In patients with rheumatoid arthritis (RA), osteoporotic vertebral fractures (OVF) are of multifactorial etiology. The main mechanism is spontaneous and therefore most are asymptomatic. The presence of VF has an impact on the quality of life of patients, and consequently on morbidity and mortality, therefore it should be systematically evaluated in this population, especially when associated factors have been reported. The main objective of this study was to identify clinical characteristics for osteoporosis and poor prognosis in rheumatoid arthritis that could be associated with the development of osteoporotic vertebral fractures identified in the lateral chest X-ray of asymptomatic patients with RA. The secondary objectives were to present the frequency, location, and severity of the fractures, as well as the inter and intra-observer correlation, when analyzing the radiographs.
MethodologyPatients with a diagnosis of RA were included, with a lateral chest X-ray and indication dissimilar to spinal symptoms. The mean age was 58 years (IQR 21–88). Three researchers evaluated 151 images in a sequenced and standardized manner using the Algorithm-Base Qualitative approach (ABQ) and Genant methods. Variables associated with the presence of osteoporosis and poor prognosis in RA were identified. Subsequently, a multivariate analysis was carried out to find an association with the presence of VF in this population.
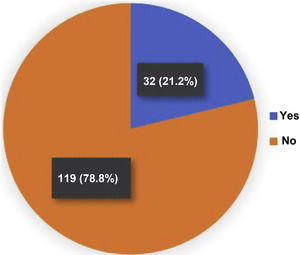
ResultsWe found 39 fractures in 32/151 patients. Identifying multiple fractures in 5 of them. The prevalence of osteoporotic vertebral fractures was 21.2%. The distribution of fractures was mainly at the level of T5, T8 and T9, with a predominance of Genant grade 1 in 46%. In the multivariate analysis, age, duration of RA (mainly greater than 10 years), rheumatoid factor, anti-citrullinated peptide antibodies, DAS28, HAQ, presence of antinuclear antibodies (ANA), smoking and being under treatment for osteoporosis showed a statistically significant association. The interobserver correlation for the ABQ and Genant methods presented a kappa index of .9 and .92, respectively.
ConclusionIn patients with RA there is a significant association with the development of VF, independent of the presence of osteoporosis. Furthermore, this research suggests that the presence of some clinical and paraclinical characteristics could be associated with the prevalence of osteoporotic vertebral fractures. Age, duration of arthritis, poor prognostic markers for RA in terms of serology and functionality, as well as being in treatment for osteoporosis had statistical significance of association. This should guide the timely detection of fractures, independent of symptoms, with the respective targeted treatment in this population and thus avoid functional complications and a decrease in quality of life.
En los pacientes con artritis reumatoide (AR), las fracturas vertebrales osteoporóticas (FVO) son de etiología multifactorial. El principal mecanismo es espontáneo y por ende la mayoría son asintomáticas. La presencia de FVO impacta en la calidad de vida de los pacientes y, en consecuencia, en la morbilidad y la mortalidad; por lo tanto, se debería evaluar de forma sistemática en esta población, más aún cuando se han reportado factores asociados. El principal objetivo de este estudio fue identificar características clínicas de osteoporosis y de mal pronóstico en AR, que podrían estar asociadas con el desarrollo de fracturas vertebrales osteoporóticas identificadas en la radiografía lateral de tórax en pacientes asintomáticos con AR. Y los objetivos secundarios fueron presentar la frecuencia, la localización y la severidad de las fracturas, como también la correlación inter e intraobservador al analizar las radiografías.
MetodologíaSe incluyeron pacientes con diagnóstico de AR, con radiografía lateral de tórax e indicación disímil a síntomas en columna. La media de edad fue 58 años (RIC 21–88). Tres investigadores evaluaron 151 imágenes, de manera secuencial y estandarizada, utilizando los métodos Algorithm-Base Qualitative approach (ABQ) y de Genant. Se identificaron variables asociadas con la presencia de osteoporosis y de pobre pronóstico en AR. Posteriormente, se hizo un análisis multivariado orientado a encontrar asociación con la presencia de FVO en esta población.
ResultadosSe encontraron 39 fracturas en 32/151 pacientes; en cinco de ellos se hallaron múltiples fracturas. La prevalencia de fracturas vertebrales osteoporóticas fue de 21,2%. La distribución de fracturas fue principalmente a nivel de T5, T8 y T9, con predominio de aquellas grado 1 de Genant (46%). En el análisis multivariado, la edad, la duración de la AR (principalmente mayor a 10 años), el factor reumatoideo, los anticuerpos antipéptidos citrulinados, el DAS28, el HAQ, la presencia de anticuerpos antinucleares (ANA), el tabaquismo y estar en tratamiento para osteoporosis presentaron una asociación estadísticamente significativa. La correlación interobservador para los métodos ABQ y Genant presentó un índice kappa de 0,9 y 0,92, respectivamente.
ConclusiónEn pacientes con AR existe una asociación significativa con el desarrollo de FVO, con independencia de la presencia de osteoporosis. Además, esta investigación sugiere que la presencia de algunas características clínicas y paraclínicas podría estar asociada con la prevalencia de fracturas vertebrales osteoporóticas. La edad, la duración de la artritis, los marcadores de mal pronóstico de la AR en cuanto a serología como funcionalidad, así como estar en tratamiento para osteoporosis tuvieron significancia estadística de asociación. Esto debería guiar una detección oportuna de las fracturas, más allá de los síntomas, con el respectivo tratamiento dirigido a esta población y así evitar complicaciones funcionales y una disminución en la calidad de vida.
Rheumatoid arthritis (RA) is a chronic and progressive autoimmune disease. Its clinical spectrum goes beyond articular involvement, being systemic extraarticular manifestations a non-negligible percentage of the disease, with the consequent impact on morbidity and mortality of these patients.1,2
An increased risk of osteoporosis (OP), and therefore of fragility fractures such as osteoporotic vertebral fractures (OVF), from two to six times, and twice for hip fractures has been documented in patients with RA, apart of the risk factors characteristic of OP.3,4 In addition to this, having a previous OVF can increase the risk of a new fracture up to four or five times, regardless of the bone mineral density.5
It should be noted that OVFs have a multifactorial etiology, from those related to osteoporosis, passing through poor prognostic factors of RA, to complications associated with therapies such as corticosteroids.6,7 The main mechanism of vertebral fractures is spontaneous and therefore they are asymptomatic in up to 70% of cases. Studies in the general population have found a prevalence of OVF of 11.18%.8 When patients with RA are evaluated, this prevalence can reach 20–30%, either clinically or as a finding in asymptomatic patients.9,10
The most well-known method for the evaluation of OVF is that of Genant.11 Nevertheless, a significant change in the vertebral morphology is necessary, limiting the results in subtle cases, added to the fact that there are no tools to differentiate between anatomical variants and true fractures, which lead to overdiagnosis or underdiagnosis. This difficulty has improved with the use of the Algorithm-Based Qualitative Approach (ABQ) method, which is fundamentally based on the evaluation of the endplate, along with the absence of confounding elements such as technical and anatomical factors, criteria of traumatic fracture, metabolic or tumor disease, which facilitates the detection of mild lesions and avoids diagnostic error.8,12
The presence of OVF impacts the quality of life of the patients, which is expressed in functionality and mortality, among other aspects, the five-year mortality is 20% higher when there is an OVF.13,14 Therefore, it is of utmost importance to be able to identify and treat these fractures, since a new one can be prevented in up to 60% of the cases.14–16 In addition, the costs derived from their management, both direct and indirect, are enormous, for which the presence of osteoporotic vertebral fractures should be evaluated systematically in this population, especially when risk or poor prognosis variables for their development are identified,17 because some of these factors, such as RA activity, habits such as smoking and other comorbidities can be modified and, in this way, it is possible to reduce the risk.18,19
The main objective of this study was to identify clinical characteristics for osteoporosis and poor prognosis in RA that could be associated with the development of osteoporotic vertebral fractures, detected in the lateral chest radiograph of asymptomatic patients with RA. The secondary objectives were to present the frequency, location and severity of the fractures, as well as the inter- and intra-observer correlation when analyzing the radiographs.
Materials and methodsDesign, population and sampleAn analytical cross-sectional study of prevalence in patients with RA was conducted in a rheumatology service between June 2015 and July 2019. The sample size was by convenience: all patients who met the criteria for the study in the time period described were recruited. Patients over 18 years of age, without distinction of sex, with a diagnosis of RA according to the ACR 1987 or ACR/EULAR 2010 criteria, who underwent a lateral chest X-ray for reasons other than back pain after the onset of the symptoms of RA were included. Patients with a known history of osteoporotic vertebral fracture before the imaging evaluation, metabolic bone disease, active neoplasm, renal lithiasis, as well as those with a history of spinal trauma or surgery prior to the start of the study were excluded.
Study protocolThe data for the different variables object of evaluation, both clinical and paraclinical, were taken from the clinical records of the patients, and all data were obtained in 100% of the patients. Demographic variables such as age, sex, body mass index, smoking status and alcoholism were included, as well as clinical and paraclinical characteristics related to RA, such as rheumatoid factor, anti-citrullinated peptide antibodies, duration of the RA, extra-articular involvement, C-reactive protein (CRP) values and erythrocyte sedimentation rate. The activity of the RA was quantified by values of the Disease Activity Score-28 (DAS28), the Clinical Disease Activity Index (CDAI) and by the functional assessment using the Health Assessment Questionnaire (HAQ). As variables associated with osteoporosis in the general population, we considered diabetes mellitus, chronic kidney disease, hepatopathy, use of glucocorticoids in the past year and, definitively, the results of Z and T-scores in the bone densitometry, according to the definitions of the World Health Organization (WHO) for osteopenia and osteoporosis. The copy of the images was made during the consultation with the patients, the x-rays have already been taken to each patient as part of routine care, and the study was approved by the ethics committee of the institution in which the work was carried out and informed consent was obtained from the patients.
Evaluation of the fracturesThe search for OVF was carried out by three researchers: a radiologist expert in images and two residents, prior to which a workshop with 10 radiographs was held in order to unify evaluation criteria. Each evaluator made the readings individually, without knowing the results of the other researchers, nor of the other study variables. A 3-megapixel Kodak screen was used and by consensus of the researchers, the lateral projections with adequate technique for visualization, at least of the vertebral bodies from T5 to L1 were selected. The OVFs were searched using the Genant and ABQ methods. If a fracture was found, it was classified by grade, according to the percentage of decrease in the height of the vertebral body, into grades 1, 2 and 3, if it was 20–25%, >25%, <40% or >40%, respectively. In case of different results in the reading, a consensus between the three researchers was reached.
Data processing and statistical analysisData were tabulated in a Microsoft Excel 2016 template. The qualitative variables were presented in absolute and relative frequencies, while measures of central tendency and dispersion were used for the quantitative variables; the choice of this statistics was oriented according to the evaluation of normality in accordance to the Shapiro-Wilk test. For the contrast of association or independence between the dependent variable (OVF) and the other qualitative variables, the X2 test was applied, and in the case of qualitative variables, the Student’s-t test. The odds ratio (OR) estimator with its respective 95% confidence interval (95% CI) was calculated in each case. The multivariate analysis was carried out using the logistic regression method, through which possible confounding variables were controlled and adjusted ORs with their respective 95% confidence intervals (95% CI) were obtained. P values <0.05, as well as 95% CIs that did not include the unit, were considered significant associations. The interobserver correlation for OVF is presented by the kappa coefficient. The statistical analyses were processed in the Stata® 15 software (Table 1).
Clinical and paraclinical characteristics of the patients with RA included in the analysis. The findings in the total population are presented and a comparison is made with statistical analysis between the subgroups with presence or absence of OVF.
| Characteristics | Total: n = 151 | OVF: n = 32 | No OVF: n = 119 | P-value |
|---|---|---|---|---|
| Age, mean (SD) years | 58 (12.8) | 64 (11.2) | 57 (12.9) | 0.009 |
| <50 years n (%) | 39 (25.8) | 4 (12.5) | 35 (29.4) | 0.06 |
| 51–70 years n (%) | 85 (52.3) | 16 (50) | 69 (57.9) | 0.23 |
| >70 years n (%) | 27 (17.9) | 17 (53.1) | 10 (8.4) | 0.02 |
| Female, n (%) | 123 (81) | 26 (81) | 97 (81.5) | 0.98 |
| BMI, mean (SD) kg/m2 | 25.2 (4.1) | 19.3 (12.2) | 25.0 (3.72) | 0.18 |
| <25 kg/m2n (%) | 78 (52) | 17 (53.1) | 61 (51.2) | 0.85 |
| 25–30 kg/m2n (%) | 57 (37.7) | 9 (28.1) | 48 (40.3) | 0.38 |
| >30 kg/m2n (%) | 16 (10.7) | 6 (18.7) | 10 (8.40) | 0.19 |
| Smoking, n (%) | 53 (35.1) | 16 (50) | 37 (31.1) | 0.04 |
| Alcoholism, n (%) | 25 (16.5) | 8 (25) | 17 (14.3) | 0.14 |
| Diabetes mellitus, n (%) | 31 (20.5) | 12 (37.5) | 19 (15.9) | 0.007 |
| Kidney disease, n (%) | 37 (24.5) | 12 (37.5) | 25 (21) | 0.06 |
| Hepatopathy, n (%) | 23 (15.2) | 7 (21.8) | 16 (13.4) | 0.23 |
| Duration of the RA, years (SD) | 13.6(11) | 19.25 (12.2) | 12.1(10.2) | 0.002 |
| Less than 10 years n (%) | 62 (41) | 5 (15.6) | 57 (47.9) | 0.01 |
| More than 10 years n (%) | 89 (59) | 27(84.4) | 62 (52.1) | 0.002 |
| Radiographic erosions n (%) | 84 (55.6) | 27 (84.4) | 57 (47.9) | 0.002 |
| Rheumatoid factor IU (SD) | 164 (242) | 334 (420) | 118 (136) | 0.002 |
| Less than 30 IU/mL n (%) | 29 (19.2) | 1 (3.1) | 28 (23.5) | 0.03 |
| 30 to 100 IU/mL n (%) | 55 (36.4) | 7 (21.8) | 48 (40.3) | 0.1 |
| More than 30 IU/mL n (%) | 67 (44.4) | 24 (75) | 43 (36.1) | 0.001 |
| Anti-CCP antibodies, IU (SD) | 197 (242) | 366 (306) | 151 (200) | <0.001 |
| Antinuclear antibodies n (%) | 79 (52.32) | 24 (75) | 55 (46.2) | 0.003 |
| CDAI, mean (SD) | 19.4 (11.2) | 22.6 (11.4) | 18.6 (11.1) | 0.0496 |
| DAS28, mean (SD) | 4.28 (1.15) | 4.91 (0.98) | 4.12 (1.14) | 0.001 |
| ESR, mean (SD) mm/h | 28.39 (17.7) | 29.5 (16.2) | 28.1 (18.1) | 0.96 |
| CRP, mean (SD) mg/L | 15.35 (17.9) | 19.6 (19.2) | 14.1 (17.4) | 0.05 |
| HAQ, mean (SD) | 0.7 (0.63) | 1.18 (0.63) | 0.57 (0.57) | <0.001 |
| Steroid dose-year, g (SD) | 1.64 (0.96) | 2.04 (1.1) | 1.53 (0.9) | 0.01 |
| Osteoporosis therapy n (%) | 48 (31.7) | 18 (56.2) | 30 (25.2) | 0.008 |
| Vitamin D levels, ng/mL (SD) | 22,15 (7.48) | 21.4 (7.2) | 22.3 (7.5) | 0.53 |
| T-score in BMD, mean (SD) | 1.78 (1.3) | 2.6 (1.2) | 1.6 (1.2) | 0.01 |
| Z-score in BMD, mean (SD) | 1.2 (1.28) | 1.6 (1.15) | 1.0 (1.3) | 0.06 |
Significant p-value <0.05; T-test for continuous variables and χ2 for categorical;
SD, standard deviation; BMI, body mass index; CCP, cyclic citrullinated peptides; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; HAQ, Health Assessment Questionnaire; DAS28, Disease Activity Score-28.
The variables with statistical significance between the groups are highlighted in bold.
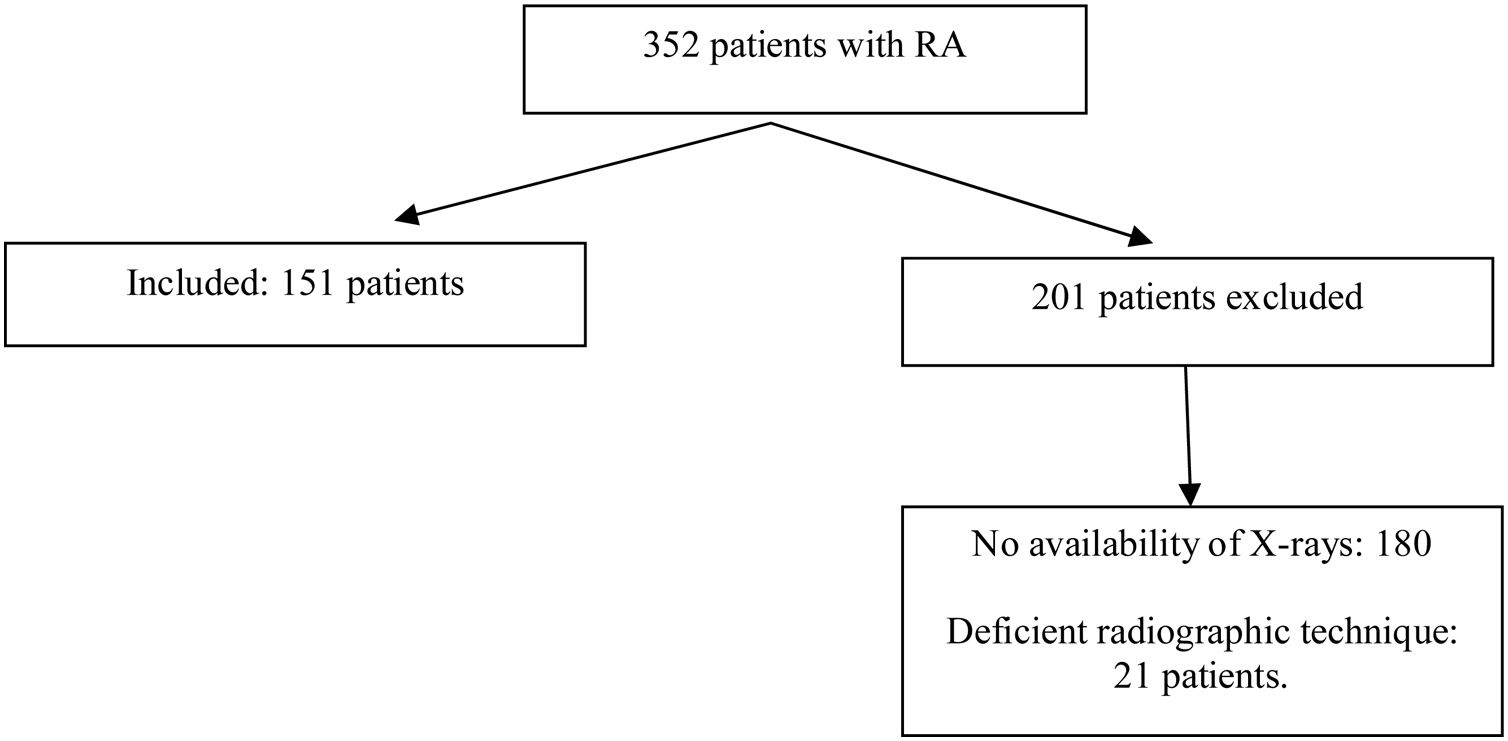
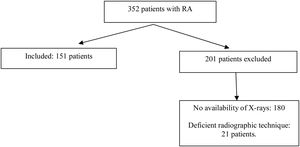
In the period between June 2015 and July 2019, 352 patients with a diagnosis of RA and PA and lateral chest X-ray attended the consultation of rheumatology. After applying the selection criteria, 151 patients were included in the study. This selection is shown in Fig. 1. The main reason for exclusion was not having a radiographic study of adequate quality for interpretation. The majority of patients (121), corresponding to 81%, were women. The median age of the total group was 58 years and only 18% corresponded to patients over 70 years of age. The average duration of RA was 15 years, while 88% of patients were seropositive. 96% of the patients were being treated with synthetic disease-modifying antirheumatic drugs (DMARDs), 8% with biologic agents, and 82% with glucocorticoids. Table 2 shows the distribution of the variables of interest, both demographic, clinical and paraclinical in the total population, as well as in the groups with and without fractures.
Multiple regression analysis for factors associated with OVF in patients with RA.
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age | 1.14 (1.02–1.17) | 0.04 |
| Duration of the RA | 1.10 (1.04–1.15) | 0.04 |
| Rheumatoid factor | 1.10 (1.07–1.14) | 0.01 |
| Anti-CCP antibodies | 1.02 (1.0036–1.029) | 0.042 |
| Antinuclear antibodies | 1.42 (1.07–6.32) | 0.031 |
| DAS28 | 3.83 (1.36–10.76) | 0.011 |
| HAQ score | 2.58 (1.12–5.95) | 0.026 |
| Smoking | 1.22 (1.08–4.62) | 0.049 |
| Osteoporosis therapy | 3.74 (1.3–10.69) | 0.014 |
RA, rheumatoid arthritis; CCP, cyclic cytrullinated peptides; DAS28, Disease Activity Score-28; HAQ, Health Assessment Questionnaire; OR, odds ratio.
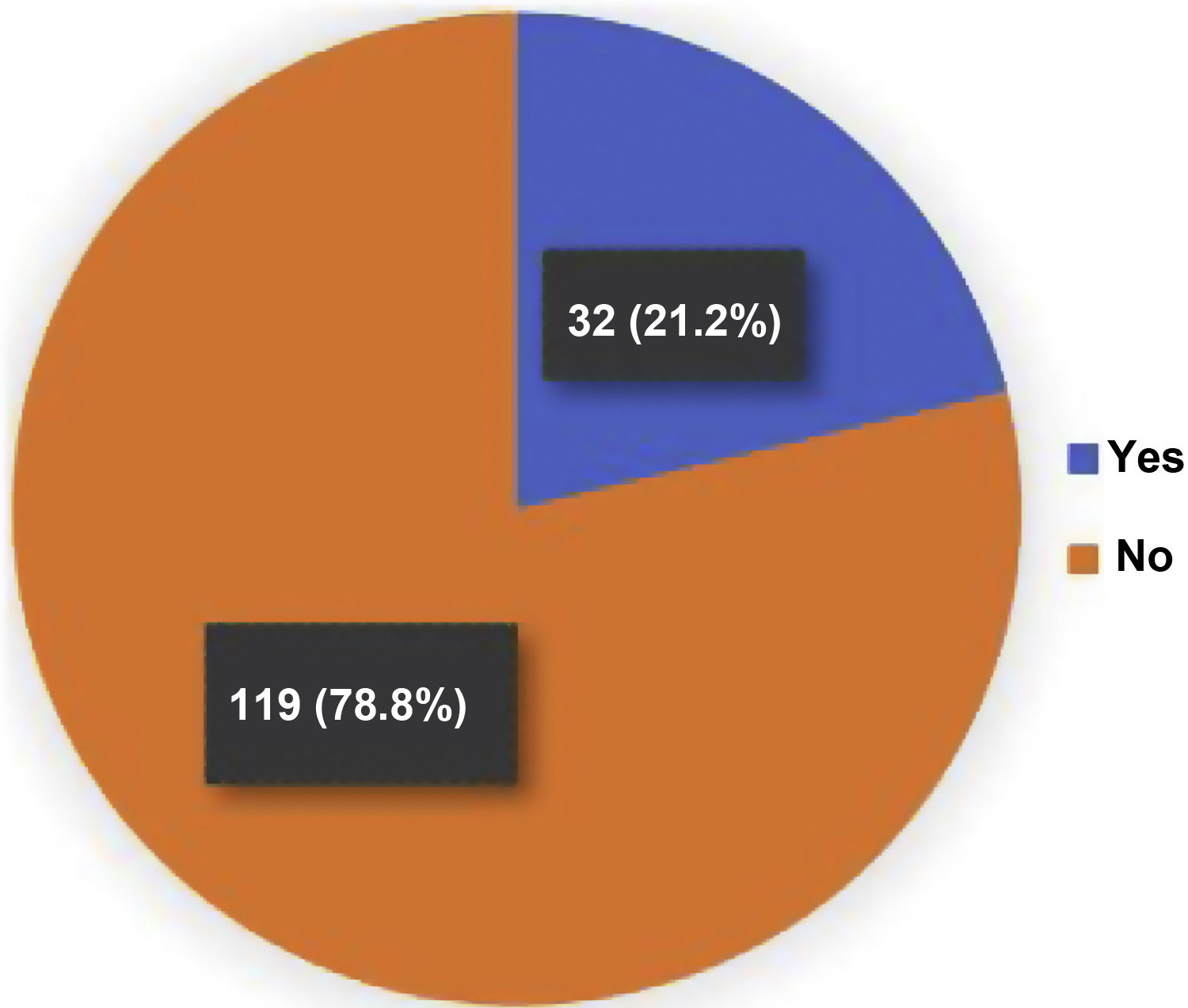
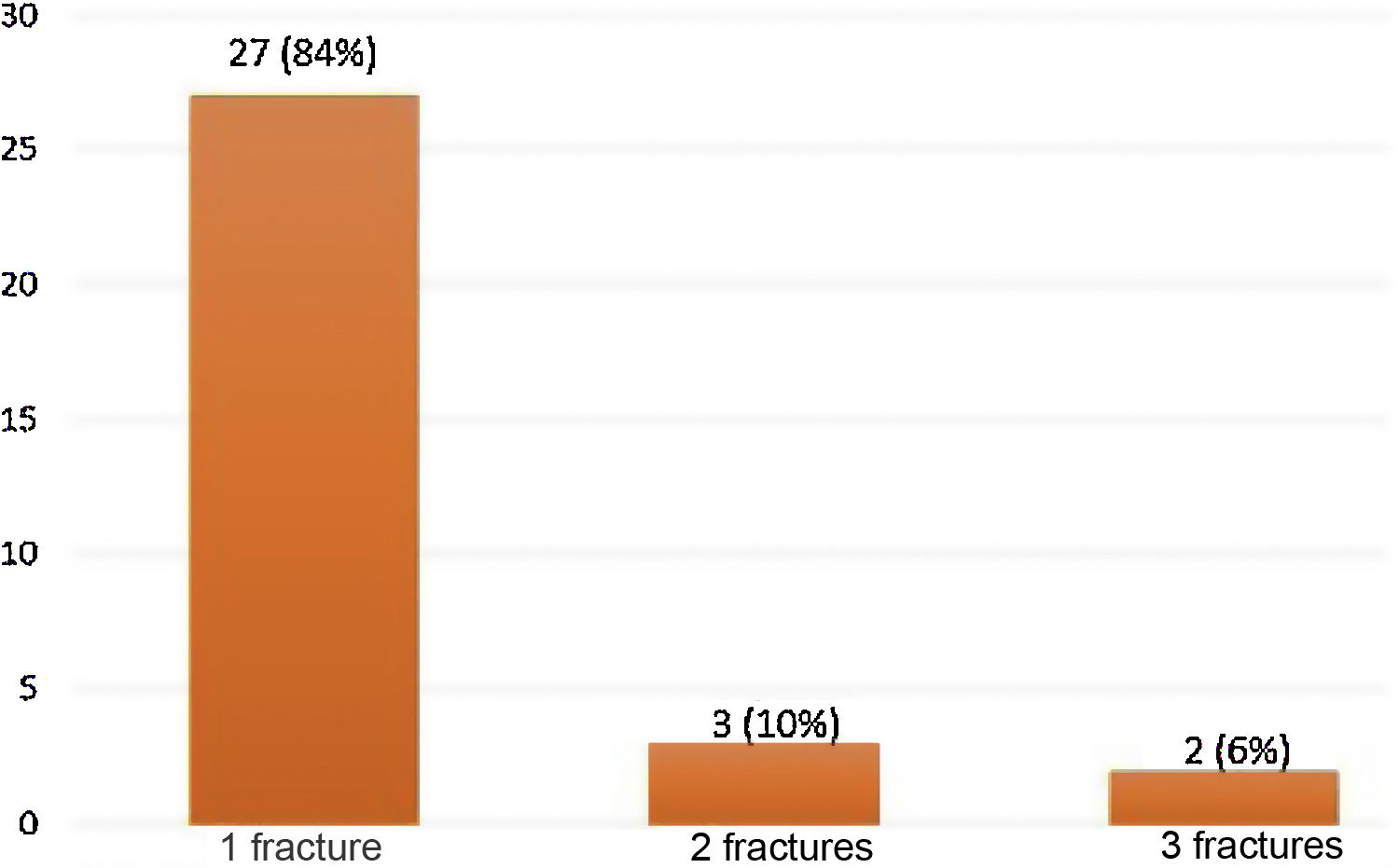
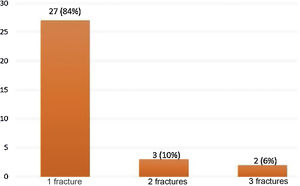
39 fractures were identified by the ABQ and Genant methods in 32/151 (21.2%) patients with RA (Fig. 1). Women represented 81% of the patients with OVF, while the mean age in the group with fractures was 64 years, with the population over 70 years of age being the most representative in relation to the number of fractures present, with respect to the total number of patients in this population (12/27; 44%). There was a longer duration of the RA (19 years) in the group with OVF and the majority (84%) had only one fracture. For two and three fractures, the distribution was 10% and 6%, respectively (Fig. 2). A fracture grade 1, 2 and 3 was identified in 46, 25 and 29%, respectively. To take into account, the patients with the highest degree of severity of the fractures were those with more than one and located primarily at the level of T8 and T5.
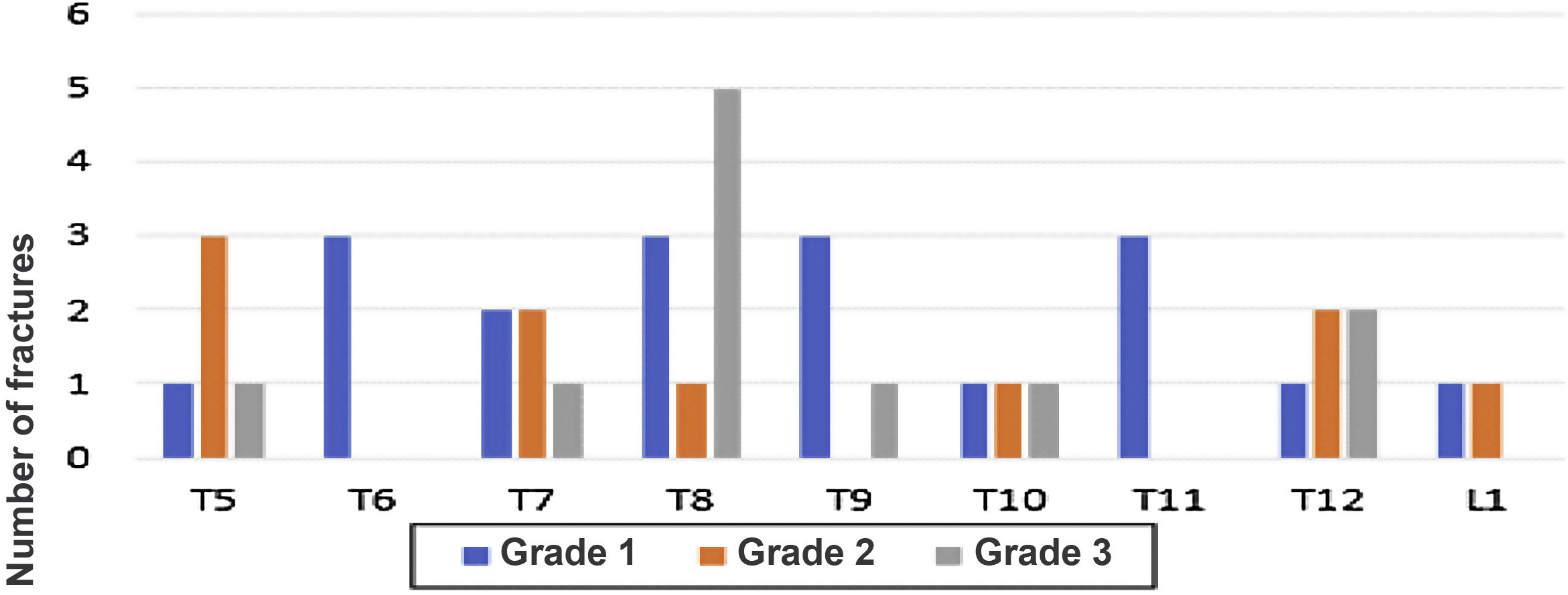
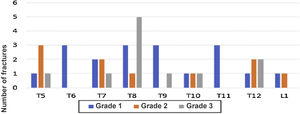
Fig. 3 shows the distribution of patients according to the number of fractures found, while Fig. 4 shows the number of fractures according to the location and severity. It should be highlighted that the presence of osteoporosis was higher in the group with OVF (59 vs. 22%) with an average T-score of -2.6 in the group with fractures vs. −1.6 in the group without fractures. 34% of the patients with OVF had levels in the osteopenia range, compared with 40% of the patients without fractures.
Number of fractures according to their location and severity. In the lateral chest image, the vertebrae from T5 to L1 were evaluated. In the same way, the degree of fractures is graphically represented, from less to more severity, according to the Genant scale, from 1 to 3, respectively, for each vertebra evaluated.
The interobserver correlation for the ABQ and Genant methods presented a kappa index of 0.9 and 0.92, respectively. When establishing the intra-observer correlation for the ABQ method, the result was 0.89, and in the case of the Genant, 0.94.
Univariate and multivariate analysis: factors associated with OVFTable 2 shows the multivariate analysis adjusted to the other variables studied: it was found a significant association with the development of OVF for age, OR 1.14, p = 0.04, fundamentally represented by the subgroup of patients over 70 years of age, duration of the arthritis, OR 1.1, p = 0.04, specifically with an evolution longer than years, rheumatoid factor values, OR 1.13, p = 0.01, with greater association with high titers, anti-cyclic citrullinated peptide antibodies, OR 1.02, p = 0.042, antinuclear antibodies, OR 1.42, p = 0.03; DAS28, OR 3.83, p = 0.011, with greater association in patients with high activity, HAQ, OR 2.58, p = 0.026 and smoking, OR 1.22, p = 0.049.
To note, in the group with treatment for osteoporosis there was a significant relationship, OR 3.74, p = 0.014, with the presence of OVF, likewise, in the univariate analysis the T-score was associated with the presence of fractures, but this association is lost in the multivariate analysis. When performing the Hosmer-Lemeshow test as a test of goodness in the logistic regression, it was found that the model fits to reality (probability > χ2 = 0.59).
Other variables such as sex, body mass index, presence of erosions, hepatopathy, chronic kidney disease, diabetes mellitus, treatment with steroids, alcoholism, erythrocyte sedimentation rate, vitamin D levels, and cumulative dose of steroids per year, were not found to be associated in the multivariate analysis, while in the univariate analysis, in the Clinical Disease Activity Index (CDAI) score and CRP it was found an association with the outcome of interest, although it is lost when adjusting for the other variables.
DiscussionOVFs constitute an important cause of morbidity and mortality. RA itself presents a high impact on the quality of life of the patients and leads to a considerable degree of disability. Added to the foregoing, multiple factors associated with osteoporosis, and other identified as of poor prognosis in relation to RA, could be associated with OVF.2,4 A frequency of osteoporotic vertebral fractures of 21% was found in this study, in agreement with studies described in other latitudes, in which it was around 16%.6,7,9 There was a higher frequency than that found in a series of patients without the presence of RA in our setting, in which it was 11%.8,20
In addition, osteoporosis was present in around 32% of the patients, which coincides with the findings of other studies, in which this condition was present in between 13 and 36%.21 According to the latter, our study confirms that the fact of presenting high-impact conditions in relation with osteoporosis, added to the presence of RA, is an independent variable of association for fractures.13 In the Tomorrow study, it was found an OR of 1.72 for the presence of RA and the development of OVF, based on a greater bone loss in relation to the chronic inflammatory state of the disease,7,22 which could explain why in our population it was found a higher proportion of patients with the outcome of fracture, compared with the general population.8,14
It is noteworthy, similarly to that was reported in other studies, that in our patients the distribution of the fractures was correlated with the segments of the spine that bear the heaviest weight, mainly T5, T8 and T12.7 The foregoing is added to the fact that up to a fifth of the patients had more than one fracture, which configures a picture of greater severity of the disease, however, 50% were grade 1, without implying not having the corresponding consideration in terms of a timely treatment as secondary prevention of OVF.23
When analyzing the age of presentation, the patients with OVF were older, especially those over 70 years of age, added to a greater association if it occurs in female patients, by adding another condition such as the postmenopausal status, which is reflected in lower bone mineral density values.24,25 This coincides with what has been previously discussed, regarding the fact that age and the female gender in patients with RA can present a significant association with the number of fractures, therefore, the systematic evaluation of the spine must be more meticulous in this population, in order to carry out a more efficient secondary prevention of the fractures.7,23,26
In the multivariate analysis, in addition to the age, an association was found with the development of OVF, the duration of the RA, smoking, the HAQ score, the DAS28 score, anti-cyclic citrullinated peptide antibodies, the rheumatoid factor and the presence of antinuclear antibodies. With reference to the duration of the RA, it is worth to discuss that the disease is dynamic over time, although severe cases may appear early, if timely therapy can be established, this can stop the progress and, therefore, in this context, the development of complications such as vertebral fractures.22 This finding is confirmed in our study: the longer the time of evolution, the greater the association with the development of fractures, specifically in patients with more than 10 years since the onset of joint symptoms, regardless of other known variables for OVF.
As for serological markers of poor prognosis in RA, such as rheumatoid factor, anti-cyclic citrullinated peptide (anti CCP) antibodies, and antinuclear antibodies, a significant association with the development of OVF was identified, in the same sense as that was found in other studies.10,12 In principle, in patients with seronegative RA, having this condition was associated as a variable of protection against the development of the fracture; however, these findings should be confirmed in other studies.
Although the DAS28 and the CDAI, together with the CRP and the erythrocyte sedimentation rate, are part of the strategies to assess the activity of the disease, these variables are dynamic over time and can depend on multiple factors, including infectious states and metabolic stress, among others, which can lead to underdiagnosis or overdiagnosis of the disease activity. The ideal would be to follow-up the patients over time to define the behavior of the activity and thus define a possible association with OVF. This is one of the limitations of the study, since serial measurements of these variables were not taken.20,21
In patients with RA, a large part of the prognostic measures and, above all, of the outcomes in clinical studies, corresponds to the degree of loss of functionality of the ndividual.21,22 The HAQ is a multidisciplinary tool that addresses a large part of these variables. In our study, it was found that having a higher HAQ score was significantly associated with the presence of osteoporotic vertebral fractures, of greater interest at values higher than 0.5. The development of a fracture can significantly increase the levels of the HAQ, being in this aspect greater the association with the presence of subsequent fractures.
When evaluating variables related to osteoporosis, it was found in the univariate analysis that the dose of steroids in the last year, smoking, the treatment for osteoporosis, being diabetic and lower levels of T-score had a probable association with the presence of fractures, however, in the multivariate analysis, only smoking and receiving treatment for osteoporosis were significantly associated. Even though the body mass index and the accumulated dose of steroids in the last year did not show statistical significance of association, in our study this should not be neglected when evaluating the probability of presenting an asymptomatic fracture.4 For future studies, the previously exposed variables should be included and an attempt should be made to detect other prognostic markers that make it possible to know in which patient is likely to develop a fracture.
In previous reviews, the two methods of evaluation of fractures have shown to be efficient altogether to identify those asymptomatic fractures.1,3,8,20 The recommendation is to apply initially the ABQ, followed by the Genant. This is based on the fact that this last method is less precise and overestimates the fractures, since it does not take into account anatomical variations, as the ABQ does.
Finally, in this study we identified limitations such as the low sample size, for which the results lose precision, with a high probability of committing a type II error due to the fact that the number of events found was less than necessary, compared with the number of predictors included in the multivariate analysis. Another limitation is the risk of selection bias, which is related to the use of 43% of the sample susceptible to be used, given that the patients who did not undergo the radiological study and therefore were not included could provide additional features. This systematic error could not be rectified because it is about retrospective observational information. According to the type of study, estimators are reported that allow to establish an association, not a causal effect for the presence of vertebral fracture, but to generate hypothesis that would be the starting point of future prospective studies in which our findings can be confirmed or refuted, or to objectify other variables such as biomarkers, both of bone metabolism and of osteocalcin, levels of pentosidin in urine, among others, and other paraclinical tests of activity of RA such as articular ultrasound and the measurement of biomarkers at the level of the joints.
It is important to highlight that throughout our work, it was possible to characterize better the population with RA, as well as the presence of asymptomatic osteoporotic vertebral fractures, in order to draw the attention among the health personal involved in the care of these patients, and thus to carry out an active search of those with a greater potential of presenting a OVF and start a timely treatment in order to reduce refractures, a condition that will significant impact the morbidity and mortality of these patients.
ConclusionsPatients with RA have a higher prevalence of osteoporotic vertebral fractures than the general population, regardless of whether they have osteoporosis as an underlying condition. The location with the greatest association with OVF corresponds to those vertebrae that bear more weight, with the exception that in a large number of patients these fractures are asymptomatic, which delays their identification.
This study suggests that the presence of some clinical and paraclinical characteristics could be associated with the prevalence of vertebral fractures. The possibility of developing OVF increases if it is accompanied by poor prognostic factors for RA, such as advanced age, duration of the RA, smoking, high disease activity and antibody positivity, in addition to those already known for osteoporosis. In this way, the systematic evaluation of radiographic studies using the ABQ and Genant methods makes it possible to identify a greater number of fractures and thus provide more timely treatment, which has an impact on the quality of life, morbidity and mortality of patients.
Although a high probability of committing a type II error was identified, added to a selection bias, the results of this investigation may be the basis for future explorations, in which the follow-up of the patients over time may make it possible to identify more precisely those with the highest risk of developing osteoporotic vertebral fractures.
Ethical considerationsThe study was approved by the ethics committee of the institution in which the work was conducted and informed consent was obtained from the patients.
FundingThis work had the financial support of the Colombian Association of Rheumatology (Asoreuma), the reception of the project took place on December 15, 2017.
Conflict of interestThe authors declare that they have no conflict of interest for the preparation of this work.