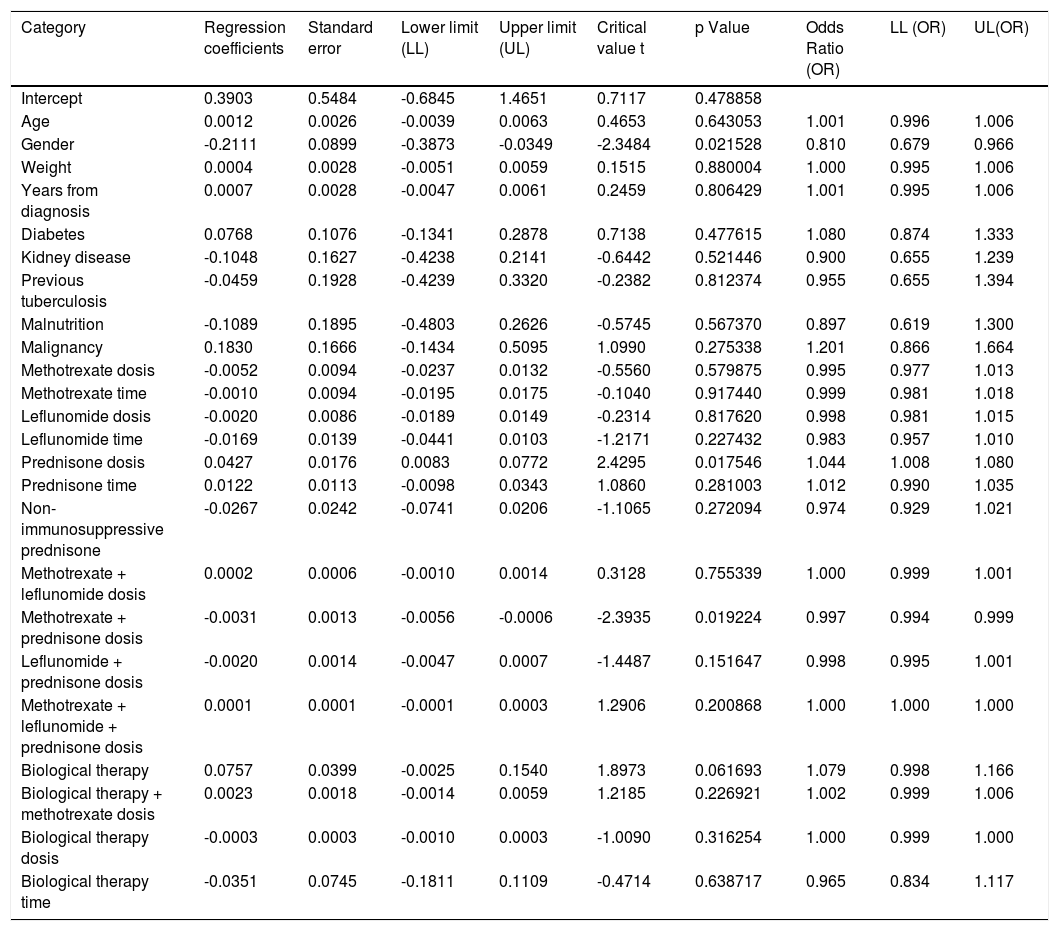
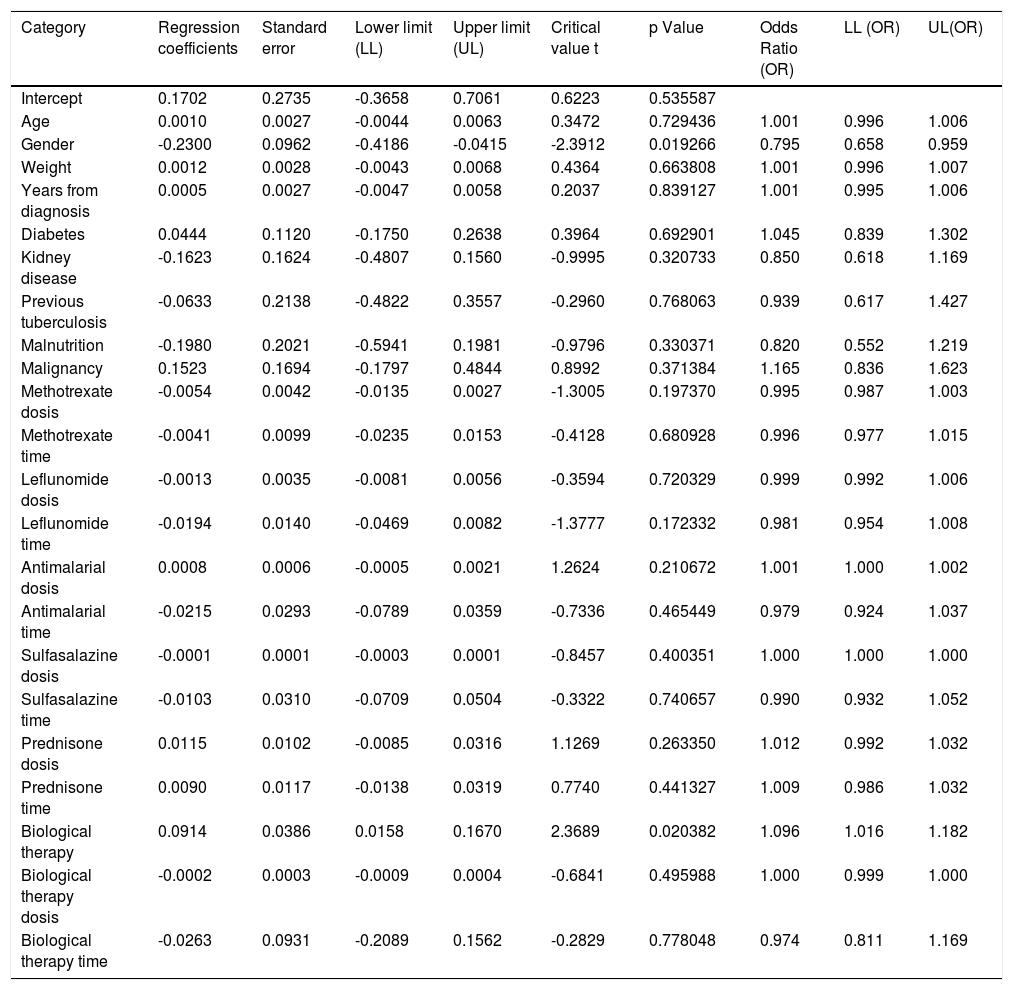
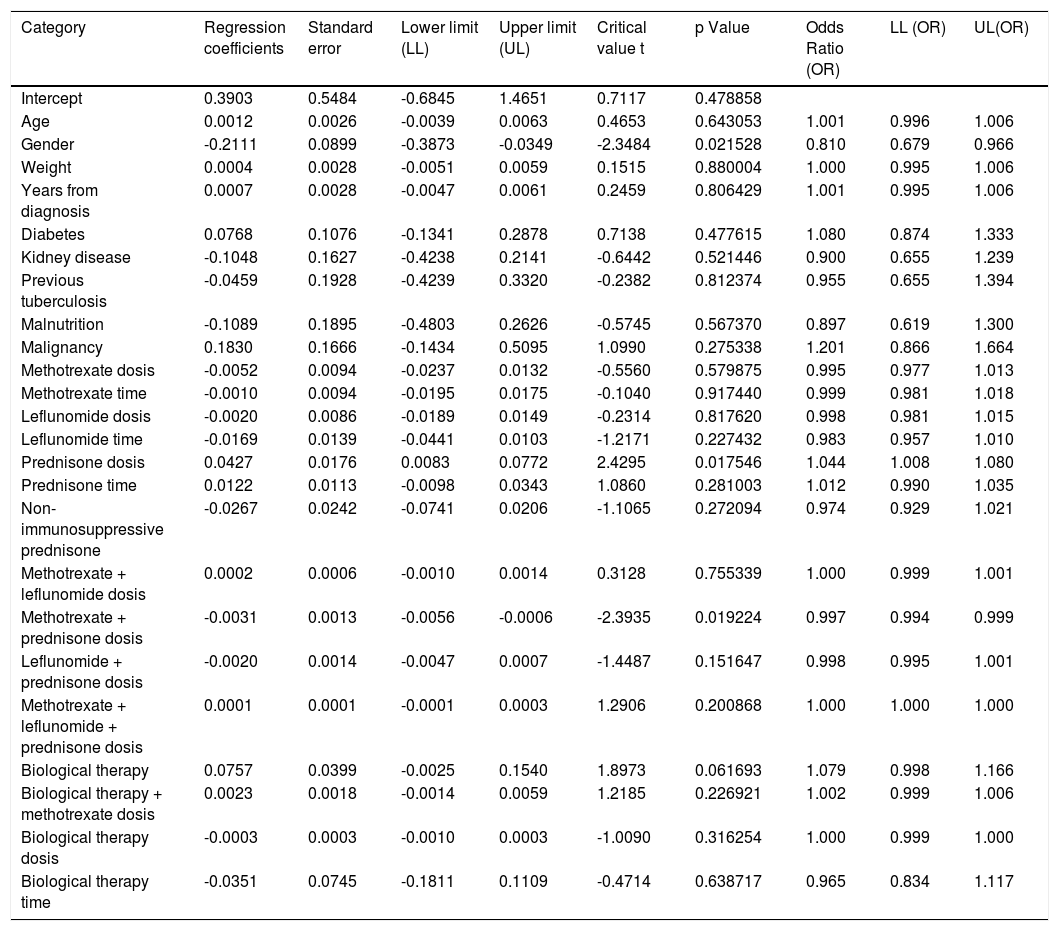
When assessing whether anergy presence is influenced by other variables, a multivariate analysis was performed with the logistic regression model. A model was designed in which the variables considered relevant were included. This model was evaluated for the presence of interactions (Comorbidities, sociodemographic variables, dose and interaction time of immunosuppressive medications). Table 3. In this model, female sex behaved as a protective variable for the generation of anergy OR 0.795 [95% CI, 0.658 - 0.959, p <0.05] and biological therapy as a risk factor OR 1.096 [95% CI, 1,016-1,182, p <0.05]. No associations with statistical significance were found in the rest of the analyzed variables.
Dependent variable logistic regression “Cutaneous anergy”.
| Category | Regression coefficients | Standard error | Lower limit (LL) | Upper limit (UL) | Critical value t | p Value | Odds Ratio (OR) | LL (OR) | UL(OR) |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.1702 | 0.2735 | -0.3658 | 0.7061 | 0.6223 | 0.535587 | |||
| Age | 0.0010 | 0.0027 | -0.0044 | 0.0063 | 0.3472 | 0.729436 | 1.001 | 0.996 | 1.006 |
| Gender | -0.2300 | 0.0962 | -0.4186 | -0.0415 | -2.3912 | 0.019266 | 0.795 | 0.658 | 0.959 |
| Weight | 0.0012 | 0.0028 | -0.0043 | 0.0068 | 0.4364 | 0.663808 | 1.001 | 0.996 | 1.007 |
| Years from diagnosis | 0.0005 | 0.0027 | -0.0047 | 0.0058 | 0.2037 | 0.839127 | 1.001 | 0.995 | 1.006 |
| Diabetes | 0.0444 | 0.1120 | -0.1750 | 0.2638 | 0.3964 | 0.692901 | 1.045 | 0.839 | 1.302 |
| Kidney disease | -0.1623 | 0.1624 | -0.4807 | 0.1560 | -0.9995 | 0.320733 | 0.850 | 0.618 | 1.169 |
| Previous tuberculosis | -0.0633 | 0.2138 | -0.4822 | 0.3557 | -0.2960 | 0.768063 | 0.939 | 0.617 | 1.427 |
| Malnutrition | -0.1980 | 0.2021 | -0.5941 | 0.1981 | -0.9796 | 0.330371 | 0.820 | 0.552 | 1.219 |
| Malignancy | 0.1523 | 0.1694 | -0.1797 | 0.4844 | 0.8992 | 0.371384 | 1.165 | 0.836 | 1.623 |
| Methotrexate dosis | -0.0054 | 0.0042 | -0.0135 | 0.0027 | -1.3005 | 0.197370 | 0.995 | 0.987 | 1.003 |
| Methotrexate time | -0.0041 | 0.0099 | -0.0235 | 0.0153 | -0.4128 | 0.680928 | 0.996 | 0.977 | 1.015 |
| Leflunomide dosis | -0.0013 | 0.0035 | -0.0081 | 0.0056 | -0.3594 | 0.720329 | 0.999 | 0.992 | 1.006 |
| Leflunomide time | -0.0194 | 0.0140 | -0.0469 | 0.0082 | -1.3777 | 0.172332 | 0.981 | 0.954 | 1.008 |
| Antimalarial dosis | 0.0008 | 0.0006 | -0.0005 | 0.0021 | 1.2624 | 0.210672 | 1.001 | 1.000 | 1.002 |
| Antimalarial time | -0.0215 | 0.0293 | -0.0789 | 0.0359 | -0.7336 | 0.465449 | 0.979 | 0.924 | 1.037 |
| Sulfasalazine dosis | -0.0001 | 0.0001 | -0.0003 | 0.0001 | -0.8457 | 0.400351 | 1.000 | 1.000 | 1.000 |
| Sulfasalazine time | -0.0103 | 0.0310 | -0.0709 | 0.0504 | -0.3322 | 0.740657 | 0.990 | 0.932 | 1.052 |
| Prednisone dosis | 0.0115 | 0.0102 | -0.0085 | 0.0316 | 1.1269 | 0.263350 | 1.012 | 0.992 | 1.032 |
| Prednisone time | 0.0090 | 0.0117 | -0.0138 | 0.0319 | 0.7740 | 0.441327 | 1.009 | 0.986 | 1.032 |
| Biological therapy | 0.0914 | 0.0386 | 0.0158 | 0.1670 | 2.3689 | 0.020382 | 1.096 | 1.016 | 1.182 |
| Biological therapy dosis | -0.0002 | 0.0003 | -0.0009 | 0.0004 | -0.6841 | 0.495988 | 1.000 | 0.999 | 1.000 |
| Biological therapy time | -0.0263 | 0.0931 | -0.2089 | 0.1562 | -0.2829 | 0.778048 | 0.974 | 0.811 | 1.169 |
Another logistic regression model was constructed by combining variables and distinguishing between immunosuppressive versus non-immunosuppressive doses of prednisone (less than or equal to 5mg in monotherapy). Table 4. No statistical significance was found in the combination of immunosuppressive treatments. Treatment with prednisone in immunosuppressive doses is associated as a risk factor for the presentation of anergy OR 1,044 [95% CI, 1,008-1080 p <0.05]. Table 5
Logistic regression combined variables.
| Category | Regression coefficients | Standard error | Lower limit (LL) | Upper limit (UL) | Critical value t | p Value | Odds Ratio (OR) | LL (OR) | UL(OR) |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.3903 | 0.5484 | -0.6845 | 1.4651 | 0.7117 | 0.478858 | |||
| Age | 0.0012 | 0.0026 | -0.0039 | 0.0063 | 0.4653 | 0.643053 | 1.001 | 0.996 | 1.006 |
| Gender | -0.2111 | 0.0899 | -0.3873 | -0.0349 | -2.3484 | 0.021528 | 0.810 | 0.679 | 0.966 |
| Weight | 0.0004 | 0.0028 | -0.0051 | 0.0059 | 0.1515 | 0.880004 | 1.000 | 0.995 | 1.006 |
| Years from diagnosis | 0.0007 | 0.0028 | -0.0047 | 0.0061 | 0.2459 | 0.806429 | 1.001 | 0.995 | 1.006 |
| Diabetes | 0.0768 | 0.1076 | -0.1341 | 0.2878 | 0.7138 | 0.477615 | 1.080 | 0.874 | 1.333 |
| Kidney disease | -0.1048 | 0.1627 | -0.4238 | 0.2141 | -0.6442 | 0.521446 | 0.900 | 0.655 | 1.239 |
| Previous tuberculosis | -0.0459 | 0.1928 | -0.4239 | 0.3320 | -0.2382 | 0.812374 | 0.955 | 0.655 | 1.394 |
| Malnutrition | -0.1089 | 0.1895 | -0.4803 | 0.2626 | -0.5745 | 0.567370 | 0.897 | 0.619 | 1.300 |
| Malignancy | 0.1830 | 0.1666 | -0.1434 | 0.5095 | 1.0990 | 0.275338 | 1.201 | 0.866 | 1.664 |
| Methotrexate dosis | -0.0052 | 0.0094 | -0.0237 | 0.0132 | -0.5560 | 0.579875 | 0.995 | 0.977 | 1.013 |
| Methotrexate time | -0.0010 | 0.0094 | -0.0195 | 0.0175 | -0.1040 | 0.917440 | 0.999 | 0.981 | 1.018 |
| Leflunomide dosis | -0.0020 | 0.0086 | -0.0189 | 0.0149 | -0.2314 | 0.817620 | 0.998 | 0.981 | 1.015 |
| Leflunomide time | -0.0169 | 0.0139 | -0.0441 | 0.0103 | -1.2171 | 0.227432 | 0.983 | 0.957 | 1.010 |
| Prednisone dosis | 0.0427 | 0.0176 | 0.0083 | 0.0772 | 2.4295 | 0.017546 | 1.044 | 1.008 | 1.080 |
| Prednisone time | 0.0122 | 0.0113 | -0.0098 | 0.0343 | 1.0860 | 0.281003 | 1.012 | 0.990 | 1.035 |
| Non-immunosuppressive prednisone | -0.0267 | 0.0242 | -0.0741 | 0.0206 | -1.1065 | 0.272094 | 0.974 | 0.929 | 1.021 |
| Methotrexate + leflunomide dosis | 0.0002 | 0.0006 | -0.0010 | 0.0014 | 0.3128 | 0.755339 | 1.000 | 0.999 | 1.001 |
| Methotrexate + prednisone dosis | -0.0031 | 0.0013 | -0.0056 | -0.0006 | -2.3935 | 0.019224 | 0.997 | 0.994 | 0.999 |
| Leflunomide + prednisone dosis | -0.0020 | 0.0014 | -0.0047 | 0.0007 | -1.4487 | 0.151647 | 0.998 | 0.995 | 1.001 |
| Methotrexate + leflunomide + prednisone dosis | 0.0001 | 0.0001 | -0.0001 | 0.0003 | 1.2906 | 0.200868 | 1.000 | 1.000 | 1.000 |
| Biological therapy | 0.0757 | 0.0399 | -0.0025 | 0.1540 | 1.8973 | 0.061693 | 1.079 | 0.998 | 1.166 |
| Biological therapy + methotrexate dosis | 0.0023 | 0.0018 | -0.0014 | 0.0059 | 1.2185 | 0.226921 | 1.002 | 0.999 | 1.006 |
| Biological therapy dosis | -0.0003 | 0.0003 | -0.0010 | 0.0003 | -1.0090 | 0.316254 | 1.000 | 0.999 | 1.000 |
| Biological therapy time | -0.0351 | 0.0745 | -0.1811 | 0.1109 | -0.4714 | 0.638717 | 0.965 | 0.834 | 1.117 |
Dependent variable logistic regression “Cutaneous energy”.
| Category | Regression coefficients | Standard error | Lower limit (LL) | Upper limit (UL) | Critical value t | p Value | Odds Ratio (OR) | LL (OR) | UL(OR) |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.1702 | 0.2735 | -0.3658 | 0.7061 | 0.6223 | 0.535587 | |||
| Age | 0.0010 | 0.0027 | -0.0044 | 0.0063 | 0.3472 | 0.729436 | 1.001 | 0.996 | 1.006 |
| Gender | -0.2300 | 0.0962 | -0.4186 | -0.0415 | -2.3912 | 0.019266 | 0.795 | 0.658 | 0.959 |
| Weight | 0.0012 | 0.0028 | -0.0043 | 0.0068 | 0.4364 | 0.663808 | 1.001 | 0.996 | 1.007 |
| Years from diagnosis | 0.0005 | 0.0027 | -0.0047 | 0.0058 | 0.2037 | 0.839127 | 1.001 | 0.995 | 1.006 |
| Diabetes | 0.0444 | 0.1120 | -0.1750 | 0.2638 | 0.3964 | 0.692901 | 1.045 | 0.839 | 1.302 |
| Kidney disease | -0.1623 | 0.1624 | -0.4807 | 0.1560 | -0.9995 | 0.320733 | 0.850 | 0.618 | 1.169 |
| Previous tuberculosis | -0.0633 | 0.2138 | -0.4822 | 0.3557 | -0.2960 | 0.768063 | 0.939 | 0.617 | 1.427 |
| Malnutrition | -0.1980 | 0.2021 | -0.5941 | 0.1981 | -0.9796 | 0.330371 | 0.820 | 0.552 | 1.219 |
| Malignancy | 0.1523 | 0.1694 | -0.1797 | 0.4844 | 0.8992 | 0.371384 | 1.165 | 0.836 | 1.623 |
| Methotrexate dosis | -0.0054 | 0.0042 | -0.0135 | 0.0027 | -1.3005 | 0.197370 | 0.995 | 0.987 | 1.003 |
| Methotrexate time | -0.0041 | 0.0099 | -0.0235 | 0.0153 | -0.4128 | 0.680928 | 0.996 | 0.977 | 1.015 |
| Leflunomide dosis | -0.0013 | 0.0035 | -0.0081 | 0.0056 | -0.3594 | 0.720329 | 0.999 | 0.992 | 1.006 |
| Leflunomide time | -0.0194 | 0.0140 | -0.0469 | 0.0082 | -1.3777 | 0.172332 | 0.981 | 0.954 | 1.008 |
| Antimalarial dosis | 0.0008 | 0.0006 | -0.0005 | 0.0021 | 1.2624 | 0.210672 | 1.001 | 1.000 | 1.002 |
| Antimalarial time | -0.0215 | 0.0293 | -0.0789 | 0.0359 | -0.7336 | 0.465449 | 0.979 | 0.924 | 1.037 |
| Sulfasalazine dosis | -0.0001 | 0.0001 | -0.0003 | 0.0001 | -0.8457 | 0.400351 | 1.000 | 1.000 | 1.000 |
| Sulfasalazine time | -0.0103 | 0.0310 | -0.0709 | 0.0504 | -0.3322 | 0.740657 | 0.990 | 0.932 | 1.052 |
| Prednisone dosis | 0.0115 | 0.0102 | -0.0085 | 0.0316 | 1.1269 | 0.263350 | 1.012 | 0.992 | 1.032 |
| Prednisone time | 0.0090 | 0.0117 | -0.0138 | 0.0319 | 0.7740 | 0.441327 | 1.009 | 0.986 | 1.032 |
| Biological therapy | 0.0914 | 0.0386 | 0.0158 | 0.1670 | 2.3689 | 0.020382 | 1.096 | 1.016 | 1.182 |
| Biological therapy dosis | -0.0002 | 0.0003 | -0.0009 | 0.0004 | -0.6841 | 0.495988 | 1.000 | 0.999 | 1.000 |
| Biological therapy time | -0.0263 | 0.0931 | -0.2089 | 0.1562 | -0.2829 | 0.778048 | 0.974 | 0.811 | 1.169 |
RA is an autoimmune, inflammatory, chronic and progressive disease, characterized mainly by the damage of small joints of the hands and feet. It is recommended that all patients diagnosed with RA begin with therapy with disease-modifying antirheumatic drugs (DMARDs) and to a large extent many require scaling up to biological therapy.73,74 RA per se has been associated with the reactivation of latent TB.1 There are multiple studies that show a decrease in tuberculin positivity in patients with RA.18,66,75–77 Immunosuppressive drug therapy, particularly iTNF, significantly increases this risk.71,78,79
Routine use of hypersensitivity tests is recommended for the diagnosis of latent TB and thus prevent reactivation. There are data that suggest that the use of a single screening test does not identify all patients at risk of TB, since false negative results are more likely in immunocompromised individuals.80,81 The dual test strategy (tuberculin + IGRA) or the use of a tuberculin booster is consistent with the recommendations of the American College of Rheumatology and other public health agencies.71,78,82
Some reports consider that the use of disease-modifying therapy and steroids are a cause of anergy without this being proven by comparison to cutaneous controls or serological methods for the diagnosis of TB.67,77,83 This is the first study that evaluates the prevalence of cutaneous anergy in patients with RA and seeks to determine if there are variables associated with its appearance. Table 6
Logistic regression combined variables.
| Category | Regression coefficients | Standard error | Lower limit (LL) | Upper limit (UL) | Critical value t | p Value | Odds Ratio (OR) | LL (OR) | UL(OR) |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 0.3903 | 0.5484 | -0.6845 | 1.4651 | 0.7117 | 0.478858 | |||
| Age | 0.0012 | 0.0026 | -0.0039 | 0.0063 | 0.4653 | 0.643053 | 1.001 | 0.996 | 1.006 |
| Gender | -0.2111 | 0.0899 | -0.3873 | -0.0349 | -2.3484 | 0.021528 | 0.810 | 0.679 | 0.966 |
| Weight | 0.0004 | 0.0028 | -0.0051 | 0.0059 | 0.1515 | 0.880004 | 1.000 | 0.995 | 1.006 |
| Years from diagnosis | 0.0007 | 0.0028 | -0.0047 | 0.0061 | 0.2459 | 0.806429 | 1.001 | 0.995 | 1.006 |
| Diabetes | 0.0768 | 0.1076 | -0.1341 | 0.2878 | 0.7138 | 0.477615 | 1.080 | 0.874 | 1.333 |
| Kidney disease | -0.1048 | 0.1627 | -0.4238 | 0.2141 | -0.6442 | 0.521446 | 0.900 | 0.655 | 1.239 |
| Previous tuberculosis | -0.0459 | 0.1928 | -0.4239 | 0.3320 | -0.2382 | 0.812374 | 0.955 | 0.655 | 1.394 |
| Malnutrition | -0.1089 | 0.1895 | -0.4803 | 0.2626 | -0.5745 | 0.567370 | 0.897 | 0.619 | 1.300 |
| Malignancy | 0.1830 | 0.1666 | -0.1434 | 0.5095 | 1.0990 | 0.275338 | 1.201 | 0.866 | 1.664 |
| Methotrexate dosis | -0.0052 | 0.0094 | -0.0237 | 0.0132 | -0.5560 | 0.579875 | 0.995 | 0.977 | 1.013 |
| Methotrexate time | -0.0010 | 0.0094 | -0.0195 | 0.0175 | -0.1040 | 0.917440 | 0.999 | 0.981 | 1.018 |
| Leflunomide dosis | -0.0020 | 0.0086 | -0.0189 | 0.0149 | -0.2314 | 0.817620 | 0.998 | 0.981 | 1.015 |
| Leflunomide time | -0.0169 | 0.0139 | -0.0441 | 0.0103 | -1.2171 | 0.227432 | 0.983 | 0.957 | 1.010 |
| Prednisone dosis | 0.0427 | 0.0176 | 0.0083 | 0.0772 | 2.4295 | 0.017546 | 1.044 | 1.008 | 1.080 |
| Prednisone time | 0.0122 | 0.0113 | -0.0098 | 0.0343 | 1.0860 | 0.281003 | 1.012 | 0.990 | 1.035 |
| Non-immunosuppressive prednisone | -0.0267 | 0.0242 | -0.0741 | 0.0206 | -1.1065 | 0.272094 | 0.974 | 0.929 | 1.021 |
| Methotrexate + leflunomide dosis | 0.0002 | 0.0006 | -0.0010 | 0.0014 | 0.3128 | 0.755339 | 1.000 | 0.999 | 1.001 |
| Methotrexate + prednisone dosis | -0.0031 | 0.0013 | -0.0056 | -0.0006 | -2.3935 | 0.019224 | 0.997 | 0.994 | 0.999 |
| Leflunomide + prednisone dosis | -0.0020 | 0.0014 | -0.0047 | 0.0007 | -1.4487 | 0.151647 | 0.998 | 0.995 | 1.001 |
| Methotrexate + leflunomide + prednisone dosis | 0.0001 | 0.0001 | -0.0001 | 0.0003 | 1.2906 | 0.200868 | 1.000 | 1.000 | 1.000 |
| Biological therapy | 0.0757 | 0.0399 | -0.0025 | 0.1540 | 1.8973 | 0.061693 | 1.079 | 0.998 | 1.166 |
| Biological therapy + methotrexate dosis | 0.0023 | 0.0018 | -0.0014 | 0.0059 | 1.2185 | 0.226921 | 1.002 | 0.999 | 1.006 |
| Biological therapy dosis | -0.0003 | 0.0003 | -0.0010 | 0.0003 | -1.0090 | 0.316254 | 1.000 | 0.999 | 1.000 |
| Biological therapy time | -0.0351 | 0.0745 | -0.1811 | 0.1109 | -0.4714 | 0.638717 | 0.965 | 0.834 | 1.117 |
Anergy is defined as the non-induration of any intradermal antigen whose immune response is of high prevalence in the general population. In this study, the tetanus toxoid concomitant with the application of tuberculin was chosen.
We found that a significant proportion of patients had anergy in up to 1 in 11 patients (9%). This is consistent with the recommendation of experts to use a combination of tests that would increase sensitivity.18,71
The main risk factors associated with cutaneous anergy in various studies are acquired immunosuppression states such as malnutrition, hematological tumors and HIV patients with low CD4 counts.37,39,51,58,71,84–86 In other diseases that receive a similar degree of immunosuppression to RA, the prevalence of anergy can reach 83%.68 In rheumatologic diseases, there are few published studies although there is clear evidence of alteration of cellular immunity.87,88 In a study conducted by Ponce de León, in patients with RA the frequency of negative PPD was 70% vs 26% in healthy controls.89
In the present study it is striking that all subjects with anergy used corticosteroids. This variable significantly increases the probability of presenting anergy and is dose dependent, presenting with doses greater than 5mg or in combination therapy. In contrast, and despite the biological plausibility, no association was found in the use of DMARDs, particularly metrotexate, the most frequent DMARD agent; as found in other studies in animals and immunosuppressed patients.90–93 In favor to this finding, there are studies in which a paradoxical effect of metrotexate has been found with an increase in false positives of PPD.94
As expected, we found that the use of biological therapy is a significant risk for the generation of anergy. This group is of special importance since there are no recommendations about screening or diagnostic studies of latent TB in patients who are already on biologic therapy.
In the present study, a third of the patients with anergy were older than 60 years. There are studies that report that older subjects may have lower antigenic reactivity.87,88,95 However, we did not find that age was a determining factor of anergy in patients. There was no anergy in subjects older than 80 years and the most prevalent group was 51 to 60 years.
The female gender behaved as a protective factor to present anergy, there's plausibility that sex hormones have a role in the cellular immune response that needs further research.96
The prevalence of positive tuberculin in patients with RA was low in this study (13%) compared to other studies in high prevalence countries where it ranges between 20 and 40%.66,94,97 In the Indian study by Agarwal et al.66 a prevalence of tuberculin positivity of 20.4% was found. Similarly to the present study, it was found that the use of steroids decreased the reaction to tuberculin (3% versus 25%, P = 0.002). They also found no association with the use of other DMARDs.
Our study has several limitations. First, the small number of subjects (n = 100) can reduce the chances of obtaining statistically significant results. Additionally, the prevalence of anergy was lower than expected, which compromises the validity of the association measures. Thirdly, we did not analyze the correlation between anergy results and disease activity.
ConclusionThe high prevalence of cutaneous anergy in patients with RA in the present study and the evidence presented here supports the recommendation of a second diagnostic test (tuberculin booster or IGRAs) for the diagnosis of latent TB in patients with RA and immunosuppressive therapy.
FundingThis work was funded through an internal call from the National University of Colombia “national call for projects to strengthen the research, creation and innovation of the national university of Colombia 2016-2018” The project identifies with the Hermes code: 37595 and QUIPU code: 201010028367. It also received financial support from the Colombian Association of Rheumatology.
Conflict interestThe authors declare that they have no financial or personal relationship with people or organizations that could give rise to a conflict of interest in relation to the present study.