Rheumatoid arthritis is a multifactorial, systemic, chronic, autoimmune, and inflammatory disease that mainly affects the joints. Ultrasound has shown to be useful in detecting subclinical synovitis; however, most of the available evidence is in patients on remission, and the evidence on a correlation with the clinical activity measured by DAS-28, in our midst, is limited.
ObjectiveTo establish the correlation between clinical activity measured by DAS-28 and ultrasound in patients with rheumatoid arthritis.
Materials and methodsA total of 40 patients diagnosed with rheumatoid arthritis who were started on biological therapy or leflunomide were included in the descriptive, longitudinal, prospective study to evaluate the correlation between DAS-28 and ultrasound at baseline visit and 4 months later.
ResultsA correlation was found between DAS-28 and ultrasound, both by using the gray-scale (r=0.943, p<0.01) and the Power Doppler (r=0.946, p<0.01). There was also a correlation between the ultrasound DAS by grayscale and ultrasound DAS by Power Doppler (r=0.953, p<0.01).
ConclusionsUltrasound is a useful tool for detecting sub-clinical inflammation and the results are conclusive when comparing the number of swollen joints in the clinical evaluation with the count obtained in the ultrasound assessment. Ultrasound evaluation suggests that the hands are the joints with better performance for measuring the grade of synovitis in rheumatoid arthritis.
La artritis reumatoide es una enfermedad multifactorial, sistémica, crónica, autoinmune e inflamatoria, que afecta fundamentalmente las articulaciones. La ultrasonografía/ecografía ha demostrado utilidad en la detección de sinovitis subclínica; sin embargo, la mayoría de la evidencia disponible es en pacientes en remisión y la evidencia para la correlación con el índice de actividad clínica (DAS-28), en Colombia, es limitada.
ObjetivosEstablecer la correlación entre la actividad clínica medida por DAS-28 y la ecografía, en pacientes con artritis reumatoide.
Materiales y métodosCuarenta pacientes con diagnóstico de artritis reumatoide que iniciaron terapia biológica o leflunomida, fueron incluidos en el estudio descriptivo, longitudinal, prospectivo para evaluar la correlación entre el DAS-28 y la ecografía, en la consulta basal y a los 4 meses.
ResultadosSe encontró correlación entre el índice de actividad clínico de la enfermedad (DAS-28) y el índice de actividad ecográfico (DAS ecográfico), tanto por escala de grises (r=0,943, p<0,01) como por Power Doppler (r=0,946, p<0,01); también se encontró correlación entre el DAS ecográfico por escala de grises y el DAS ecográfico por Power Doppler (r=0,953, p<0,01).
ConclusionesLa ecografía es de utilidad en la detección de inflamación subclínica y los resultados son concluyentes cuando se compara el número de articulaciones inflamadas en la evaluación clínica, con el conteo obtenido en la evaluación ecográfica. La evaluación ecográfica sugiere que las manos son las articulaciones con mejor rendimiento para la medición del grado de sinovitis en la artritis reumatoide.
Rheumatoid arthritis (RA) is a multifactorial, systemic, chronic, autoimmune and inflammatory disease that affects the diarthrodial joints.1 The clinical manifestations of the disease include arthralgia, morning stiffness and synovitis, causing diverse degrees of alterations and functional limitation. The disease has a progressive course; therefore, the lack of treatment and control of the disease activity leads to structural joint damage, causing deformity of the joint. The physical and functional deterioration, in the short and long term, causes decrease in the quality of life and increases morbidity and mortality.2–7
The ideal therapeutic objective in RA seeks to achieve and maintain clinical remission, and to stop the erosive progression while preserving the physical function and the mobility of the patient.4,6,8 In this context, the assessment of the achievement of the therapeutic objectives requires the determination of the disease activity level, its progression and the response obtained with a particular intervention. In this sense, different clinical tools (composite activity indexes) blood biomarkers and radiographic evaluation techniques9 that are frequently used in clinical trials and in clinical practice, and which allow to evaluate the degree of inflammatory activity, the functional disability and the residual structural damage, have been validated.
The composite indexes of clinical activity include the disease activity score (DAS-28), the Simplified Disease Activity Index, and the clinical disease activity score, among others. The radiological aids include conventional radiography (X-rays), which is useful in the follow up of the radiological progression, through the application of indexes that evaluate the erosions and the reduction of the joint space. Meanwhile, echography (ECHO) is a technique of recent use that includes the use of grayscale (GS) images that evaluate the anatomical structures and the Doppler modalities (Power Doppler – PD) that show the blood flow, allowing to: (1) localize the increase of the synovial vascularization related with the inflammatory activity, and (2) detect erosions more easily than with conventional radiography. On the other hand, it is a rapid non-invasive technique, more economic than magnetic resonance imaging (MRI), and it can be carried out easily in routine clinical practice.9,10 Different studies have demonstrated a high sensitivity in the detection of signs of inflammation in comparison with the clinical assessment11–13 and in the evaluation of the bone erosion, compared with X-rays.14
The usefulness of DAS-28 has been questioned due, among other aspects, to (1) the subjectivity of the clinical evaluation, (2) the little concordance between the number of swollen joints (NSJ) and the ESR values, (3) the omission of the foot joints, (4) the insufficient ability to define the remission of the disease, (5) the high count of painful joints with small changes in the ESR and (6) the joint count is an indirect evaluation of the inflammation in a joint and it can hardly be considered as an objective measure.13,15 In addition, in some patients in apparent remission or low disease activity by DAS-28, activity and radiological progression have been documented when they are assessed by imaging techniques such as ECHO or MRI.16–19 In this sense, there is increasing evidence that the radiological assessment methods, especially the high-resolution ECHO and MRI, are more sensitive in the detection of synovitis and in the evaluation of the progression of joint damage.13
Blood biomarkers, clinical assessment and activity indexes such as the DAS-28, the Simplified Disease Activity Index and the clinical disease activity score are mainly used in routine clinical practice for the follow-up of patients with RA.4,20 On the other hand, the ECHO has shown to be useful in the detection of subclinical synovitis related to the clinical disease-activity.21 However, most of the available evidence comes from patients in clinical remission of the disease12,22; while the information related to the correlation between the activity measured by DAS-28 and ECHO and the radiological progression, in our environment, is limited.9 Therefore, the purpose of this work was to evaluate the correlation between the clinical activity measured by DAS-28 and ECHO, and as secondary objective, the radiological progression (measured by X-rays) in patients with RA.
Materials and methodsA descriptive, longitudinal, prospective study in patients with RA was conducted in the IPS Medicarte in Medellin, Colombia. The study included patients with diagnosis of RA, older than 18 years, who met the ACR classification criteria of 1987 (in patients with an evolution of RA≥2 years) or the ACR and EULAR criteria of 2010 (in patients with an evolution of the RA<2 years) who attended the IPS Medicarte in the period between 15/12/2013 and 27/05/2015, to initiate biological therapy or leflunomide within a comprehensive care program. Patients with overlap syndromes, active infectious processes, pregnancy or recent or scheduled surgical procedures were excluded.
A non-probability sample was defined for convenience. The patients entered the study after meeting the selection criteria (dynamic cohort) and signing the informed consent.
The variables were defined according to the 3 follow-up measures studied: disease activity by DAS-28, disease activity by ECHO, and radiological progression according to the Sharp/Van der Heidje (SVdH) index. The patients were evaluated when they entered the study and 4 months later.
Assessment of DAS-28: in order to determine the disease activity, the DAS-28 was applied to all patients in the baseline visit and then at 4 months. The DAS-28 was conducted by a rheumatologist of the IPS Medicarte who was unaware of the echographic results. During the clinical evaluation, the number of painful joints (NPJ) and the NSJ were determined through the count of 28 joints, which included the wrists, metacarpophalangeal (MCP), proximal interphalangeal, elbows, shoulders and knees. In addition, it was recorded the global health assessment carried out by the patient (PGE) on a visual analog scale from 0 to 100 and the erythrocyte sedimentation rate (ESR). The DAS-28 was calculated with these variables according to the international guidelines.23,24
The results obtained were interpreted according to the following categories2,4: values <2.6 were defined as remission of the disease, values ≥2.6 and <3.2 were defined as low disease activity, values ≥3.2 and <5.1 were defined as moderate disease activity, and values ≥5.1 were defined as high disease activity.
Echographic evaluation: US was carried out to all patients at the baseline visit and at 4 months. The ECHO was carried out by a rheumatologist specialized in the technique (Carmen Ceron Villaquiran) and certified by the EULAR for the performance of musculoskeletal ECHO and who was unaware of the results of the DAS-28. An Esaote MyLab™ Class C ultrasound system was used. The images were analyzed for synovitis by GS in order to determine the degree of synovial hypertrophy and the PD technique was associated to determine the degree of inflammatory activity related with the disease activity.
ECHO was performed on the joints of the hands (wrists, MCP and proximal interphalangeal) using a high-frequency linear probe (6–18MHz). Also, ECHO of the elbows and knees was performed using a high-frequency linear probe (4–13MHz). The recommendations of the EULAR guidelines for the performance of musculoskeletal ECHO in rheumatology were followed.11,25
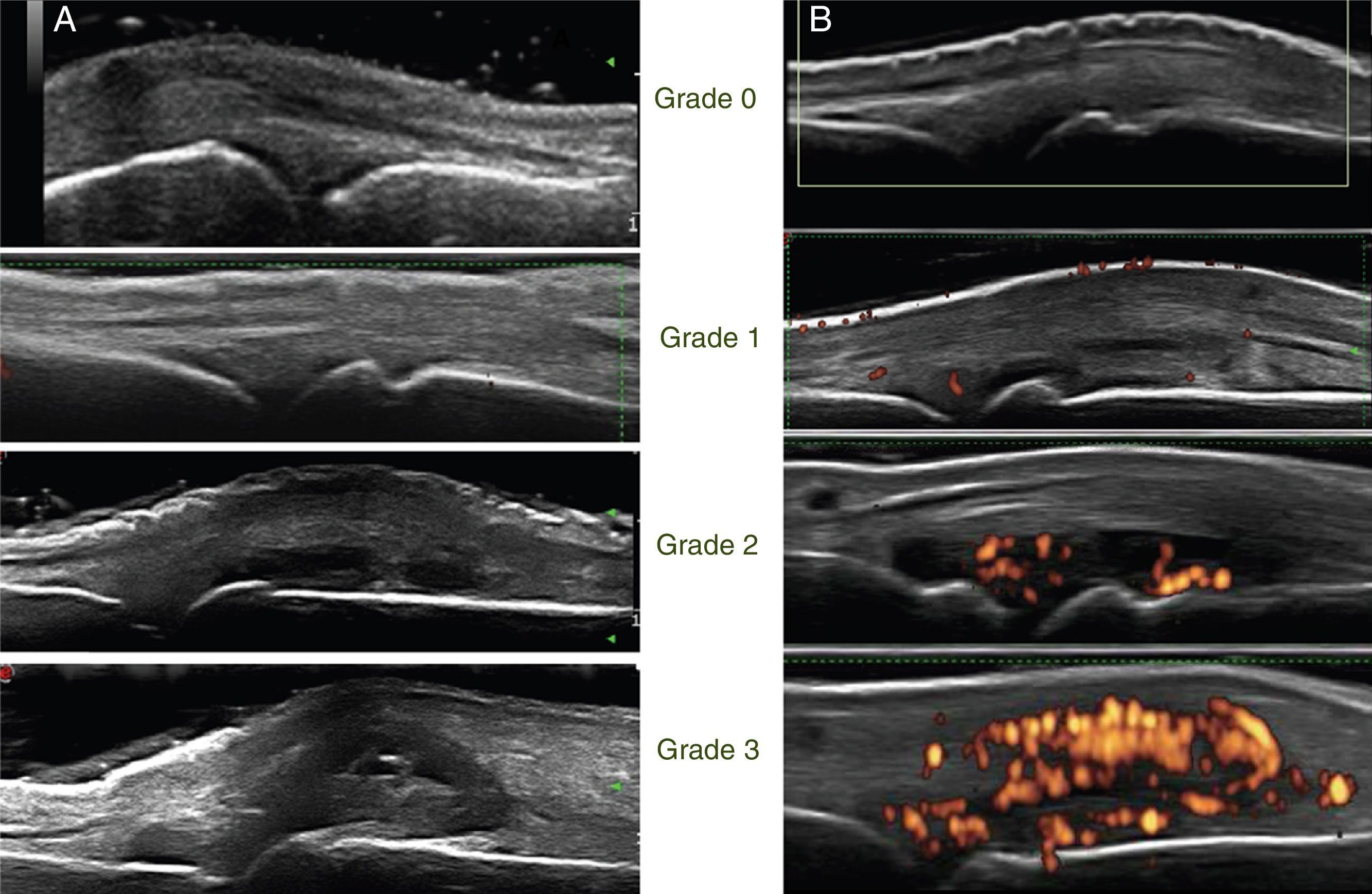
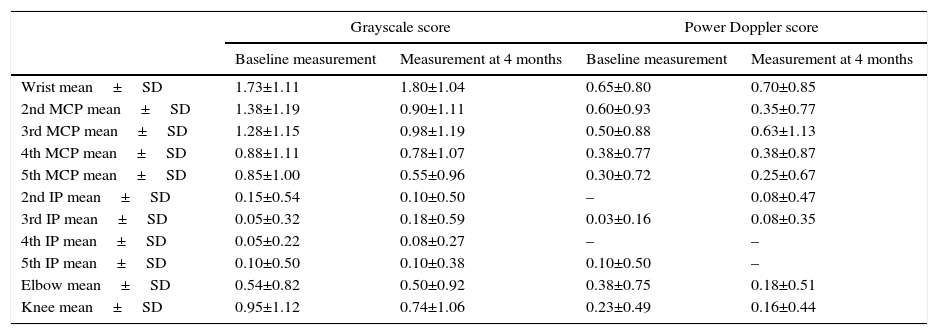
The images were stored digitally and then, the same rheumatologist who took them made their reading. Synovitis was evaluated for each joint, both with GS (synovial hypertrophy) and PD (inflammatory activity). The following semiquantitative scoring scale was used for both techniques (Fig. 1).9
Grayscale: semi-quantitative scale from 0 to 3, where grade 0: normal, there is no synovial hypertrophy, absence of anechoic, hyperechoic or hypoechoic structures; grade 1: mild synovial hypertrophy (small intra-articular anechoic or hypoechoic area that discreetly displaces the joint capsule); grade 2: moderate synovial hypertrophy (intra-articular anechoic or hypoechoic area that discreetly displaces the joint capsule without bulging it); grade 3: severe synovial hypertrophy (intra-articular anechoic or hypoechoic area that discreetly displaces the joint capsule generating bulging thereof).
Power Doppler: semi-quantitative scale from 0 to 3, where grade 0: normal, absence of signal, associated with absence of inflammatory activity; grade 1: mild, one to 3 signal points, associated with mild inflammatory activity; grade 2: moderate, more than 3 points that occupy less than 50% of the articular synovial surface, associated with moderate inflammatory activity; grade 3: severe, occupies more than 50% of the articular synovial surface, associated with high inflammatory activity.
After the semi-quantitative scoring of each joint, it was carried out the echographic count of the number of joints (articulations) with synovitis by grayscale (NAS-GS), for synovial hypertrophy, and the number of joints with synovitis by PD (NAS-PD), for inflammatory activity. A joint to which it had been assigned a score different from 0 (score from 1 to 3) was considered as a joint with synovitis. In order to calculate the echographic DAS by grayscale (DASECHO-GS) and the echographic DAS by PD, the variables, NAS-GS and NAS-PD, were replaced in the formula of DAS-28 by the NSJ. The results obtained were interpreted in the same way as the DAS-28.26,27
Assessment by conventional radiography (X-rays): comparative bilateral X-rays of hands and feet were carried out as secondary objective at the baseline visit and at 4 months of follow-up, in the reference center designated by the institution. The digital radiographic readings were analyzed and interpreted by a radiologist who assigned the score of the SVdH index and who was unaware of the clinical and echographic results of the patients. Erosions were evaluated in 32 joints of the hands and wrists, and in 12 joints of the feet. The scores of the erosions per joint were from 0 to 5 in the hands and the wrists, and from 0 to 10 in the feet, with a maximum total score of 280. The narrowing of the joint space was evaluated in 30 joints of the hands and wrists, and in 12 joints of the feet. The scores were assigned per joint in a range from 0 to 4 with a maximum total score of 168.28
Data analysis: it was made the descriptive analysis of the quantitative variables (NPJ, NSJ, ESR, PGE, DAS-28, NAS-GS, NAS-PD, DASECHOGS, DASECHOPD, SVdHI). Subsequently, the validation of normality assumptions and homogeneity of variances was carried out using the Shapiro Wilk test for n<50. The non-parametric Wilcoxon test for related samples (repeated measures) was applied. Finally, the correlation between the clinical, echographic and radiological variables was performed using the Spearman's correlation test according to the distribution of the variables and the sample number (samples smaller than or equal to 50). The statistical analysis was performed with the SPSS statistical software, version 22.0 (SPSS Inc., Chicago, Illinois).
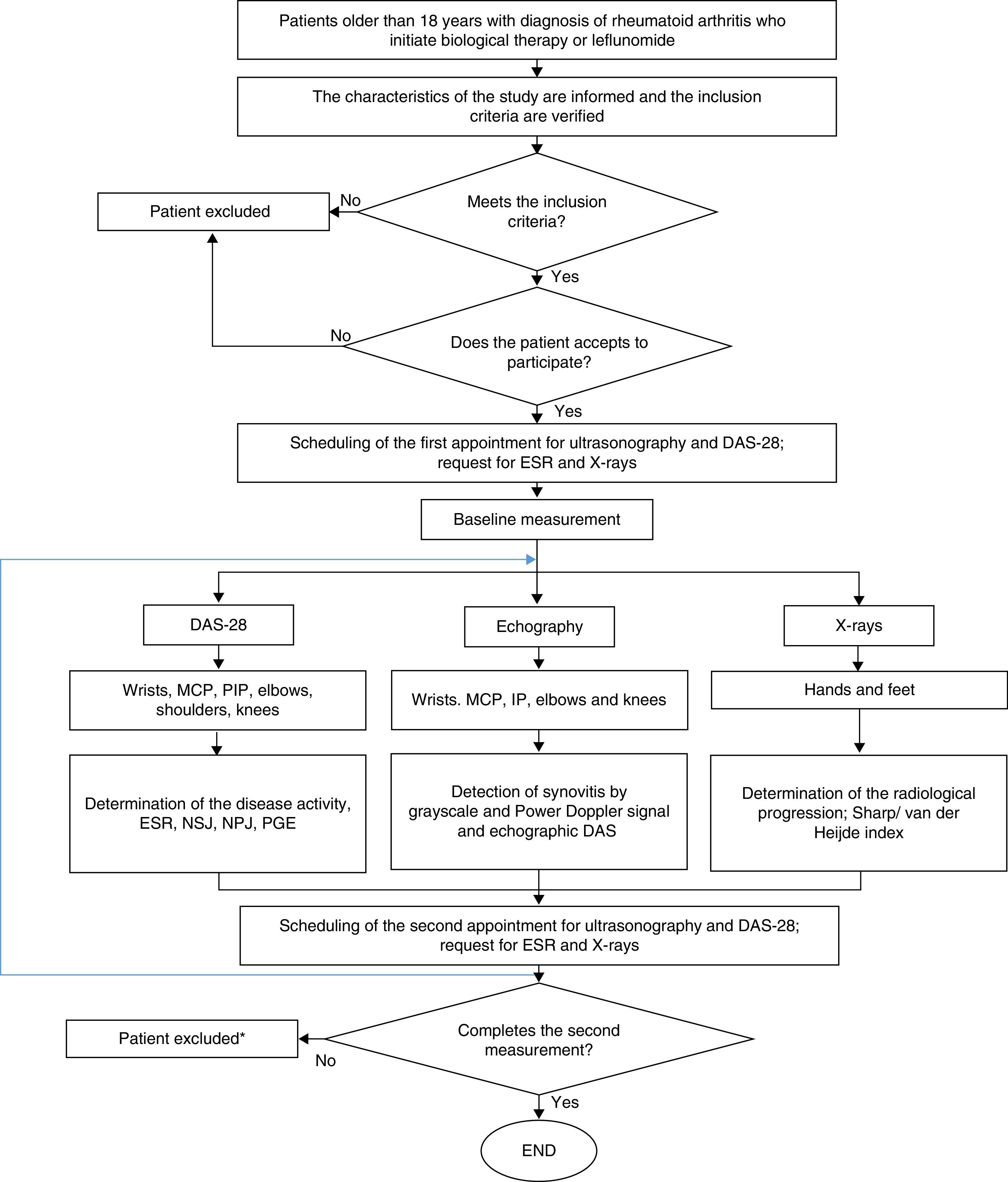
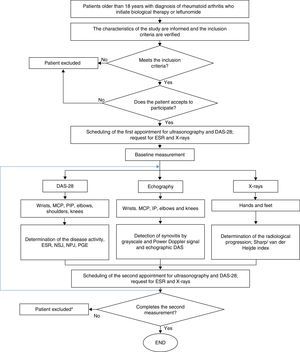
Ethical considerationsThe protocol of this study was in compliance with that was established by Resolution 8430 of 1993, from the Ministry of Health of Colombia, being valued as of low risk for the patients. In this sense, it was approved by the Technical Committee of Research of the Faculty of Pharmaceutical and Food Sciences of the University of Antioquia and by the Ethics Committee of the institution where the patients were treated. The participation in the study was voluntary and the patients signed informed consent. Fig. 2 shows the general algorithm for the study, which is based on the considerations described above.
General procedure for the study. DAS-28: disease activity score; PGE: patient's global evaluation; PIP: proximal interphalangeal; MCP: metacarpophalangeal; NPJ: number of painful joints; NSJ: number of swollen joints; ESR: erythrocyte sedimentation rate. * Only the patients who did not complete the 2 measurements of echography and DAS28 were excluded.
In the study were included 48 patients, who entered between 15/12/2013 and 27/05/2015. Complete follow-up data for DAS-28 and US were obtained in 40 patients. The remaining 8 patients only completed one measurement of DAS-28 and US, and were excluded. In this sense, the data analysis was performed with the 40 patients who completed the clinical and echographic evaluation.
X-rays of the hands and feet were taken in 34 patients; baseline measurement and at 4 months were obtained in 18 patients; 16 patients had only one baseline and comparative X-ray measurement, and of follow-up (7 baseline measurement and 9 at 4 months).
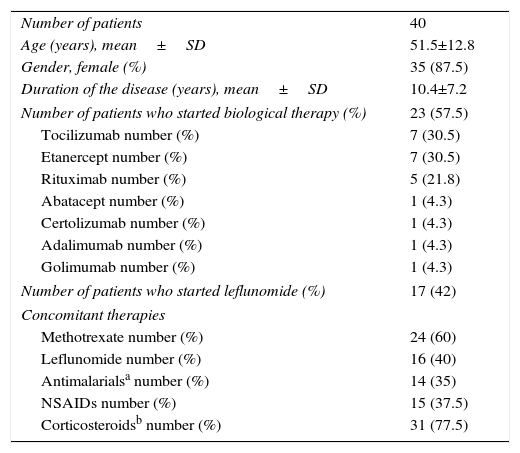
Table 1 shows the summary of the sociodemographic characteristics of the patients. 87.5% of the patients were women and 12.5% were men, with an age (mean±SD) of 51.5±12.8 years and a time of duration of the disease (mean±SD) of 10.4 years±7.2. Regarding the pharmacological treatment, 23 patients (57.5%) were under treatment with biological therapy and 17 patients (42.5%) were under treatment with leflunomide. Of the patients under biological therapy 4 (17.4%) were in monotherapy and 19 patients (82.6%) received combination therapy with DMARDs.
General characteristics of the patients.
| Number of patients | 40 |
| Age (years), mean±SD | 51.5±12.8 |
| Gender, female (%) | 35 (87.5) |
| Duration of the disease (years), mean±SD | 10.4±7.2 |
| Number of patients who started biological therapy (%) | 23 (57.5) |
| Tocilizumab number (%) | 7 (30.5) |
| Etanercept number (%) | 7 (30.5) |
| Rituximab number (%) | 5 (21.8) |
| Abatacept number (%) | 1 (4.3) |
| Certolizumab number (%) | 1 (4.3) |
| Adalimumab number (%) | 1 (4.3) |
| Golimumab number (%) | 1 (4.3) |
| Number of patients who started leflunomide (%) | 17 (42) |
| Concomitant therapies | |
| Methotrexate number (%) | 24 (60) |
| Leflunomide number (%) | 16 (40) |
| Antimalarialsa number (%) | 14 (35) |
| NSAIDs number (%) | 15 (37.5) |
| Corticosteroidsb number (%) | 31 (77.5) |
NSAIDs: non-steroidal anti-inflammatory drugs; SD: standard deviation.
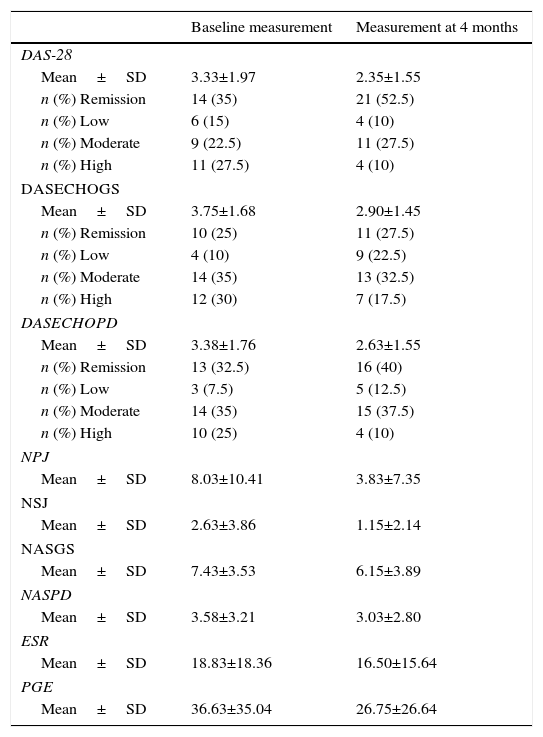
Evolution of the clinical and echographic parameters: Table 2 shows the course of the clinical, echographic and radiological progression parameters at the baseline evaluation and at 4 months. When the disease activity index was evaluated it was found a mean±SD for the DAS-28 of 3.33±1.97 at the baseline measurement and 2.35±1.55 at 4 months; for the DASECHOGS of 3.75±1.68 at the baseline measurement and 2.90±1.45 at 4 months and for the DASECHOPD of 3.38±1.76 at the baseline measurement and 2.63±1.65 at 4 months. In general, it was observed an improvement in the scoring of these scales at 4 months and a favorable change regarding the classification of the disease activity. In this sense, at 4 months, the majority of patients were in remission and low disease activity.
Course of the clinical and echographic parameters in the baseline evaluation and at 4 months.
| Baseline measurement | Measurement at 4 months | |
|---|---|---|
| DAS-28 | ||
| Mean±SD | 3.33±1.97 | 2.35±1.55 |
| n (%) Remission | 14 (35) | 21 (52.5) |
| n (%) Low | 6 (15) | 4 (10) |
| n (%) Moderate | 9 (22.5) | 11 (27.5) |
| n (%) High | 11 (27.5) | 4 (10) |
| DASECHOGS | ||
| Mean±SD | 3.75±1.68 | 2.90±1.45 |
| n (%) Remission | 10 (25) | 11 (27.5) |
| n (%) Low | 4 (10) | 9 (22.5) |
| n (%) Moderate | 14 (35) | 13 (32.5) |
| n (%) High | 12 (30) | 7 (17.5) |
| DASECHOPD | ||
| Mean±SD | 3.38±1.76 | 2.63±1.55 |
| n (%) Remission | 13 (32.5) | 16 (40) |
| n (%) Low | 3 (7.5) | 5 (12.5) |
| n (%) Moderate | 14 (35) | 15 (37.5) |
| n (%) High | 10 (25) | 4 (10) |
| NPJ | ||
| Mean±SD | 8.03±10.41 | 3.83±7.35 |
| NSJ | ||
| Mean±SD | 2.63±3.86 | 1.15±2.14 |
| NASGS | ||
| Mean±SD | 7.43±3.53 | 6.15±3.89 |
| NASPD | ||
| Mean±SD | 3.58±3.21 | 3.03±2.80 |
| ESR | ||
| Mean±SD | 18.83±18.36 | 16.50±15.64 |
| PGE | ||
| Mean±SD | 36.63±35.04 | 26.75±26.64 |
DAS-28: disease activity score; DASECHOGS: echographic DAS by grayscale; DASECHOPD: echographic DAS by Power Doppler; PGE: patient's global evaluation; NPJ: number of painful joints; NSJ: number of swollen joints; NASGS: number of joints with synovitis (synovial hypertrophy) by grayscale; NASPD: number of joints with synovitis (inflammatory activity) by Power Doppler; ESR: erythrocyte sedimentation rate.
Regarding the joint count, the mean±SD of the NSJ in the clinical assessment was 2.63±3.86 at the baseline measurement and 1.15±2.14 at 4 months of follow-up; while the mean±SD for the NASGS and NASPD was 7.43±3.53 and 3.58±3.21, respectively, at the baseline measurement and 6.15±3.89 and 3.03±2.80, respectively, at 4 months of follow-up.
In the echographic evaluation is observed that the wrists are the joints with the highest score by GS with a mean±SD of 1.73±1.11 for the right wrist and 1.93±1.12 for the left wrist at the baseline measurement and of 1.80±1.04 for the right wrist and 1.80±1.20 for the left wrist at 4 months. Something similar occurs at the evaluation with PD where it was found a mean±SD of 0.65±0.80 for the right wrist and 0.75±0.81 for the left wrist at the baseline measurement and of 0.70±0.85 for the right wrist and 0.78±0.83 for the left wrist at 4 months. Among the MCP joints, it is observed that the highest score was assigned to the second MCP, both left and right, in GS as well as with PD. Table 3 shows the results of the echographic evaluation in relation with the semi-quantitative scoring assigned to the joints evaluated both with grayscale and with PD, the results of the joints of the right side are shown; similar results were obtained for the joints of the left side.
Results of the echographic evaluation.
| Grayscale score | Power Doppler score | |||
|---|---|---|---|---|
| Baseline measurement | Measurement at 4 months | Baseline measurement | Measurement at 4 months | |
| Wrist mean±SD | 1.73±1.11 | 1.80±1.04 | 0.65±0.80 | 0.70±0.85 |
| 2nd MCP mean±SD | 1.38±1.19 | 0.90±1.11 | 0.60±0.93 | 0.35±0.77 |
| 3rd MCP mean±SD | 1.28±1.15 | 0.98±1.19 | 0.50±0.88 | 0.63±1.13 |
| 4th MCP mean±SD | 0.88±1.11 | 0.78±1.07 | 0.38±0.77 | 0.38±0.87 |
| 5th MCP mean±SD | 0.85±1.00 | 0.55±0.96 | 0.30±0.72 | 0.25±0.67 |
| 2nd IP mean±SD | 0.15±0.54 | 0.10±0.50 | – | 0.08±0.47 |
| 3rd IP mean±SD | 0.05±0.32 | 0.18±0.59 | 0.03±0.16 | 0.08±0.35 |
| 4th IP mean±SD | 0.05±0.22 | 0.08±0.27 | – | – |
| 5th IP mean±SD | 0.10±0.50 | 0.10±0.38 | 0.10±0.50 | – |
| Elbow mean±SD | 0.54±0.82 | 0.50±0.92 | 0.38±0.75 | 0.18±0.51 |
| Knee mean±SD | 0.95±1.12 | 0.74±1.06 | 0.23±0.49 | 0.16±0.44 |
SD: standard deviation; IP: interphalangeal; MCP: metacarpophalangeal. The values in which (–) is indicated correspond to variables that had a score of 0 in all the measurements.
The normality assumptions of the variables were checked by the Shapiro–Wilk test used for n<50. The number of joints with synovitis by GS (NASGS) was the only variable that fulfilled the normality assumption (p=0.222 at the baseline measurement and p=0.075 at 4 months). Since the majority of the variables did not fulfill the normality assumption, it was applied the non-parametric Wilcoxon test for related samples (repeated measures).
Table 4 shows the results of the Wilcoxon test. Statistically significant results were found for the clinical DAS-28, the DASECHOGS, the DASECHOPD ant the NSJ (p=0.009; p=0.009, p=0.026 and p=0.017, respectively). On the other hand, in the count of joints with synovitis by GS (NASGS) and by PD (NASPD) no statistically significant differences were found (p=0.071 and p=0.132, respectively).
Wilcoxon test for the clinical, echographic and radiological progression variables.
| Variable | Baseline measurement | 4 months | Wilcoxon (p-value) | ||
|---|---|---|---|---|---|
| Median | Interquartile range (25–75%) | Median | Interquartile range (25–75%) | ||
| DAS-28 | 3.0 | 1–4 | 2.0 | 1–4 | 0.009 |
| DASECHOGS | 3.5 | 2–5 | 3.0 | 2–5 | 0.009 |
| DASECHOPD | 3.0 | 2–4 | 2.0 | 2–4 | 0.026 |
| NSJ | 0.50 | 0–2 | 0 | 0–2 | 0.017 |
| NASGS | 7.0 | 4–9 | 6 | 4–9 | 0.071 |
| NASPD | 3.0 | 1–5 | 2 | 1–5 | 0.132 |
| ESR | 15 | 7–21 | 14.5 | 7–21 | 0.472 |
| SVdHI | 29 | 16.25–43.50 | 22 | 16.25–43.50 | 0.407 |
DAS: disease activity score; GS: grayscale; SVdHI: Sharp/Van der Heidje index; NSJ: number of swollen joints; PD: Power Doppler; ESR: erythrocyte sedimentation rate.
Spearman's correlation was determined for the clinical, echographic and radiological variables in the total time. A high positive correlation was found between the DAS-28 and the echographic DAS in grayscale (DASECHOGS), r=0.943, p<0.01; between DAS-28 and the echographic DAS by PD (DASECHOPD), r=0.946, p<0.01 and between the DASECHOGS and the DASECHOPD, r=0.953, p<0.01. A regular positive correlation was found between the DAS-28 and the NSJ detected in the clinical evaluation, r=0.740, p<0.01 and the number of joints with synovitis by GS (NASGS) and by PD (NASPD), r=0.570, p<0.01.
As an additional measure, the correlation with the radiological progression measured by the SVdH index was determined in 34 patients. However, no correlation was found with any of the clinical or echographic variables.
In addition, the Spearman correlation was determined for the clinical, echographic and radiological variables at baseline and at 4 months. At the baseline measurement, a regular positive correlation was found between the DAS-28 and the NSJ detected in the clinical evaluation, r=0.710, p<0.01; a regular positive correlation was also found for the number or joints with synovitis by GS (NASGS) and by PD (NASPD), r=0.495, p<0.01.
In the follow-up at 4 months, a moderate positive correlation was found between the DAS-28 and the NSJ detected in the clinical evaluation, r=0.749, p<0.01; a moderate positive correlation was also found for the number of joints with synovitis by GS (NASGS) and by PD (NASPD), r=0.570, p<0.01.
Table 5 shows the results of Spearman's correlation in the total time, at baseline and at 4 months for the clinical, echographic and radiological progression variables.
Spearman's correlation for the clinical, echographic and radiological variables.
| Variables | Total | Baseline | At 4 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | N | r | p | N | r | p | N | |
| DAS-28-DASECHOGS | 0.943 | <0.01* | 80 | ||||||
| DAS-28-DASECHOPD | 0.946 | <0.01* | 80 | ||||||
| DAS-28-NSJ | 0.740 | <0.01* | 80 | 0.710 | <0.01* | 40 | 0.749 | <0.01* | 40 |
| DAS-28-NASGS | 0.082 | 0.467 | 80 | −0.163 | 0.315 | 40 | 0.275 | 0.086 | 40 |
| DAS-28-NASPD | 0.071 | 0.529 | 80 | −0.075 | 0.647 | 40 | −0.160 | 0.426 | 40 |
| DASECHOGS-DASECHOPD | 0.953 | <0.01* | 80 | ||||||
| NSJ-NASGS | 0.115 | 0.308 | 80 | −0.161 | 0.321 | 40 | 0.381 | 0.015** | 40 |
| NSJ-NASPD | 0.211 | 0.060 | 80 | 0.152 | 0.348 | 40 | 0.231 | 0.151 | 40 |
| NASGS-NASPD | 0.570 | <0.01* | 80 | 0.495 | <0.01* | 40 | 0.666 | <0.01* | 40 |
| DAS-28-SVDHI | −0.069 | 0.628 | 52 | −0.065 | 0.757 | 25 | −0.160 | 0.426 | 27 |
| DASECHOGS-SVDHI | 0.000 | 0.999 | 52 | ||||||
| DASECHOPD-SVDHI | −0.020 | 0.889 | 52 | ||||||
| NSJ-SVDHI | −0.105 | 0.461 | 52 | −0.028 | 0.894 | 25 | −0.324 | 0.099 | 27 |
| NASGS-SVDHI | 0.153 | 0.279 | 52 | 0.300 | 0.145 | 25 | 0.067 | 0.738 | 27 |
| NASPD-SVDHI | 0.133 | 0.349 | 52 | 0.186 | 0.372 | 25 | −0.017 | 0.933 | 27 |
DAS-28: disease activity score; SVdHI: Sharp/Van der Heijde Index; NSJ: number of swollen joints; NASGS: number of joints with synovitis (synovial hypertrophy) by grayscale; NASPD: number of joints with synovitis (inflammatory activity) by Power Doppler.
The development of new reliable methods to evaluate the synovial inflammation and the response to treatment in patients with RA is a challenge in routine clinical practice and a relevant field of research in rheumatology. In the last decade there has been an increasing use of musculoskeletal ECHO in the evaluation of patients with RA, and especially the use of techniques of PD to evaluate the joint inflammatory activity in patients with RA.11
The DAS-28 is the most used clinical tool for the evaluation and monitoring of the patients with RA. Among the methods of radiological evaluation stand out the advantages and the usefulness of the ECHO in the diagnosis and follow-up of patients with RA and its use in clinical practice is currently common in countries as the United States and Spain.29 These tools can be used as measures of disease progression, at the articular level and in the planning of therapeutic strategies to modify the disease activity and improve the quality of life of the patients.
In this study were included 40 patients who completed follow-up at baseline and at 4 months; it can be observed that most of the patients are women (87.5%), which is related with the incidence or RA that has shown to be higher in women. In Spain, the data indicate that the disease affects 0.5% of the general population, 0.8% of women and 0.2% of men.30 Studies conducted in Latin America have shown prevalences of 0.46% for Brazil, 0.3% for Mexico and 0.2% for Argentina.31 Currently, a study on the prevalence of rheumatic diseases in Colombian population is being carried out with data not published yet. It was found a duration of the disease in years of 10.4±7.22 (mean±SD) which indicates that the patients included in the study are patients with established RA, in whom a greater extension of the sequelae and the synovial proliferation is expected, and it becomes important to determine the degree of activity of the lesions.4
The DAS-28 was calculated in order to establish the clinical disease activity at the baseline visit and at 4 months and echographic assessment was carried out, both by GS (to determine the presence of joint effusion or synovial hypertrophy) and by PD (to evaluate the inflammatory activity) at the baseline visit and at 4 months.
Related with the clinical evaluation of the disease activity using the DAS-28, at the baseline visit 50% of patients were in remission and low disease activity; while at 4 months the value reached 62% of the patients. The results for the echographic DAS were similar, highlighting an increase in the percentage of patients with improvement in this scale at 4 months of follow-up. However, although the clinical and echographic parameters improved at 4 months of follow-up, the Wilcoxon test only yielded statistically significant results for DAS-28, DASECHOGS, DASECHOPD and the NSJ, suggesting that these monitoring tools were sensitive to the change and, therefore, useful in the evaluation of the improvement in clinical activity at 4 months of follow-up. These results differ from those found for the variables related with the number of joints with synovitis by GS and PD, which did not show statistically significant results in the Wilcoxon test, suggesting that the echographic joint count could be useful in the identification of patients with inflamed joints that are not detected in the clinical evaluation, which would provide the specialist with information for the therapeutic interventions that are necessary to achieve the therapeutic objectives. However, this result would require to be verified in a study with a larger number of patients and a longer follow-up period.
The correlation between the disease activity variables, measured by DAS-28 and US, and the radiological progression, measured by the SVdH index was evaluated, finding an excellent positive correlation between the DAS-28 and the echographic DAS, both in grayscale (DASECHOGS) and PD (DASECHOPD), but no correlation was found with the radiographic progression of the disease, explained, in part, by the short follow-up time of the study, which makes difficult the detection of radiological changes and by the small number of patients included in the study. These results are similar to those reported by Mandl et al.,26 who evaluated the metrological properties of the composite disease activity indexes for RA, using information of the clinical and echographic evaluation in GS and PD, and thereby, they classified the patients according to the disease activity. This study also did not find significant differences between the classic DAS-28 and the echographic indexes, in which the count of inflamed joints was replaced by the count of joints with synovitis by GS and PD. This could be explained by the inconveniences that have been questioned to DAS-28 and, therefore, the counts of the NSJ and painful joints do not generate major modifications of the final result of the DAS.15,29
On the other hand, when the correlation between the NSJ at clinical evaluation and the echographic count of joints with synovitis was made, no correlation was found between these variables. Therefore, it could be established that US could detect joints with synovitis (both at the evaluation by GS and at the evaluation by PD) not detected during the clinical assessment; characteristic that has been recognized as one of the main advantages of US, favoring the detection of inflammation more easily than the composite indexes. In this direction, several studies have shown the usefulness of US, especially associated with PD, in the detection of subclinical synovitis,21 which is useful in therapeutic decision making. In addition, it has demonstrated a better capacity to discriminate patients with a progression in the structural damage from those who do not present it, acting as an instrument to predict relapse among the patients with RA in remission, which could have very important implications in therapy adjustments, reductions in medication doses and withdrawal of therapies in search of a better cost-effectiveness ratio.32 Similarly, there have been described in ultrasound high scores in the GS, the PD signal and even erosions in early stages in undifferentiated inflammatory arthritis, which have a higher risk of developing RA in the presence of one or more of these echographic signs.9
Naredo et al.,11 found in their longitudinal study that the echographic findings by PD, accumulated over time and related with the inflammatory activity, had a strong correlation with the progression of the radiological damage, as well as with the radiographic erosion and the total scores after a year of follow-up in patients with early RA. Another study evaluating the validity, the sensitivity and the predictive value of the ECHO-PD in the follow-up of the response to the antagonists of the tumor necrosis factor alpha (anti-TNFα) in RA, showed the usefulness of US to monitor the response of inflammatory activity. The persistence of synovial PD signal would have a predictive value for the radiological progression in patients with established RA, who are treated with anti-TNFα agents.33
The echographic evaluation showed that the wrists and the second MCP are the joints that exhibit better performance in the evaluation of the synovitis, both by GS and by PD. These results coincide with the characterization of RA as an inflammatory disease that mainly affects the small joints of the hands and feet.4,7 Ceponis et al.,34 evaluated the usefulness of US in the hands and wrists of patients with RA to establish the inflammatory activity in this disease and they found that the performance of US in the wrists and in the second and third MCP was useful and clinically significant in the follow-up of the disease activity in patients with RA, as well as in the therapeutic decision making.
On the other hand, the early signs of inflammation of the extensor carpi ulnaris have a high predictive value for the development of erosive damage in RA.35 In addition, the use of US in early RA can be useful in the definition of clinical remission, due to its ability to identify subclinical inflammation in patients considered in remission; moreover, the presence of positive PD signal indicates an increased risk of relapses and progression of the disease. This indicates that US can be very useful in the classification of patients according to their inflammatory activity, which will allow the instauration of pharmacological measures in order to fulfill the therapeutic objectives for the disease.9
Although the usefulness of US in the evaluation of structural and inflammatory changes has been described, most studies have focused on the evaluation of synovitis and erosions, ignoring other fundamental aspects in RA, such as tenosynovitis, bursitis, and changes in the cartilage, among others. Moreover, there is currently no consensus on a single optimal scoring system or on the joints that should be evaluated; the semi-quantitative scale is one of the most used in the majority of the studies and the joints commonly evaluated include the second metacarpophalangeal and the wrists. That is why US, being a tool with a great capacity to evaluate more accurately the joint abnormalities, would favor the classification of apparent clinical remissions. The presence of subclinical synovitis can be considered as a sign of possible progression of RA that needs to be monitored in order to provide the rheumatologist with useful information for therapeutic decision-making.15,30
The results and conclusions of this work should be interpreted with caution, due to the limitations of the study. In this sense, our study is observational, with a cohort of 40 patients and with a short-term follow-up despite of the fact that US is not a widely extended diagnostic technique in our environment and that is not used routinely in clinical practice, our study reflects the high sensitivity to change of this diagnostic technique and invites to a more generalized use of US in the ambulatory practice of rheumatology.
It is important to highlight that this is a descriptive study of 2 measurements and it is necessary to carry out similar studies with long-term follow-up and that include a larger number of patients to evaluate the behavior of these variables, especially in relation to radiological progression. However, the present study stands out for the inclusion of patients with RA independently of its clinical activity and the follow-up during a period of 4 months using 3 tools that are important in rheumatology practice and with a methodological rigor adequate for the variables analyzed during the follow-up.
ConclusionsUS in grayscale and associated with PD identifies a greater NSJ (with synovitis) than the clinical evaluation, and could be useful in the detection and follow-up of inflamed joints that are not detected in the clinical evaluation in patients with established RA.
In terms of correlation, no correlation was found between the number of swollen joints in the clinical evaluation and the joints inflamed by US. But there was correlation when the inflamed joints detected by US were replaced in the formula of the DAS-28.
DAS-28, DASECHOGS, DASECHOPD and the NSJ are useful tools to evaluate the change in the disease activity at 4 months. On the other hand, no correlation was identified between the clinical and echographic results and the radiological progression, so long-term studies that evaluate this relationship are necessary.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestNone declared.
José A. Gómez-Puerta receives support from Colciencias (Convocation 656 of 2014).
Please cite this article as: Uribe L, Cerón C, Amariles P, Llano JF, Restrepo M, Montoya N, et al. Correlación entre la actividad clínica por DAS-28 y ecografía en pacientes con artritis reumatoide. Rev Colomb Reumatol. 2016;23:159–169.