Giant cell arteritis is a vasculitis that predominantly affects large caliber vessels, and usually appears in people over 50 years-old. Its clinical presentation includes headache, hearing impairment, or polymyalgia rheumatica-like symptoms. In its most severe form, it can cause uni- or bilateral vision loss secondary to arteritic ischemic optic neuropathy. Currently, the gold standard for its diagnosis is the temporal artery biopsy, a procedure that is not harmless and may have complications such as infection, nerve injury, bleeding, among others. Among non-invasive diagnostic methods, the ultrasound and temporal artery Doppler have gained a predominant role in the diagnosis of giant cell arteritis, as it is a benign test with no adverse effects. Through strategies such as “fast-track” clinics, supported by this diagnostic method, a reduction has been achieved in ischemic complications of the disease.
La arteritis de células gigantes es una vasculitis que afecta de manera predominante a vasos de gran calibre y aparece en personas mayores de 50 años. Su presentación clínica incluye cefalea, alteraciones auditivas o síntomas similares a polimialgia reumática. En su forma más grave puede causar pérdida de visión uni o bilateral, secundaria a neuropatía óptica isquémica de tipo arterítico. En la actualidad, el estándar de referencia para su diagnóstico es la biopsia de arterias temporales, procedimiento que no es inocuo y que puede tener como complicaciones infección, lesión nerviosa o sangrado, entre otras. Entre las técnicas no invasivas de diagnóstico, el ultrasonido y el Doppler de arterias temporales han tomado un rol cada vez más importante en el diagnóstico de esta patología, dado que son pruebas benignas, con nulos efectos adversos, y a través de estrategias como las clínicas fast-track apoyadas en este método diagnóstico se ha logrado la reducción de complicaciones isquémicas de la enfermedad.
Giant cell arteritis (GCA) is a vasculitis that affects medium and large caliber vessels (the aorta and its proximal branches), predominantly in people over 50 years of age. Its prevalence is low and has been estimated at 2.2 per 10,000 patients in the United Kingdom,1 with an incidence in populations of Scandinavian descent of up to 19.8 per 100,000.2 These data have not been established in Colombia. In the study conducted by Ochoa et al. in 2009, of 857 cases of vasculitis reported in Colombia, only 9 corresponded to GCA.3 The inflammation of the vessels wall leads to a thickening of the intima that causes the reduction of blood flow with secondary ischemic phenomena.
Histologically, it is characterized by the vascular infiltration of CD4+ T cells and macrophages that subsequently fuse to form the so-called giant cells.4
Clinically, 2 phenotypes of presentation have been defined according to the affected arteries and the territory irrigated by these vessels: a cranial phenotype, in which the characteristic symptoms are headache, especially in the temporal area, hypersensitivity of the scalp and jaw claudication. This subtype is the one that most frequently includes, as a serious and catastrophic manifestation, visual loss secondary to ischemic optic neuritis (anterior or posterior) of arteritic type and usually affects the older age group. On the other hand, the extracranial phenotype, also called large-vessel GCA, affects the aorta and its main proximal branches, especially of the upper extremities; it occurs with constitutional symptoms, fever, claudication of the upper limbs and aortitis, and patients are usually younger than those with the intracranial phenotype.5
Polymyalgia rheumatica is closely related to GCA, it is associated in up to 40%–50% of patients, while GCA can be found in 10% of patients with a previous diagnosis of polymyalgia rheumatica.6 Early and timely diagnosis and treatment of patients with GCA is essential to prevent serious vascular complications of the disease, particularly blindness. Even though it can be suspected clinically, the gold standard for diagnosis continues to be the temporal artery biopsy; however, it can be negative in a significant percentage of patients and is not exempt from complications, which makes it necessary to use non-invasive diagnostic methods, such as Doppler ultrasound of the temporal arteries.7
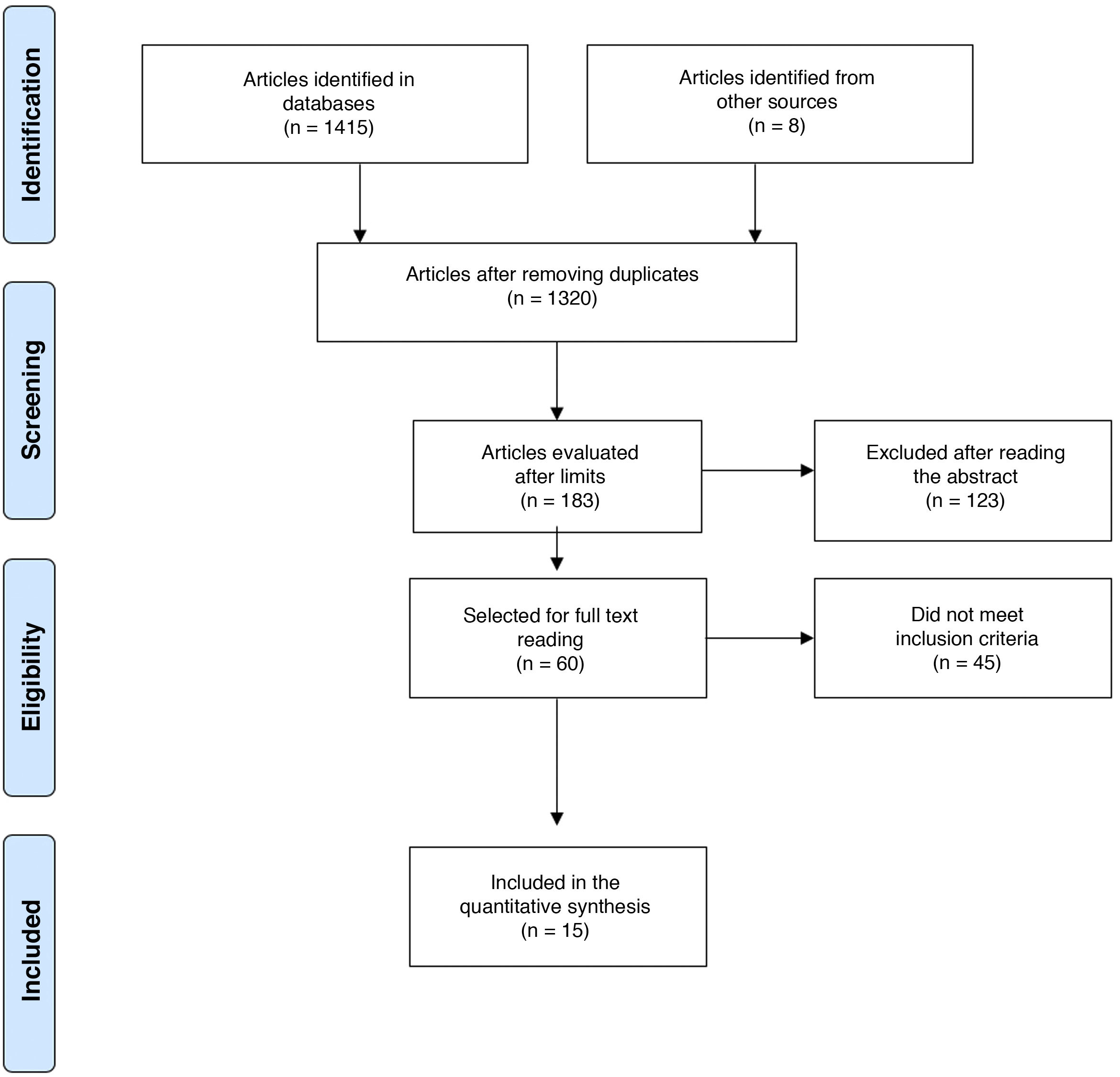
Material and methodsA bibliographic search was systematized to answer, in a qualitative manner, the question about the usefulness of ultrasonography and Doppler in the diagnosis of GCA (Annex 1, Supplementary data). The search was conducted in PubMed, Embase, Web of Science, Google Scholar, Cochrane and Epistemonikos, using the search terms defined for each database and the different Boolean connectors (Annex 2, Supplementary data). It was limited to English and Spanish. The included studies should ideally compare ultrasonography with the reference standard (temporal artery biopsy), or assess the operational characteristics of this diagnostic test compared to other diagnostic strategies such as nuclear magnetic resonance image, or clinical criteria.
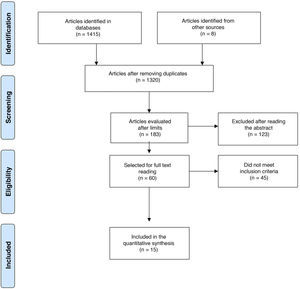
The defined period was from January 1, 1990, until March 31, 2020. The search was conducted by 4 of the authors in an autonomous manner (DJA, AV, CHMV, MPT). In case of disagreements, these were settled by one of the authors (DJA) with thematic and methodological expertise (Fig. 1).8 For the evaluation of the risk of bias of the publications, the 6 domains of the Cochrane Collaboration tool for assessing the risk of bias were considered (Table 1).
Data extraction on the performance of temporal arteries ultrasound.
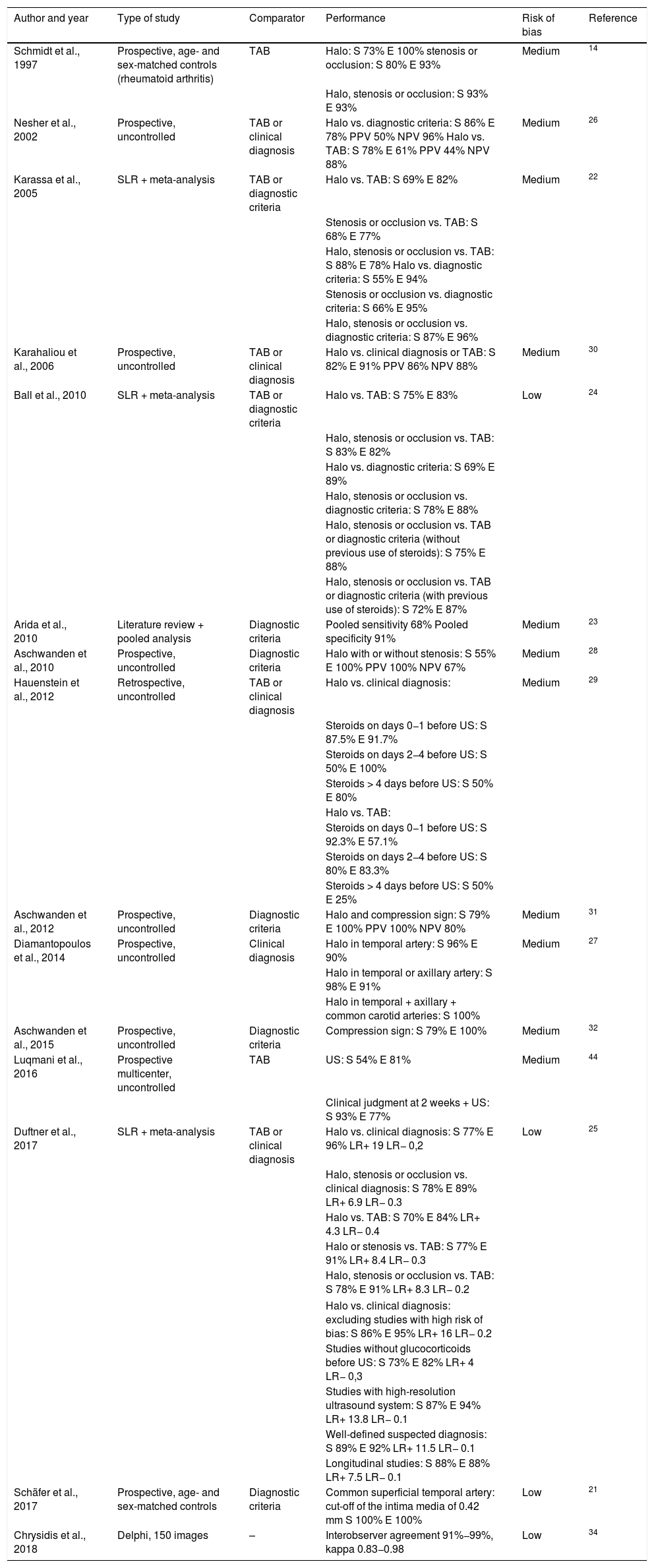
| Author and year | Type of study | Comparator | Performance | Risk of bias | Reference |
|---|---|---|---|---|---|
| Schmidt et al., 1997 | Prospective, age- and sex-matched controls (rheumatoid arthritis) | TAB | Halo: S 73% E 100% stenosis or occlusion: S 80% E 93% | Medium | 14 |
| Halo, stenosis or occlusion: S 93% E 93% | |||||
| Nesher et al., 2002 | Prospective, uncontrolled | TAB or clinical diagnosis | Halo vs. diagnostic criteria: S 86% E 78% PPV 50% NPV 96% Halo vs. TAB: S 78% E 61% PPV 44% NPV 88% | Medium | 26 |
| Karassa et al., 2005 | SLR + meta-analysis | TAB or diagnostic criteria | Halo vs. TAB: S 69% E 82% | Medium | 22 |
| Stenosis or occlusion vs. TAB: S 68% E 77% | |||||
| Halo, stenosis or occlusion vs. TAB: S 88% E 78% Halo vs. diagnostic criteria: S 55% E 94% | |||||
| Stenosis or occlusion vs. diagnostic criteria: S 66% E 95% | |||||
| Halo, stenosis or occlusion vs. diagnostic criteria: S 87% E 96% | |||||
| Karahaliou et al., 2006 | Prospective, uncontrolled | TAB or clinical diagnosis | Halo vs. clinical diagnosis or TAB: S 82% E 91% PPV 86% NPV 88% | Medium | 30 |
| Ball et al., 2010 | SLR + meta-analysis | TAB or diagnostic criteria | Halo vs. TAB: S 75% E 83% | Low | 24 |
| Halo, stenosis or occlusion vs. TAB: S 83% E 82% | |||||
| Halo vs. diagnostic criteria: S 69% E 89% | |||||
| Halo, stenosis or occlusion vs. diagnostic criteria: S 78% E 88% | |||||
| Halo, stenosis or occlusion vs. TAB or diagnostic criteria (without previous use of steroids): S 75% E 88% | |||||
| Halo, stenosis or occlusion vs. TAB or diagnostic criteria (with previous use of steroids): S 72% E 87% | |||||
| Arida et al., 2010 | Literature review + pooled analysis | Diagnostic criteria | Pooled sensitivity 68% Pooled specificity 91% | Medium | 23 |
| Aschwanden et al., 2010 | Prospective, uncontrolled | Diagnostic criteria | Halo with or without stenosis: S 55% E 100% PPV 100% NPV 67% | Medium | 28 |
| Hauenstein et al., 2012 | Retrospective, uncontrolled | TAB or clinical diagnosis | Halo vs. clinical diagnosis: | Medium | 29 |
| Steroids on days 0−1 before US: S 87.5% E 91.7% | |||||
| Steroids on days 2−4 before US: S 50% E 100% | |||||
| Steroids > 4 days before US: S 50% E 80% | |||||
| Halo vs. TAB: | |||||
| Steroids on days 0−1 before US: S 92.3% E 57.1% | |||||
| Steroids on days 2−4 before US: S 80% E 83.3% | |||||
| Steroids > 4 days before US: S 50% E 25% | |||||
| Aschwanden et al., 2012 | Prospective, uncontrolled | Diagnostic criteria | Halo and compression sign: S 79% E 100% PPV 100% NPV 80% | Medium | 31 |
| Diamantopoulos et al., 2014 | Prospective, uncontrolled | Clinical diagnosis | Halo in temporal artery: S 96% E 90% | Medium | 27 |
| Halo in temporal or axillary artery: S 98% E 91% | |||||
| Halo in temporal + axillary + common carotid arteries: S 100% | |||||
| Aschwanden et al., 2015 | Prospective, uncontrolled | Diagnostic criteria | Compression sign: S 79% E 100% | Medium | 32 |
| Luqmani et al., 2016 | Prospective multicenter, uncontrolled | TAB | US: S 54% E 81% | Medium | 44 |
| Clinical judgment at 2 weeks + US: S 93% E 77% | |||||
| Duftner et al., 2017 | SLR + meta-analysis | TAB or clinical diagnosis | Halo vs. clinical diagnosis: S 77% E 96% LR+ 19 LR− 0,2 | Low | 25 |
| Halo, stenosis or occlusion vs. clinical diagnosis: S 78% E 89% LR+ 6.9 LR− 0.3 | |||||
| Halo vs. TAB: S 70% E 84% LR+ 4.3 LR− 0.4 | |||||
| Halo or stenosis vs. TAB: S 77% E 91% LR+ 8.4 LR− 0.3 | |||||
| Halo, stenosis or occlusion vs. TAB: S 78% E 91% LR+ 8.3 LR− 0.2 | |||||
| Halo vs. clinical diagnosis: excluding studies with high risk of bias: S 86% E 95% LR+ 16 LR− 0.2 | |||||
| Studies without glucocorticoids before US: S 73% E 82% LR+ 4 LR− 0,3 | |||||
| Studies with high-resolution ultrasound system: S 87% E 94% LR+ 13.8 LR− 0.1 | |||||
| Well-defined suspected diagnosis: S 89% E 92% LR+ 11.5 LR− 0.1 | |||||
| Longitudinal studies: S 88% E 88% LR+ 7.5 LR− 0.1 | |||||
| Schäfer et al., 2017 | Prospective, age- and sex-matched controls | Diagnostic criteria | Common superficial temporal artery: cut-off of the intima media of 0.42 mm S 100% E 100% | Low | 21 |
| Chrysidis et al., 2018 | Delphi, 150 images | – | Interobserver agreement 91%−99%, kappa 0.83−0.98 | Low | 34 |
E: specificity; LR: Likelihood ratio; SLR: systematic literature review; S: sensitivity; TAB: temporal artery biopsy; US: temporal artery ultrasound; NPV: negative predictive value; PPV: positive predictive value.
In 1990, the American College of Rheumatology defined 5 classification criteria for the disease: age over 50 years, new-onset localized headache, tenderness in the temporal artery or decreased temporal artery pulse, erythrocyte sedimentation rate higher than 50 mm/h and suggestive findings in the temporal artery biopsy: mononuclear infiltrate or granulomatous process with multinucleated giant cells. If 3 of these criteria were met, the patient was classified as having GCA, with a sensitivity of 93.5% and a specificity of 91.2%.9
The disease can be suspected with the symptoms described in a patient over 50 years of age with elevated acute phase reactants; however, the gold standard for the diagnosis is the temporal artery biopsy, despite its variable sensitivity between 49 and 85% depending on the sample length, the involvement of arteries other than the temporal, the segmental nature of the inflammation and the time of treatment with glucocorticoids.10,11 The complications from this procedure are not frequent, but may include arterial or venous bleeding, infection of the surgical site, and injury to the auriculotemporal nerve or to branches of the facial nerve.12
Role of the Doppler ultrasound of the temporal arteriesSince the 1970s, the Doppler ultrasound of the temporal arteries has been used, initially to locate them before the biopsy.13 In 1997, Schmidt et al. proposed this exam as a diagnostic tool14 for this disease since it was a non-invasive, quick and inexpensive technique, usually well tolerated, without exposure to contrast media, which provides information on the presence of edema of the vessel wall and allows a dynamic evaluation around its full extension, which entails an advantage over the biopsy, since it overcomes the inconvenience of the fact that the affection might be segmental15; in addition, it allows the evaluation of the superficial temporal artery and other cranial branches, as well as of extracranial vessels such as the axillary arteries.16
Its main disadvantage is that it is an operator-dependent technique. The expertise of the operator, especially an adequate learning curve in positive cases, and the optimal quality of the ultrasound system influence the performance of the study. The European League Against Rheumatism (EULAR) recommends ultrasound of the temporal arteries as the first imaging technique in patients with suspected GCA with predominantly cranial involvement and highlights the importance of an optimal ultrasonography assessment performed by a trained specialist and with an appropriate technical equipment.17
TechniqueUltrasound and Doppler techniques for the assessment of temporal and axillary arteries should be performed with a linear transducer; there are 3 Doppler techniques: Spectral Doppler, which consists in a curve of velocity in the Y axis and time in the X axis, which represents the variation in flow; Color Doppler, which, according to the mean flow velocity assigns a color on a predetermined scale, and that overlaps the B-mode; finally, the Power Doppler observes the flow intensity, unlike the previous ones that determine the velocity. The most widely used Doppler technique for the assessment of the flow of the temporal arteries is Color Doppler.
With regard to Color Doppler, it should be performed at a frequency of at least 10 MHz with a vascular pre-set. The evaluation of the temporal arteries should be done in the supine position. The first portion of the temporal artery is visualized in the tragus. The transducer should be in a transverse plane, and later in a longitudinal plane. After completing an initial scan with one plane, the transducer is rotated 90 degrees to evaluate the opposite plane. The level of the bifurcation between the frontal and parietal branches is used as a reference point to define the origin of the 2 branches. The entire course of the common temporal artery and its branches should be evaluated.
The axillary artery is evaluated with the transducer on the mid-axillary line and a scan is made following its anatomical trajectory. The transducer must be applied both in a longitudinal and a transverse plane. This technique can be used by rheumatologists, radiologists and specialists in vascular medicine, with the particularity that whoever does it must have adequate training in echography and ultrasound, especially of the temporal arteries. The minimum recommended technical characteristics of the equipment are detailed below (it should be noted that these specifications have been proposed by consensus of experts),15,17,18
GrayscaleFrequency: ≥15 MHz for temporal arteries and between 7–15 MHz for extracranial arteries.
Focus: it should be on the artery. In general, 5 mm for temporal arteries, 20–30 mm for axillary arteries.
Depth: in general, 10–20 mm for temporal arteries, 30–40 mm for axillary arteries.
B-mode gain: on average, 35−45 dB.
Linear density: 3.
Dynamic range: 40−60 dB.
Color DopplerFrequency: Between 7 and 12 MHz for temporal arteries and between 4 and 8 MHz for extracranial arteries.
Pulse repetition frequency: 2–3.5 kHz for temporal arteries and between 3 and 4 kHz for extratemporal arteries.
Wall filter: the least possible filter.
Color box: it should be at an insonation angle ≤ 60°.
Color gain: between 2 and 18.
Pathological ultrasonographic findings in the patient with giant cell arteritis4 pathological findings can be documented in the ultrasound of the temporal arteries of a patient with GCA16:
- 1
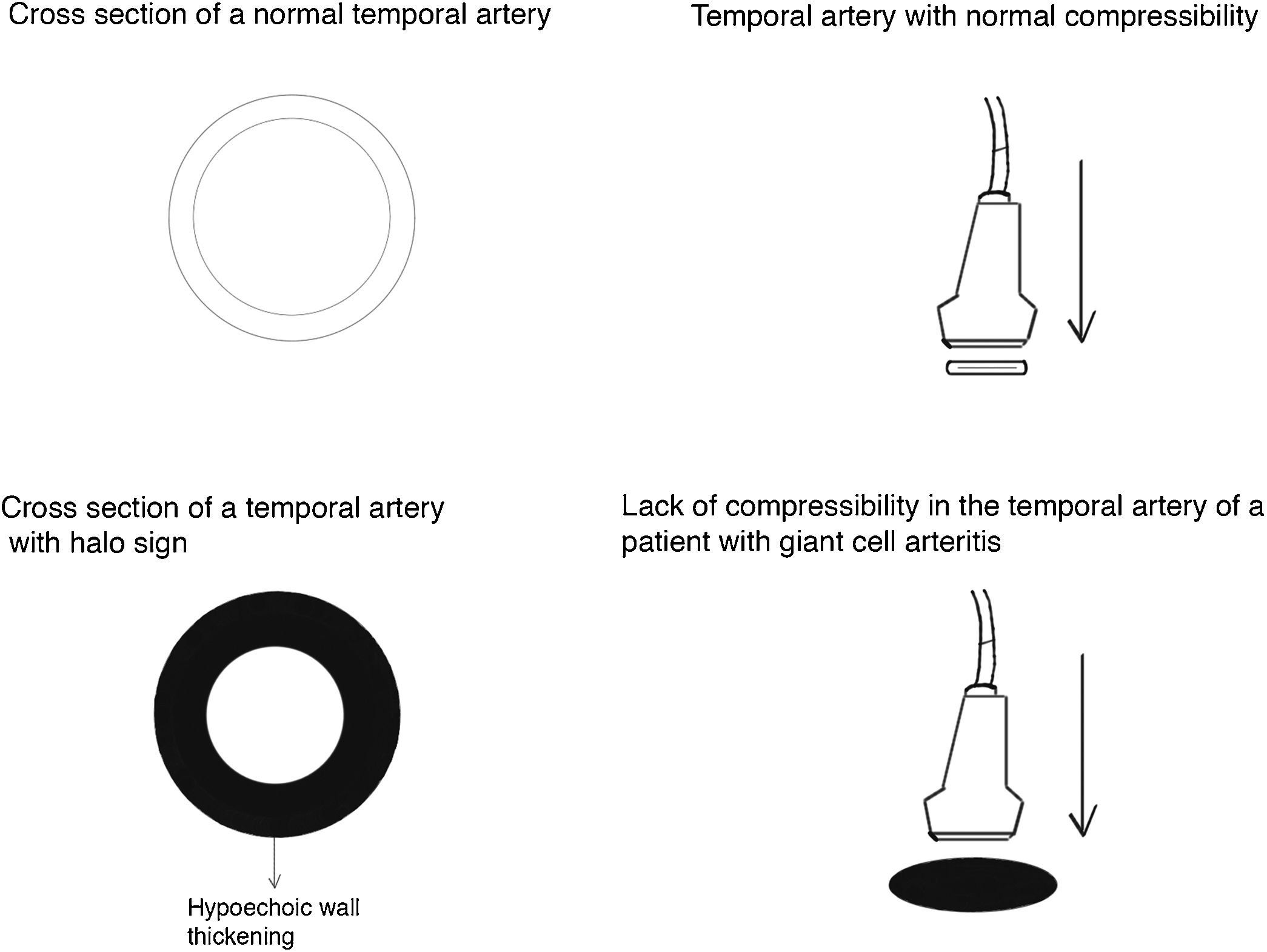
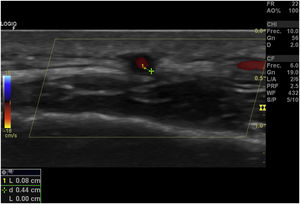
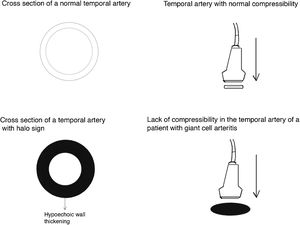
Halo sign (Fig. 2): it is a homogeneous thickening of the wall, hypoechoic (in mode B), with defined edges (it is possible to delimit the tissue adjacent to the vessel), visible in the transverse (usually concentric) and longitudinal planes. It reflects the edema of the vessel wall and it is the most relevant finding for this disease. Some studies defined cut-off points of the diameter of the halo for temporal arteries between 0.3 and 1.0 mm and for axillary arteries between 1.0 and 2.0 mm.19,20 Schafer et al., in a prospective study comparing 40 patients with GCA and healthy controls, found that the normal diameter of the intima-media thickness (IMT) was on average 0.2 mm in the temporal arteries and 0.6 mm in the axillary arteries, and they established cut-off points for the IMT of patients with GCA in the common superficial temporal arteries of 0.65 mm (sensitivity and specificity of 100%), in the frontal branch of 0.54 mm (sensitivity and specificity of 100%), in the parietal branch of 0.50 mm (sensitivity of 97.2% and specificity of 98.7%), in the facial artery of 0.53 mm (sensitivity of 87.5% and specificity of 98.8%) and in the axillary arteries of 1.7 mm (sensitivity and specificity of 100%).21 Several meta-analyses have reported a sensitivity of 68%–77% and a specificity of 83%–96% for the unilateral halo sign and a sensitivity of 43% and a specificity of 100% for the bilateral halo sign,22–25 with a positive predictive value of 50% and a negative predictive value of 96%.26 The sensitivity of Doppler ultrasound only increases by 2% when the evaluation of the axillary arteries is added to the study of the temporal arteries27. Different studies have shown that in the majority of patients the halo sign is not visible after 2–4 weeks following the start of the therapy with glucocorticoids, because the inflammatory changes disappear and the sensitivity of the study decreases to 50% from the 4th day of treatment, so early evaluation with Doppler ultrasound is recommended in the first 2 days after starting the treatment.28–30
- 2
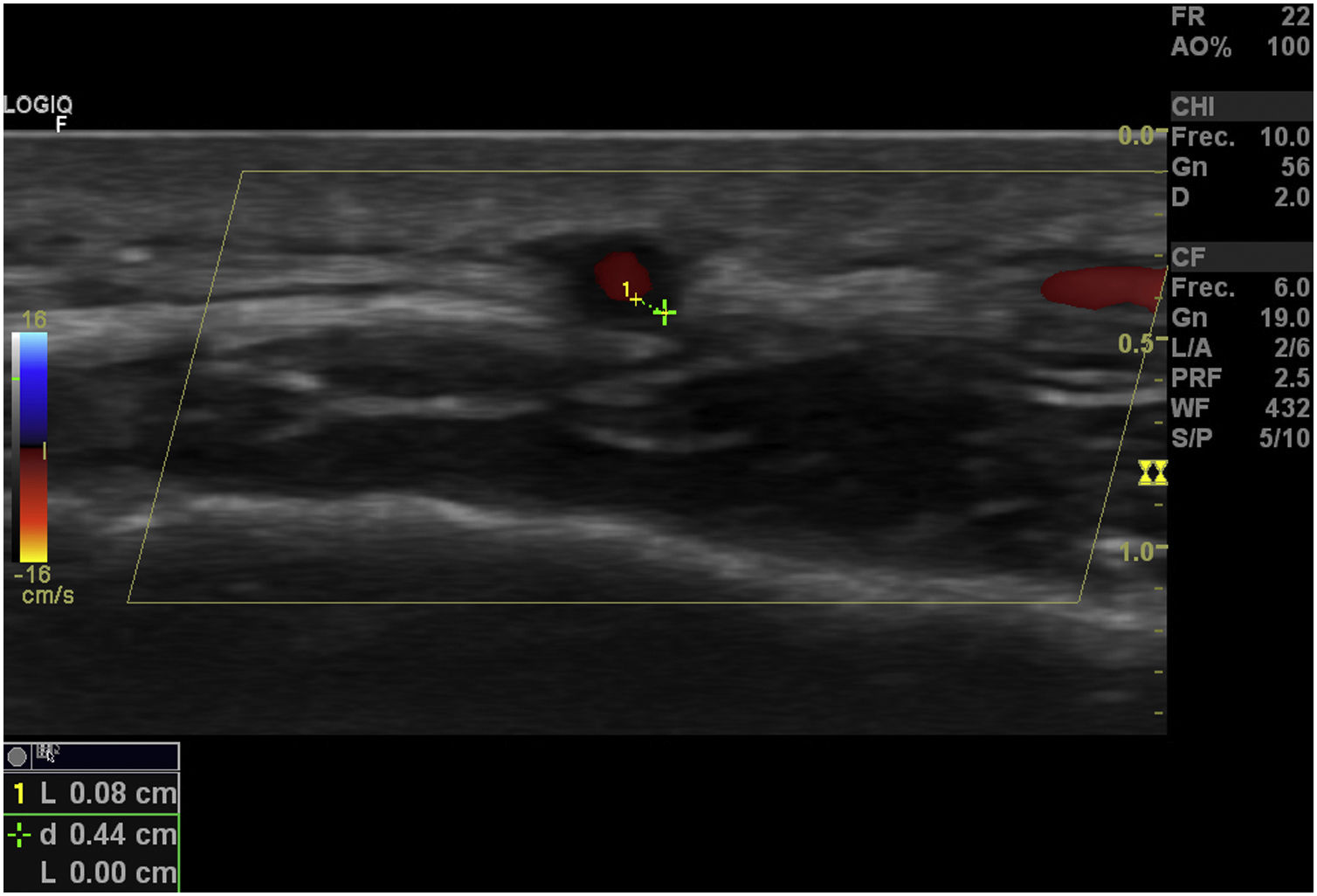
Temporal artery compressibility sign (Fig. 3): it is the persistence of the hypoechoic halo despite extrinsic compression of the arterial lumen with the ultrasound transducer («sign of compressibility»); it is a variant of the halo with a sensitivity of 77%–79% and a specificity of 100%,31,32
- 3
Stenosis: it occurs when the blood flow velocity is more than twice the recorded in the immediately anterior area, with waveforms which demonstrate turbulence and reduction in velocity behind the area of stenosis16.
- 4
Occlusion: complete absence of flow. In the study conducted by Schmidt et al. both stenosis and occlusions had a sensitivity of 80% and a specificity of 93%16. The finding of arterial stenosis or occlusion does not increase the diagnostic performance of the halo sign25.
In order to standardize and evaluate the performance of Doppler ultrasound in the diagnosis of large vessel vasculitis, the OMERACT LV-US (Outcome Measures in Rheumatology Large Vessel Ultrasound) working group was constituted within the framework of the annual congress of the American College of Rheumatology in Boston in 2014.16 Based on this strategy, it was published a first study whose objective was to define the fundamental ultrasound findings of GCA and evaluate its performance according to these findings. 25 experts in Doppler ultrasound in CGA participated obtaining a total of 9 definitions on findings consisting in normal appearance, vasculitis and atherosclerosis of cranial and extracranial arteries. It was found that halo and compression signs are the key findings on Doppler ultrasound of patients with GCA.
The definitions of normal temporal and axillary arteries, halo and compressibility signs showed an interobserver agreement of 91%−99%, with a mean kappa value of 0.83−0.98 among the expert evaluators.33 In a recent case-control study, it was demonstrated an adequate inter-and intra-observer concordance of the compressibility and halo signs among previously trained operators (more than 300 studies), with a standardized study protocol of at least 15−20 min per study and using transducers of more than 15 MHz.34
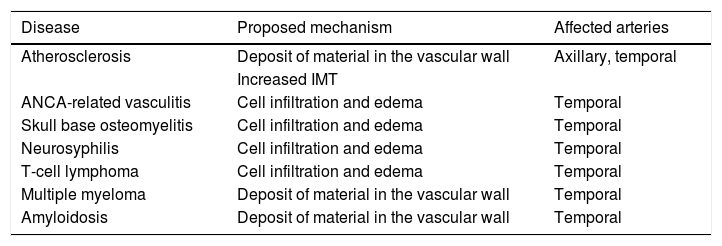
False positives of the halo sign on Doppler ultrasoundFalse positives of the halo sign have been described in other forms of vasculitis (such as polyarteritis nodosa and the anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, amyloidosis, neoplasms, infections and atherosclerosis.26,35–38Table 2 summarizes the possible mechanisms implied in the presence of the halo sign in diseases other than GCA.
False positives of the halo sign in Doppler ultrasound.
| Disease | Proposed mechanism | Affected arteries |
|---|---|---|
| Atherosclerosis | Deposit of material in the vascular wall | Axillary, temporal |
| Increased IMT | ||
| ANCA-related vasculitis | Cell infiltration and edema | Temporal |
| Skull base osteomyelitis | Cell infiltration and edema | Temporal |
| Neurosyphilis | Cell infiltration and edema | Temporal |
| T-cell lymphoma | Cell infiltration and edema | Temporal |
| Multiple myeloma | Deposit of material in the vascular wall | Temporal |
| Amyloidosis | Deposit of material in the vascular wall | Temporal |
ANCA: antineutrophil cytoplasmic antibodies; IMT: intima-media thickness.
The foregoing demands that the results of the Doppler ultrasound be interpreted in the context of the clinical and laboratory findings, and also requires the optimization and standardization of the imaging protocols in the diagnostic process, as well as the establishment of cut-off points (hopefully in the different populations) to reduce false positive rates.18,20,21,39
Both atherosclerosis and CGA increase in frequency with age. In atherosclerosis, calcified plaques of hyperechoic appearance are produced or an increase in the IMT is generated, which is visualized hyperechoic on Doppler ultrasound.40,41 Until now, there is no consensus to properly differentiate between an inflammatory involvement of the vascular wall and atherosclerotic changes, especially when there is no predominant calcium component. Some authors suggest that the carotid IMT shows a significant correlation with the temporal IMT: A carotid IMT > 0.9 mm was associated with a temporal IMT > 0.3 mm, simulating a halo sign, so they propose a cut-off point of IMT > 0.34 in at least 2 branches of the temporal artery to reduce the number of false positives42. More studies are needed to validate these findings.
Could the temporal artery biopsy be replaced by Doppler ultrasound in the diagnosis of patients with giant cell arteritis?Ultrasound-guided biopsy of the temporal arteries does not appear to increase the sensitivity of the histological study for the diagnosis of the disease. In the presence of a positive halo sign, the probability of having a positive biopsy is equally high, regardless of whether the biopsy is guided by ultrasound or by clinical evaluation.30 The use of Doppler ultrasound has been proposed as a possible diagnostic tool to prescind from the biopsy in selected patients,14,43 although some studies report contradictory results in which Doppler ultrasound does not surpass the clinical evaluation39 and it has a better performance when it is negative to rule out the disease, due to its high negative predictive value.26 However, works published after 2010, in which ultrasound systems of higher resolution were used, demonstrated performances comparable with the temporal artery biopsy for the diagnosis of GCA.25
The TABUL study assessed the performance and cost-effectiveness of Doppler ultrasound of the axillary and temporal arteries compared with temporal artery biopsy in the diagnosis of GCA in 381 patients. The results showed a sensitivity of the Doppler ultrasound of 54% and of the temporal artery biopsy of 39%, with specificities of 81% and 100%, respectively. The interobserver correlation of the sonographers in a post hoc evaluation was comparable with that of the pathologists (intraclass correlation coefficient: 0.61 vs. 0.62, respectively).44
The EULAR recommends to evaluate the temporal arteries initially; and if the study is negative and clinical suspicion persists, additional vessels such as the axillary arteries and other cranial or extracranial arteries should be evaluated.17 Patients with clinical signs of GCA and a positive halo sign on Doppler ultrasound could be treated without the need for a temporal artery biopsy, unless there is suspicion of another type of vasculitis (such as the ANCA-associated, which would show a different histopathology). Those who have strong clinical evidence of GCA, but only have stenoses or occlusions, should undergo a temporal artery biopsy. Patients with clinical signs of polymyalgia rheumatica without symptoms of GCA but who have abnormal findings on Doppler ultrasound (halo, stenosis or occlusion) should undergo biopsy and receive treatment with glucocorticoids while the results of the biopsy are known in order to avoid visual loss.14
The fast-track ultrasound clinics for the early diagnosis of GCA were created with the aim of reducing the ischemic complications of the disease, especially the permanent visual loss, since it has been demonstrated that the early detection and initiation of glucocorticoid treatment improves outcomes.45 Outpatients with clinical suspicion of GCA undergo rapid clinical and ultrasound evaluation by trained specialists, and glucocorticoid therapy is promptly initiated if indicated. In a reference center which uses this modality of care, the evaluation by a fast-track ultrasound model in patients with CGA demonstrated a reduction of 88% in the risk of permanent visual loss, compared with the conventional model (RR 0.12; 95% CI: 0.01−0.97; P = .01) and the mean difference in days of hospitalization between the patients evaluated in a conventional manner and the patients evaluated by fast-track ultrasound was 3 days (3.6 vs. 0.6 days; P < .0005).46
ConclusionsGiant cell arteritis, although infrequent, is an entity that generates high morbidity. Timely recognition and early treatment can prevent serious complications, such as visual loss. The clinical challenge represented by its diagnosis and the limitations of the gold standard have opened the door to new imaging techniques. Color Doppler is an inexpensive, fast, and easily available tool with good diagnostic performance. However, it is essential to have trained personnel, appropriate ultrasound equipment and to use the adequate programming.
Doppler findings, such as the halo sign, the lack of compressibility of the temporal artery, the stenosis and occlusion are powerful evidence for the diagnosis of GCA. Among these findings, the most studied sign is the halo, which has a good diagnostic performance. However, this sign has findings that can lead to false positives and that must be taken into account in a differential diagnosis. Lastly, the fast-track clinics supported by ultrasound and Doppler have managed to reduce the ischemic complications of the disease. In our environment is essential to foster research and training of the personnel who will be in charge of these patients.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Urrego-Callejas T, Jaramillo-Arroyave D, Vanegas-García A-L, Muñoz-Vahos CH, Tenorio MP. Ultrasonido Doppler de arterias temporales en pacientes con arteritis de células gigantes: estado del arte y revisión sistemática de la literatura. Rev Colomb Reumatol. 2021;28:203–212.