The Colombian Osteoporosis and Mineral Metabolism Association met in early 2017 to update the Colombian Consensus on Osteoporosis. This was first issued in 2005, and is seen as a necessary step in view of the underdiagnosed status of this disease, and the expected impact of population aging. A technical team was formed with specialists with long experience across multiple disciplines, who were assigned to four working groups: definitions and epidemiology, diagnosis, pharmacological treatment, and non-pharmacological treatment. After a scientific literature review and a series of meetings, the definitions and recommendations are summarized in this article.
La Asociación Colombiana de Osteoporosis y Metabolismo Mineral se reunió a principios de 2017 para actualizar el Consenso Colombiano de Osteoporosis, elaborado por primera vez en 2005, un paso que se consideró necesario en vista del subdiagnóstico de esta enfermedad, el impacto esperado del envejecimiento poblacional y los cambios en el tratamiento farmacológico que ha habido desde entonces. Se seleccionó un equipo técnico con especialistas de múltiples áreas y amplia trayectoria, repartidos en 4 grupos de trabajo: definición y epidemiología, diagnóstico, tratamiento farmacológico y medidas no farmacológicas. Luego de una revisión de la literatura científica, en reuniones de trabajo se generaron las definiciones y recomendaciones que se resumen en este documento.
The Colombian Consensus on the Management of Postmenopausal Osteoporosis compiles the available evidence for the prevention, diagnosis, and management of the disease, with a view to reducing the risk of fragility fractures in this population, improving the quality of life of the patients affected by the disease, and optimizing the healthcare resources for managing this condition.
General objectiveTo assist in the identification of patients with osteoporosis, in order to initiate timely and effective treatment leading to a decrease in the risk of fragility fractures and associated morbidity/mortality.
Specific objectives- -
To promote the early diagnosis of osteoporosis through clinical factors (history and findings), laboratory tests, and imaging studies.
- -
To highlight the importance of preventing fragility fractures in osteoporosis through the implementation of pharmacological and non-pharmacological measures.
- -
To recommend the most appropriate therapeutic option, based on the best available evidence and on the patient's characteristics to avoid fragility fractures.
- -
To generate an impact on the healthcare system in order for osteoporosis to be recognized as a public health issue that must be approached by the Ministry of Health and Social Welfare.
Developing an osteoporosis consensus in Colombia is a need to improve the knowledge of healthcare professionals, in view of the under-diagnosis of the disease and the aging of the population, leading to a significant increase in the number of patients affected by fragility fractures that deteriorates their quality of life and shortens their life expectancy. Since 2005, when the first national consensus was developed, no updates have been made, despite the arrival of new therapeutic options.
MethodologyProcess descriptionThe starting point was the selection of the technical team for the development of the consensus; the team included specialists from multiple areas of interest, with a vast experience in the management of patients with osteoporosis. Once the team was appointed, the methodological advisor standardized the evidence-based medicine methodology, who then designed the questions and the consensus approach.
4 work groups were set up to deal with the different aspects of osteoporosis: epidemiology, diagnosis, pharmacological and non-pharmacological treatment. When completing the design of the questions, scientific evidence was searched, simultaneously with the construction of the conceptual framework. Each theme group was responsible for reviewing the literature, based on the methodology provided by the methodological advisor. At subsequent group meetings, the answers to the questions were discussed, and the recommendations to be submitted by the consensus were generated.
Description of the strategy used to generate the consensus questionsA document was prepared defining the essential aspects in the approach of a postmenopausal patient with osteoporosis, to understand and ensure comprehensive treatment of this pathology. Based on this, and with the support of the methodological advisor, the PICOS (Patient, Intervention, Comparison, Outcome, Studies) methodology was followed to prepare a comprehensive list of questions related to each of the central themes (epidemiology, diagnosis, pharmacological and non-pharmacological treatment). Once the questions were chosen, a search was conducted to identify the available evidence for each one of them.
Description of the strategies used for searching the evidenceThe standard strategies designed by the methodological advisor and developed jointly with the consensus technical team were used. The first step was to separate each question into its component parts to define the descriptors and their respective Mesh terms, to subsequently establish the search strategies. Searches were made in Medline (PubMed), Embase, and Lilacs. Only articles written in English, Spanish, and Portuguese were considered, and there were no limitations in terms of date of publication.
The titles and abstracts of each article identified were reviewed, pursuant to the questions asked by the authors; the papers with the best answers were selected. The type of design and the usual criteria regarding quality of the evidence were taken into consideration. In case of doubt, the article was selected and its bibliography was submitted. Likewise, the authors of this consensus conducted Internet searches and contributed with the documents they had available.
Description of the process for selecting, reviewing, and summarizing the evidenceTo implement the process, the people responsible for answering each question were appointed. Each one of them selected, assessed, and summarized the most relevant evidence to answer the question and present a summary of the evidence identified.
Drafting of recommendationsThe technical team in charge of each one of the themes of the consensus developed the recommendations based on the synthesis prepared by the authors and on their own experience. The text was then reviewed by the other members of the technical team who gave their feedback about the process. Once a consensus was reached, the final recommendations were established, with their corresponding support documents. The text was subsequently reviewed by other members of the technical team, who were in charge of giving their feedback about the process. The complete document was then reviewed by each one of the team members, and any concerns and questions were discussed and clarified at a work meeting.
Upon completion of the process, a final evaluation was conducted. A Delphi methodology adapted to this consensus was followed to develop all of these steps. The recommendations issued by the work group that developed the consensus felt that by following the recommendation, the benefits for the target population would outweigh any harm.
Definition and epidemiologyWhat is osteoporosis?Osteoporosis is a systemic skeletal disease characterized by low bone mass and micro-architectural disruption of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture.1
More recently, osteoporosis has been defined as a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of two primary factors: density and bone quality.2
Epidemiology of osteoporosisOsteoporosis and fragility fractures mainly affect postmenopausal women. The morbidity associated with these fractures goes beyond the individual's health decline, with significant economic impact due to hospitalization costs, surgeries, home care, disability, and death.
The average life expectancy in Colombia is 78 years.3 In Latin America, the incidence of hip fracture ranges between 40 and 362 per every 100,000 inhabitants over 50 years old, with a ratio of 3 women affected per 1 affected man. The mortality from hip fractures ranges between 1.02 and 10% during hospitalization, and between 23 and 30% over the next year after the fracture.4–6
The 2017 forecast in Colombia indicated a total of 49,291,609 inhabitants, of which 10,913,693 (22%) are over 50 years old, and 7,037,283 (14%) are over 65 years old.7
The Audit Latin America of the International Osteoporosis Foundation (IOF) was published in 2012. Of the 14 countries studied, the percentage of individuals who were over 50 years old was estimated between 13 and 29%. The estimated increase for the year 2050 ranges between 28 and 49%. The increase in the percentage of people over 70 years old between 2011 and 2050 could average 280%. The impact of population aging will increase the incidence of osteoporosis, and subsequently, of fragility fractures.8 In Colombia, the estimates for 2012 were 2,609,858 and 1,423,559 women with osteopenia and osteoporosis, respectively. By 2050, these figures could raise to 3,852,000 and 2,101,000, respectively.9
In the osteoporosis prevalence registries, a study conducted in Bogotá in subjects over 50 years old, showed a prevalence of spinal osteoporosis of 15.7% and of hip osteoporosis of 11.4%. The reported levels of spinal and hip osteopenia were 49.7% and 47.5%, respectively.10 Another report from the National Police Central Hospital in Bogotá in 2072 bone scans, of which 95% were from women between 50 and 70 years old, showed 32% of osteoporosis in any location, and 42% of osteopenia among the women studied.11
With regards to the incidence of hip fractures, a prospective study for standardizing the Fracture Risk Assessment Tool (FRAX®) model for Colombia, conducted in Barranquilla between 2004 and 2006, found 676 hip fractures in subjects over 50 year old and 458 in women; according to the prevailing demographic characteristics at the time, these figures represented an incidence of 78 per 100,000 inhabitants in males and 127 per every 100,000 in females.12
Colombia participated in the Latin American Vertebral Osteoporotic Study (LAVOS) which found a prevalence of vertebral fractures of 11.18%, in a radiological sample of 1922 women from 5 Latin American countries. This number was similar to the prevalence found in China, and in countries of the Mediterranean basin, but is slightly higher than the number reported for the United States. The women randomized were over 50 years old, and came from Argentina, Brazil, Colombia, Mexico and Puerto Rico. The prevalence was similar in the 5 countries, and increased as age progressed: from 6.9% in the group between 50 and 59 years, to 27% in the group of women 80 years and older.13
What is the impact of osteoporosis on the social context?The social impact of osteoporosis should be assessed from different angles: costs, direct and indirect healthcare expenditures, quality of life disruption for the patient, quality of life disruption for third parties involved with the patient, loss of earnings of the patient and loss of earnings of the caregiver.
In Colombia, osteoporosis is not yet considered a priority disease under the public health policy, and therefore there are no specific primary prevention programs in our healthcare system. Also for this reason, statistics on osteoporosis in our country are nor reliable or clear.
Patients with osteoporosis that sustain a vertebral fracture are more prone to be hospitalized for any other reason, as compared against patients that have not experienced a vertebral fracture.14 According to a study conducted in Colombia by members of the Colombian Association of Osteoporosis and Mineral Metabolism (ACOMM), on the cost of fractures in women with osteoporosis, the cost of follow-up of a patient with osteoporosis in 2014 was $622,588. In 2014, the cost of operating a hip fracture in Colombia was $8,687,829.21; operating on a vertebral fracture represented a cost of $11,348,379.90 and the cost of operating on a distal radius fracture was $2,319,111.67. In case of a vertebral fracture that did not require surgical management, the cost was estimated at $5,034,055.60 for one-year follow-up. It was estimated that in 2015 the economic impact of treating hip fractures in the country was $205,602,914,414, the cost of surgery for vertebral fractures was $1,370,947,862 and for non-surgical management the cost was $11,653,771,426; in terms of distal radius fractures, the cost was $122,858,360,231.15
If we estimate the total cost of these interventions based on direct costs only, the amount expend by the healthcare system was $341,485,994,433; consequently, osteoporosis should be considered a high cost pathology, and a priority public health program should be made available.15
With regards to indirect healthcare expending, rehabilitation, loss of earnings of the patient and the caregiver, no local data were found. Spain reports indirect costs exceeding €420 million per year, based on 100,000 fractures experienced over that time. In Canada, the estimated indirect cost is US$4218 per fracture, in patients between 65 and 69 years old and of US$1158 per fracture in patients over 75 years old.16
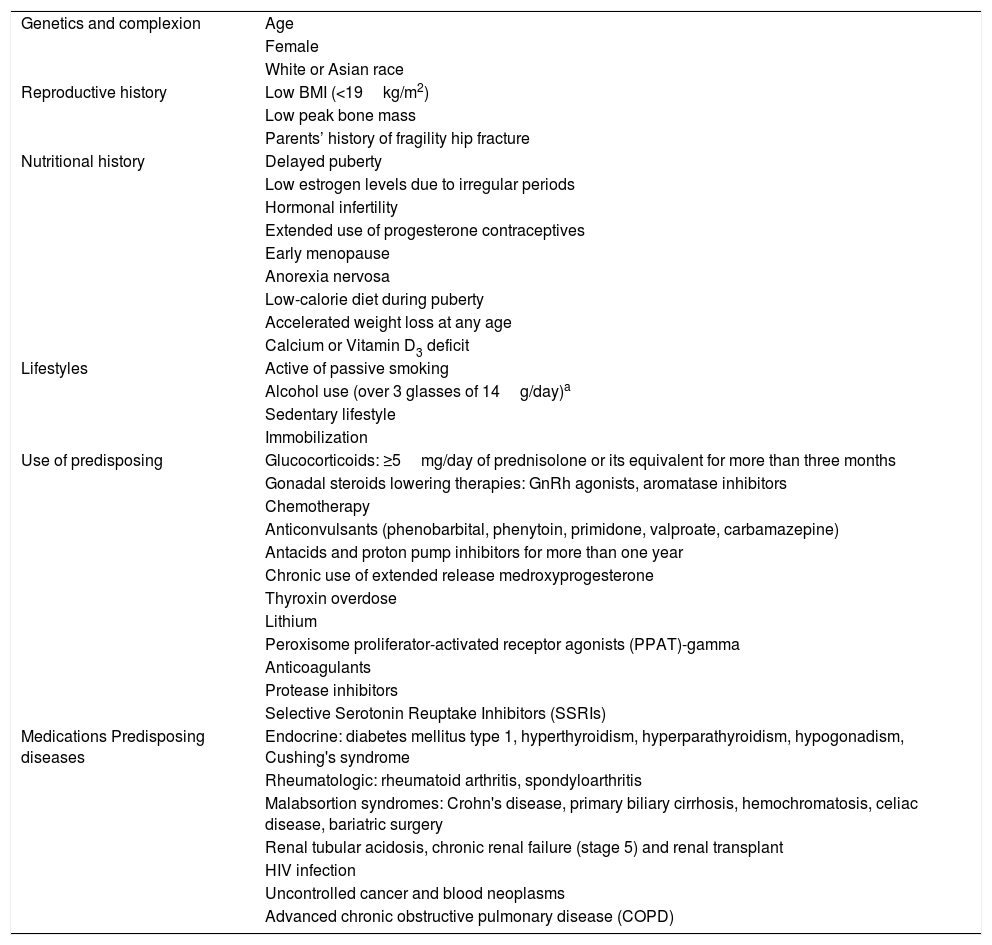
Which are the risk factors for osteoporosis and fractures in adult patients?As the population ages, the prevalence of osteoporosis is growing around the world. Old age is the most important risk factor to predict osteoporosis. A previous low impact fracture after 40 years of age, early menopause, a family history of hip fracture, a low body mass index (BMI), and the presence of diseases or use of predisposing medications or substances, represent the primary risk factors for osteoporosis and fragility fractures.
The world Health Organization (WHO) in its recommendations about developing algorithms to establish the risk of fracture, included these factors as the determining factors of risk and on that basis developed the FRAX® tool, an algorithm to establish the 10-year risk of hip fracture and major fracture (hip, vertebra, proximal humerus, and distal radius). Table 1 lists the risk factor for osteoporosis.
Risk factors for osteoporosis.
| Genetics and complexion | Age |
| Female | |
| White or Asian race | |
| Reproductive history | Low BMI (<19kg/m2) |
| Low peak bone mass | |
| Parents’ history of fragility hip fracture | |
| Nutritional history | Delayed puberty |
| Low estrogen levels due to irregular periods | |
| Hormonal infertility | |
| Extended use of progesterone contraceptives | |
| Early menopause | |
| Anorexia nervosa | |
| Low-calorie diet during puberty | |
| Accelerated weight loss at any age | |
| Calcium or Vitamin D3 deficit | |
| Lifestyles | Active of passive smoking |
| Alcohol use (over 3 glasses of 14g/day)a | |
| Sedentary lifestyle | |
| Immobilization | |
| Use of predisposing | Glucocorticoids: ≥5mg/day of prednisolone or its equivalent for more than three months |
| Gonadal steroids lowering therapies: GnRh agonists, aromatase inhibitors | |
| Chemotherapy | |
| Anticonvulsants (phenobarbital, phenytoin, primidone, valproate, carbamazepine) | |
| Antacids and proton pump inhibitors for more than one year | |
| Chronic use of extended release medroxyprogesterone | |
| Thyroxin overdose | |
| Lithium | |
| Peroxisome proliferator-activated receptor agonists (PPAT)-gamma | |
| Anticoagulants | |
| Protease inhibitors | |
| Selective Serotonin Reuptake Inhibitors (SSRIs) | |
| Medications Predisposing diseases | Endocrine: diabetes mellitus type 1, hyperthyroidism, hyperparathyroidism, hypogonadism, Cushing's syndrome |
| Rheumatologic: rheumatoid arthritis, spondyloarthritis | |
| Malabsortion syndromes: Crohn's disease, primary biliary cirrhosis, hemochromatosis, celiac disease, bariatric surgery | |
| Renal tubular acidosis, chronic renal failure (stage 5) and renal transplant | |
| HIV infection | |
| Uncontrolled cancer and blood neoplasms | |
| Advanced chronic obstructive pulmonary disease (COPD) |
Risk factors included in the FRAX® tool to predict a fracture17,19,20:
- •
Age
- •
Sex
- •
Body mass index
- •
Family history of hip fracture
- •
Personal history of fractures
- •
Chronic use of glucocorticoids
- •
Rheumatoid arthritis
- •
Alcohol abuse
- •
Smoking
- •
Secondary osteoporosis
- •
Bone Mineral Density (BMD) of the femoral neck (not mandatory to use the algorithm).
A fragility fracture is defined as a fracture resulting from any fall from standing height while conducting a physical daily life activity or as a result of minimal trauma.17 Typically, these fractures develop in the spine, the hip and the forearm. Vertebral fractures may occur without trauma, as a characteristic of bone fragility and may be asymptomatic. Imaging studies are required to identify these fractures.
Which are the risk factors for fragility fractures?Fragility fractures depend on a combination of various factors such as age, decreased BMD, occurrence of previous fractures, bone quality, and the intensity of trauma.
BMD is the primary predictor of factures, but not the only one. The lower the BMD, the higher the risk of fracture.21
The risk of fracture doubles per each one-point reduction in the standard deviation (SD) of the BMD, regardless of the type of fracture or the site where the BMD was measured; however, an inverse relationship between the reduction in the risk of fracture and increased BMD has not been shown in clinical trials of treatments against osteoporosis.22
Age as a risk factor for fragility fractures is even more obvious when considering the absolute risk of fracture within a particular time period, as recommended by the IOF.23,24 The risk of sustaining a fracture increases considerably with age.25
The most relevant risk factors for fractures from the clinical point of view have been established by the National Osteoporosis Foundation (NOF)26:
- •
Low BMD
- •
Prior history of fractures after 40 years of age
- •
Family history of fractures
- •
Low weight
- •
Active smoking
A history of previous fractures is the most relevant clinical factor: approximately 19% of the people that sustain a vertebral fracture will experience a new fracture over the course of the next year, in addition to having an increased risk of sustaining a hip fracture.27 After experiencing a hip fracture, there is an increased risk of sustaining a second fracture over the first year, and particularly during the first 3 months.28 Likewise, any distal radius fracture increases the risk of sustaining new fragility fractures, both in the spine and hip, in both males and females.29
What is, and how is a prevalent fracture defined?This is a pre-existing fracture before the clinical evaluation or a fracture seen on the imaging studies, even if the patient is not aware of it. In terms of vertebral fractures, this means a loss of over 20% in the height of the vertebral body with respect to the posterior wall (wedge fracture) or with respect to the adjacent vertebra (compression fracture).30,31
DiagnosisIs bone densitometry measured according to Dual Energy X-Rays (DXA) absorptiometry the method of choice for the diagnosis of osteoporosis?Yes, in patients with a clinical suspicion for osteoporosis, based on risk factors, the recommendation is to do a DXA bone scan, with spine and hip measurements.32
DXA measures the BMD using low radiation dose, for a low cost, is easy to use, requires short measurement times, and is widely available in most cities.33–35
BMD of the femoral neck is a strong predictor of the risk of hip fracture, both in males and females. At 65 years of age, the relative risk increased by 2.94 (95% CI: 2.04–4.27) in males and by 2.88 (95% CI: 2.31–3.59) in females, per each reduction of one standard deviation in BMD. This effect is age-dependent: the absolute risk of fracture increased notable with age.
The risk gradient for any fragility fracture was lower than for hip fractures. However, the risk of fragility fractures in males increased by 1.41 per every SD reduction in BMD (95% CI: 1.33–1.51) and in females increased 1.38 per every SD reduction (95% CI: 1.28–1.48). BMD is then a significant factor in determining the risk of fracture and is similar for both genders. Its international validation enables the use of BMD in strategies to do case search.36
In patients with a fragility fracture, a bone scan is not mandatory to start therapy. However, a DXA bone scan measurement helps not just in making a diagnosis, but also in monitoring treatment and establishing the risk of fracture of the patient.37
In which cases should a bone scan be required in women?Based on the WHO recommendations38:
- •
Women aged 65 or older
- •
Women less than 65 years old, in the presence of a low bone mass risk factor, such as:
- •
Low body weight (BMI <19kg/m2)
- •
Prior fragility fracture
- •
Use of high-risk medications
- •
Disease or condition associated with bone loss (for instance, early menopause, HIV infection)
- •
- •
Any person undergoing therapy intended to monitor the effect of osteoporosis treatment.
The criterion to define and diagnosing osteoporosis in postmenopausal women is a T-score of less than or equal to −2.5 in the lumbar spine, the femoral neck, the hip or the radius. A T-score between −1.0 and −2–5 in the lumbar spine, the femoral neck, or the radius is considered osteopenia.2,38–40
The radius shall only be considered when the spine or the femoral neck cannot be interpreted. If the above-mentioned sites of interest can be interpreted, the radius shall not be measured routinely.
Which are the clinical criteria for osteoporosis?In the opinion of this consensus, an individual that sustains a fragility fracture of the spine or the hip may be diagnosed with osteoporosis, regardless of the BMD value. Nevertheless, the BMD shall be subsequently measured to assess the effectiveness of the treatment administered. A fragility fracture other that the above should undergo a diagnostic assessment for osteoporosis.41
In which patients should a vertebral morphometry be conducted?Vertebral morphometry is a quantitative method to identify the presence of vertebral fractures based on the measurement of the height of the vertebral bodies. The morphometric definition of vertebral fracture is based on the difference between the height of the vertebral body – in its anterior, medial, and posterior segments – and the adjacent vertebra.42 The morphometry may be performed using conventional radiology or DXA imaging.
The vertebral morphometry shall be performed in patients with a T-score <−1.0 and with one or more of the following conditions43:
- •
Women ≥70 years old
- •
Height loss of more than 4cm
- •
Report or personal history of a non-documented vertebral fracture
- •
Use of glucocorticoids (≥5mg/day of prednisolone or its equivalent) for more than 3 months
- •
Any patient with osteoporosis shall have a lateral X-ray of the dorsolumbar spine.32
- •
Patients with a T-score <−1, associated with one or more of the following parameters32–34,44:
- •
Women ≥70 years old
- •
Height loss of more than 4cm
- •
A history of spinal fracture according to the clinic, though not documented
- •
Glucocorticoid therapy (>5mg/day of prednisone or its equivalent), for at least 3 months
- •
Hyperkyphosis
- •
Thoracic or lumbar spine pain for more than 15 days, without apparent cause
- •
A history of hip fracture secondary to low intensity trauma. Plain X-rays of the thoracolumbar spine should not be used as a diagnosis of osteoporosis if not associated with spinal fractures
- •
Symptomatic vertebral fractures, with neurological involvement or increased kyphosis shall undergo an MRI.45 If after 6 weeks of a vertebral fracture the patient continues experiencing disabling lumbar or dorsal pain, an MRI shall be ordered to consider the possibility of percutaneous surgery (vetebroplasty or kyphoplasty).46 The MRI may yield findings that differentiate a benign from a malignant compression fracture.47
Should bone turnover markers be measured routinely in patients with osteoporosis?No. This consensus does not recommend a routine measurement of bone turnover markers in patients with a diagnosis of postmenopausal osteoporosis, since the standardized techniques for measuring and reporting bone turnover markers are not yet available in our setting.
In some cases, bone resorption markers could be helpful for follow-up of antirresoprtive therapy and compliance.48–50 This decision is based on the request and interpretation of the treating physician.
Should serum calcium be measured in patients with osteoporosis prior to the initiation of treatment?Yes, this consensus recommends measuring total serum calcium (non ionized) and correcting calcium with albumin in cirrhosis, nephrotic syndrome, malnutrition, malabsorption syndrome and paraproteinemias.
Should phosphorus be measured in patients with osteoporosis before the start of treatment?No. This consensus does not recommend routine phosphorus measurements. It should only be ordered in special cases to study phosphorus-associated metabolism pathologies.
Should a CBC be done on patients with osteoporosis prior to initiating therapy?Yes, it is recommended to do a blood test including sedimentation rates, since this helps to suspect secondary and occult causes such as multiple myeloma or leukemia.
Should 24-h urine calcium be measured in patients with osteoporosis prior to initiating treatment?Yes, 24-h urine calcium orients toward pathologies such as idiopathic hypercalciuria and familial hypercalcemia hypercalciuria, supports the diagnosis of hyperparathyroidism, and is found in Hypovitaminosis D, the initial phases of hyperparathyroidism, or renal failure. Hypercalciuria is defined as >4mg/kg.
Should 25-hydroxyvitamin D be measured in patients with osteoporosis prior to the initiation of treatment?Yes, this consensus recommends measuring the levels of 25-hydroxyvitamin D, in view of the high prevalence of low vitamin D levels and its negative impact on hone health. The decision to start any therapy for osteoporosis is based on the assumption that the patient already has adequate Vitamin D levels.51
Should creatinine be measured in patients with osteoporosis prior to initiating treatment?Yes, medications for osteoporosis treatment, with the exception of denosumab, are excreted through the kidney. It is recommended to also do a creatinine clearance measurement in renal patients, since patients with chronic nephropathy exhibit calcium metabolism disorders that affect the bone mass. Creatinine also helps in estimating the glomerular filtration rate (GFR); if the value is below 30mg/ml/min/1.73m2, the use of some anti-osteoporotic therapies is contraindicated.
Should transaminases be measured in patients with osteoporosis before initiating treatment?Yes, this test helps to rule out any underlying liver disease and in patients with multiple drug therapy, medications for osteoporosis are metabolized in the liver.
Should alkaline phosphatase be measured in patients with osteoporosis before starting treatment?Yes, this consensus recommends the routine measurement of alkaline phosphatase in all patients starting osteoporosis treatment, in order to help in ruling out primary biliary cirrhosis, autoimmune liver disease, infiltrative liver diseases, and osteomalacia, Paget's disease and hypophosphatasia, among other conditions.
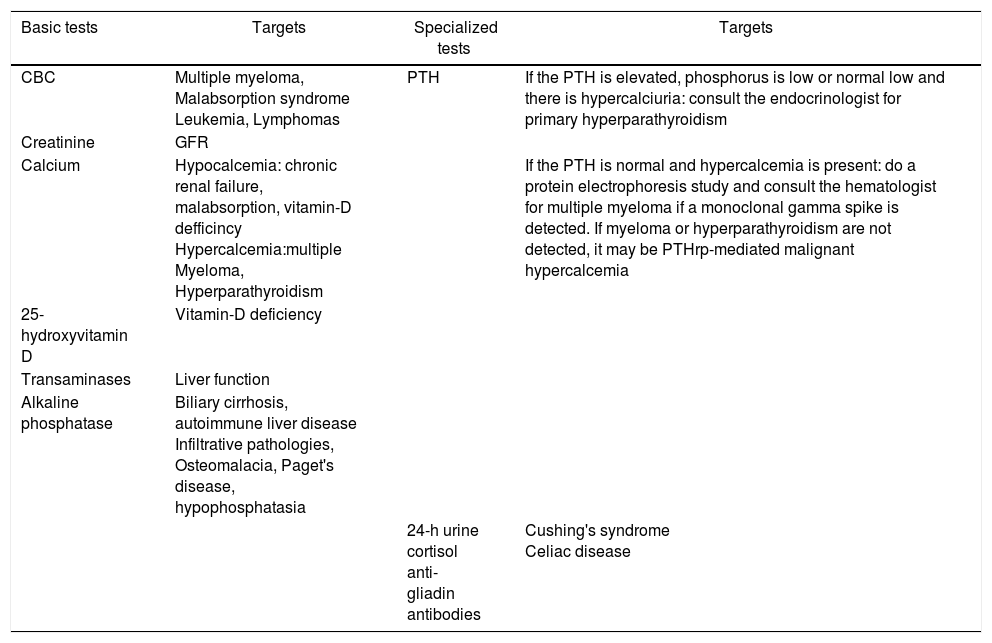
What other tests shall be ordered in patients suspicious for secondary osteoporosis?In patients that are suspicious for secondary osteoporosis, an electrophoresis protein test shall be conducted if multiple myeloma is suspected; 24-h urine free cortisol when suspecting Cushing's syndrome, and parathyroid hormone (PTH) to study hyper or hypoparathyroidism. Table 2 shows a detailed list of diagnostic tests.
Laboratory tests in osteoporosis.
| Basic tests | Targets | Specialized tests | Targets |
|---|---|---|---|
| CBC | Multiple myeloma, Malabsorption syndrome Leukemia, Lymphomas | PTH | If the PTH is elevated, phosphorus is low or normal low and there is hypercalciuria: consult the endocrinologist for primary hyperparathyroidism |
| Creatinine | GFR | ||
| Calcium | Hypocalcemia: chronic renal failure, malabsorption, vitamin-D defficincy Hypercalcemia:multiple Myeloma, Hyperparathyroidism | If the PTH is normal and hypercalcemia is present: do a protein electrophoresis study and consult the hematologist for multiple myeloma if a monoclonal gamma spike is detected. If myeloma or hyperparathyroidism are not detected, it may be PTHrp-mediated malignant hypercalcemia | |
| 25-hydroxyvitamin D | Vitamin-D deficiency | ||
| Transaminases | Liver function | ||
| Alkaline phosphatase | Biliary cirrhosis, autoimmune liver disease Infiltrative pathologies, Osteomalacia, Paget's disease, hypophosphatasia | ||
| 24-h urine cortisol anti-gliadin antibodies | Cushing's syndrome Celiac disease |
PTHrp: PTH-associated protein.
FRAX® is a tool to estimate the risk of sustaining a fracture, based on a number of clinical risk factors, with or without bone densitometry data. Depending on the quality of the data available to do the estimates and the methodology currently used, FRAX is probably the most recommended method for determining the risk of fracture.
FRAX® is used to estimate the absolute risk of an osteoporotic fracture in general and of a hip fracture in particular, over the next 10 years, in different populations, including the Colombian population, in people aged 40–90, who are not receiving osteoporosis treatment.52
When should FRAX® be used?If the patient has osteoporosis there is no need to use the FRAX® tool. FRAX® is indicated in patients with osteopenia to determine the risk of fracture when a bone scan is not available.
Which is the FRAX® value established to start treating a patient in Colombia?Although it is desirable to measure the BMD which is in itself a determining factor for fractures, most fragility fractures present in patients that have not reach the cut point for osteoporosis (54% in the United States,53 56% in Australia,54 48% in France,55 53% in Mexico13). For this reason, the systematic use of risk factors is needed.
FRAX® is an easy and accessible instrument, and since 2017 we have available the thresholds for evaluation and intervention in the Colombian population. FRAX® was designed to be used in primary care settings in countries where the availability of densitometry is limited; therefore, it can be used for screening purposes as a decision-making tool. Specialists having access to bone scanners may include those results, but should keep in mind that some risk factors have proven to be BMD-independent.52
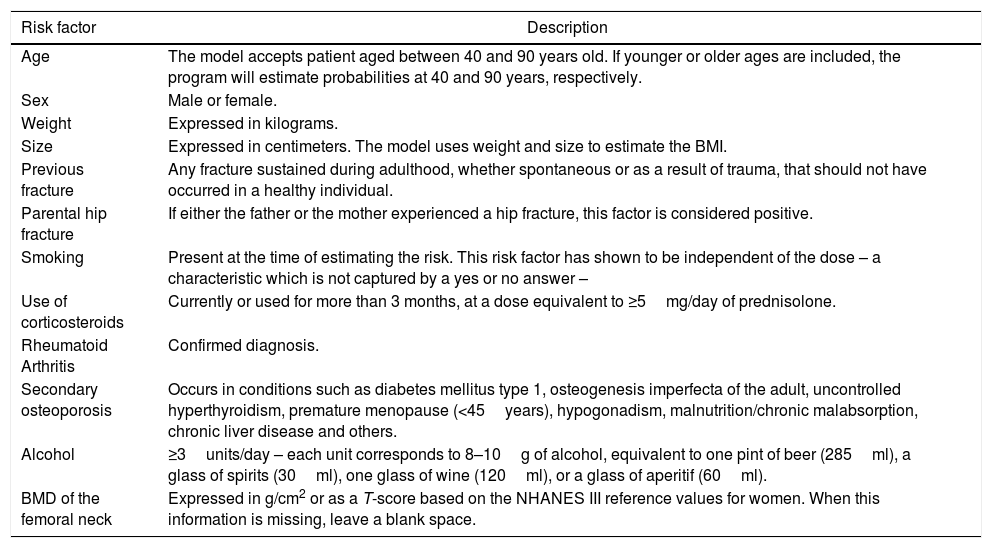
FRAX® only considers 7 risk factors (Table 3), chosen on the basis of a meta-analysis that established their validity, their strength, and relative weight. For instance, a previous low impact fracture after 40 years of age is a powerful predictor.56
Risk factors included in FRAX®.
| Risk factor | Description |
|---|---|
| Age | The model accepts patient aged between 40 and 90 years old. If younger or older ages are included, the program will estimate probabilities at 40 and 90 years, respectively. |
| Sex | Male or female. |
| Weight | Expressed in kilograms. |
| Size | Expressed in centimeters. The model uses weight and size to estimate the BMI. |
| Previous fracture | Any fracture sustained during adulthood, whether spontaneous or as a result of trauma, that should not have occurred in a healthy individual. |
| Parental hip fracture | If either the father or the mother experienced a hip fracture, this factor is considered positive. |
| Smoking | Present at the time of estimating the risk. This risk factor has shown to be independent of the dose – a characteristic which is not captured by a yes or no answer – |
| Use of corticosteroids | Currently or used for more than 3 months, at a dose equivalent to ≥5mg/day of prednisolone. |
| Rheumatoid Arthritis | Confirmed diagnosis. |
| Secondary osteoporosis | Occurs in conditions such as diabetes mellitus type 1, osteogenesis imperfecta of the adult, uncontrolled hyperthyroidism, premature menopause (<45years), hypogonadism, malnutrition/chronic malabsorption, chronic liver disease and others. |
| Alcohol | ≥3units/day – each unit corresponds to 8–10g of alcohol, equivalent to one pint of beer (285ml), a glass of spirits (30ml), one glass of wine (120ml), or a glass of aperitif (60ml). |
| BMD of the femoral neck | Expressed in g/cm2 or as a T-score based on the NHANES III reference values for women. When this information is missing, leave a blank space. |
When introducing these risk factors, the FRAX® algorithm determines the absolute risk of fracture in 10 years, both of major fracture (vertebra, wrist, hip, and proximal humerus) or just the hip. Since 2011, the Colombian FRAX® has been calibrated with the epidemiological data of fractures and mortality, so it may be used with confidence (available in: https://www.shef.ac.uk/FRAX®/tool.jsp).
After 2017 we also have evaluation and intervention thresholds for the Colombian population, using the methodology described by Kanis et al.,57 in the United Kingdom and by Clark et al.52 in México.
2 thresholds were estimated52,54,56,57:
- •
Evaluation threshold, which does not take BMD into consideration and is recommendable in countries without broad availability of densitometry equipment and for screening purposes at the primary care level.
- •
Intervention threshold, to be used when central densitometry equipment is available. Adding this factor increases the accuracy of the measurement.
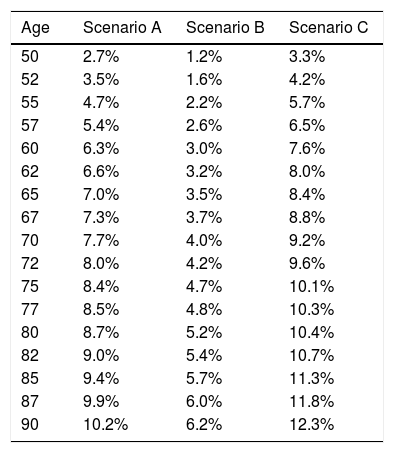
Three clinical scenarios were used to establish these thresholds, as described in Table 452,54,56,57:
- •
Scenario A: women ≥50 years old, with a BMI of <25, a previous fracture, with no BMD data. This is the intervention threshold, since this is the high risk group.
- •
Scenario B: women >50 years old, with a BMI of <25, with no previous fractures, without BMD data. This is the lowest evaluation threshold. Cases with a lower risk as compared with this profile should not be intervened or referred for densitometry.
- •
Scenario C: a profile with a 1.2-fold risk versus the upper threshold of evaluation shall be intervened, regardless of the densitometry. Any cases between the 2 evaluation thresholds require densitometry to recalculate the risk of FRAX® incorporating the BMD of the femoral neck.
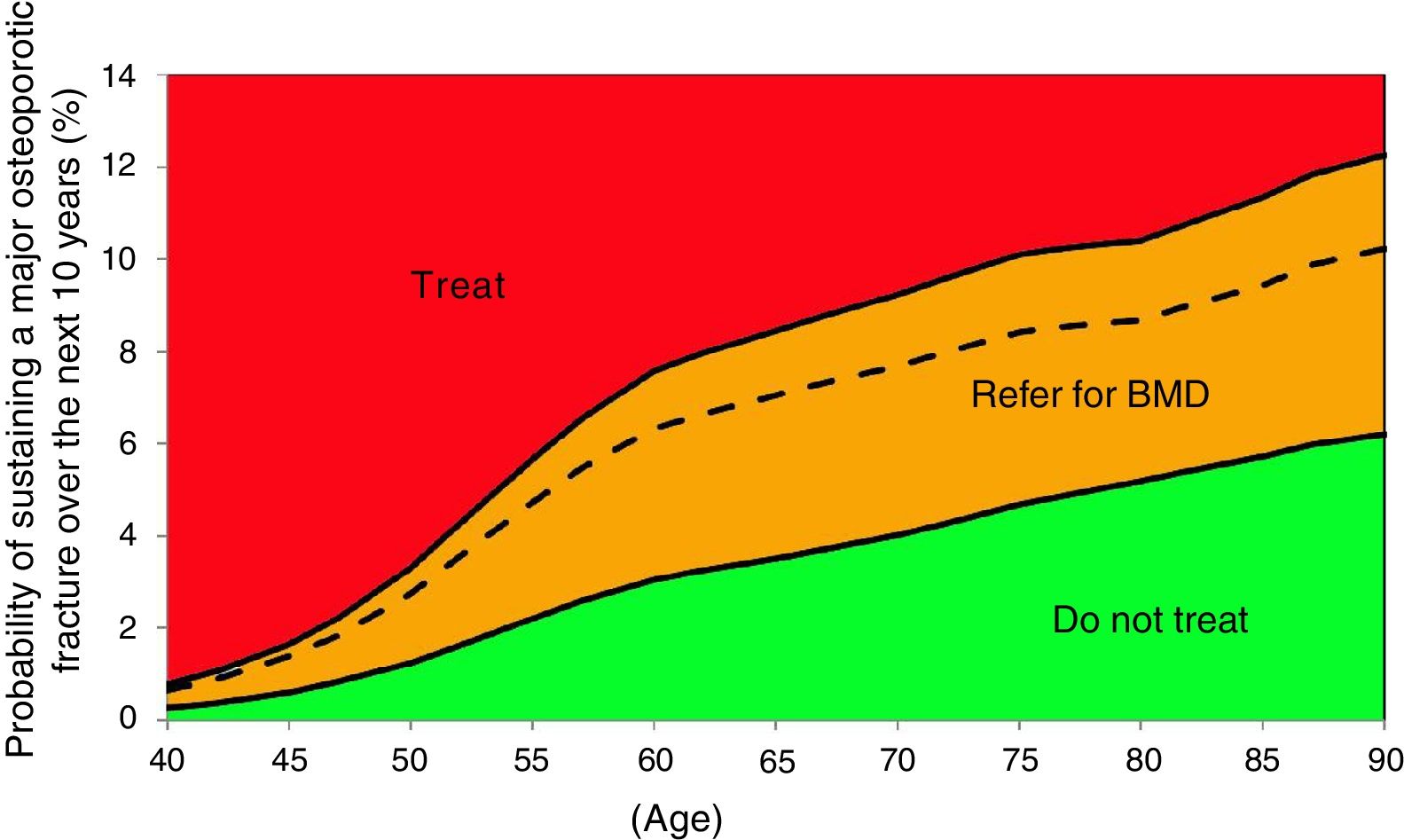
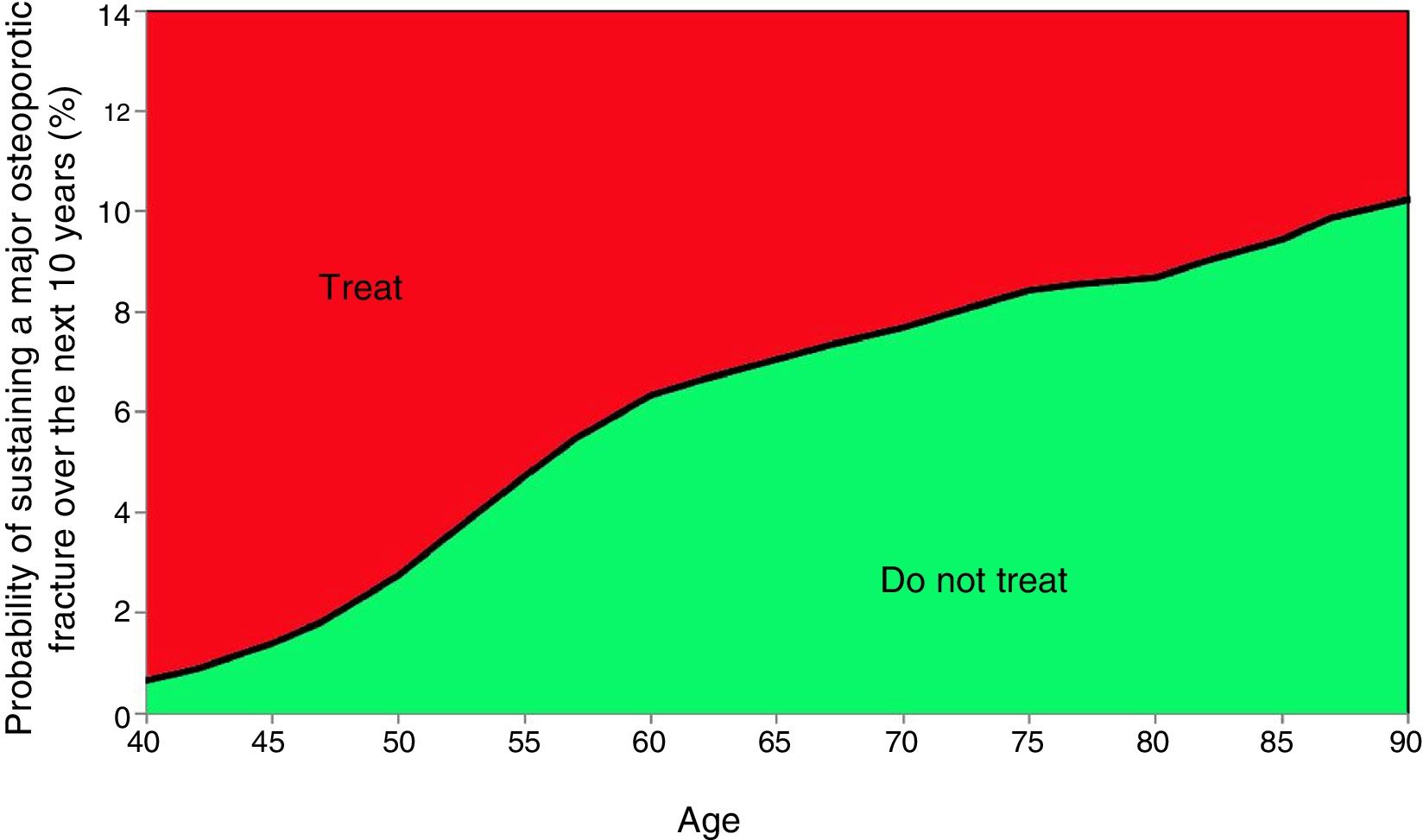
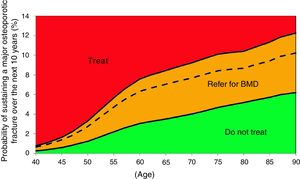
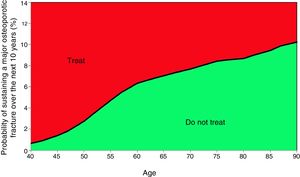
Probability of major fracture.
| Age | Scenario A | Scenario B | Scenario C |
|---|---|---|---|
| 50 | 2.7% | 1.2% | 3.3% |
| 52 | 3.5% | 1.6% | 4.2% |
| 55 | 4.7% | 2.2% | 5.7% |
| 57 | 5.4% | 2.6% | 6.5% |
| 60 | 6.3% | 3.0% | 7.6% |
| 62 | 6.6% | 3.2% | 8.0% |
| 65 | 7.0% | 3.5% | 8.4% |
| 67 | 7.3% | 3.7% | 8.8% |
| 70 | 7.7% | 4.0% | 9.2% |
| 72 | 8.0% | 4.2% | 9.6% |
| 75 | 8.4% | 4.7% | 10.1% |
| 77 | 8.5% | 4.8% | 10.3% |
| 80 | 8.7% | 5.2% | 10.4% |
| 82 | 9.0% | 5.4% | 10.7% |
| 85 | 9.4% | 5.7% | 11.3% |
| 87 | 9.9% | 6.0% | 11.8% |
| 90 | 10.2% | 6.2% | 12.3% |
Based on these probabilities, the graphs corresponding to the intervention and evaluation thresholds were developed (Figs. 1 and 2).
For example, one patient who has no densitometry data and falls within the orange zone, shall be re-assessed, including densitometry data; in some cases, this could lower the risk down to the green zone and hence no intervention shall be required.
FRAX® has a few limitations:
- •
Since its main objective is to identify high risk individuals for timely treatment, it may only be used in first-time treatment-naïve patients.
- •
Not applicable for pre-menopausal women.
- •
Does not include all the clinical risk factors evaluated during consultation, such as the risk of falls.
- •
Does not establish a risk gradient regarding the use of substances. For instance, it may underestimate the risk of patients receiving long-term high-dose glucocorticoids.
As a result of these limitations, the treatment decision shall not be based on one single tool. Clinical judgment is still the determining factor.
What is the trabecular bone score (TBS) and when should it be measured in patients with postmenopausal osteoporosis?TBS is a non-invasive, non-standardized method in our population that evaluates the bone microarchitecture, and in combination with BMD, increases the sensitivity to detect the risk of fracture. If available, it may be used as an additional tool in conjunction with FRAX®, considering its higher value in special populations such as glucocorticoid users and patients with diabetes type 2, in whom the risk of fracture seems to be less associated with the loss of BMD.58–60
TreatmentObjectives of osteoporosis treatmentThe objectives of osteoporosis treatment are to prevent fractures, improve the bone density and quality, and correcting any modifiable risk factors.61,62
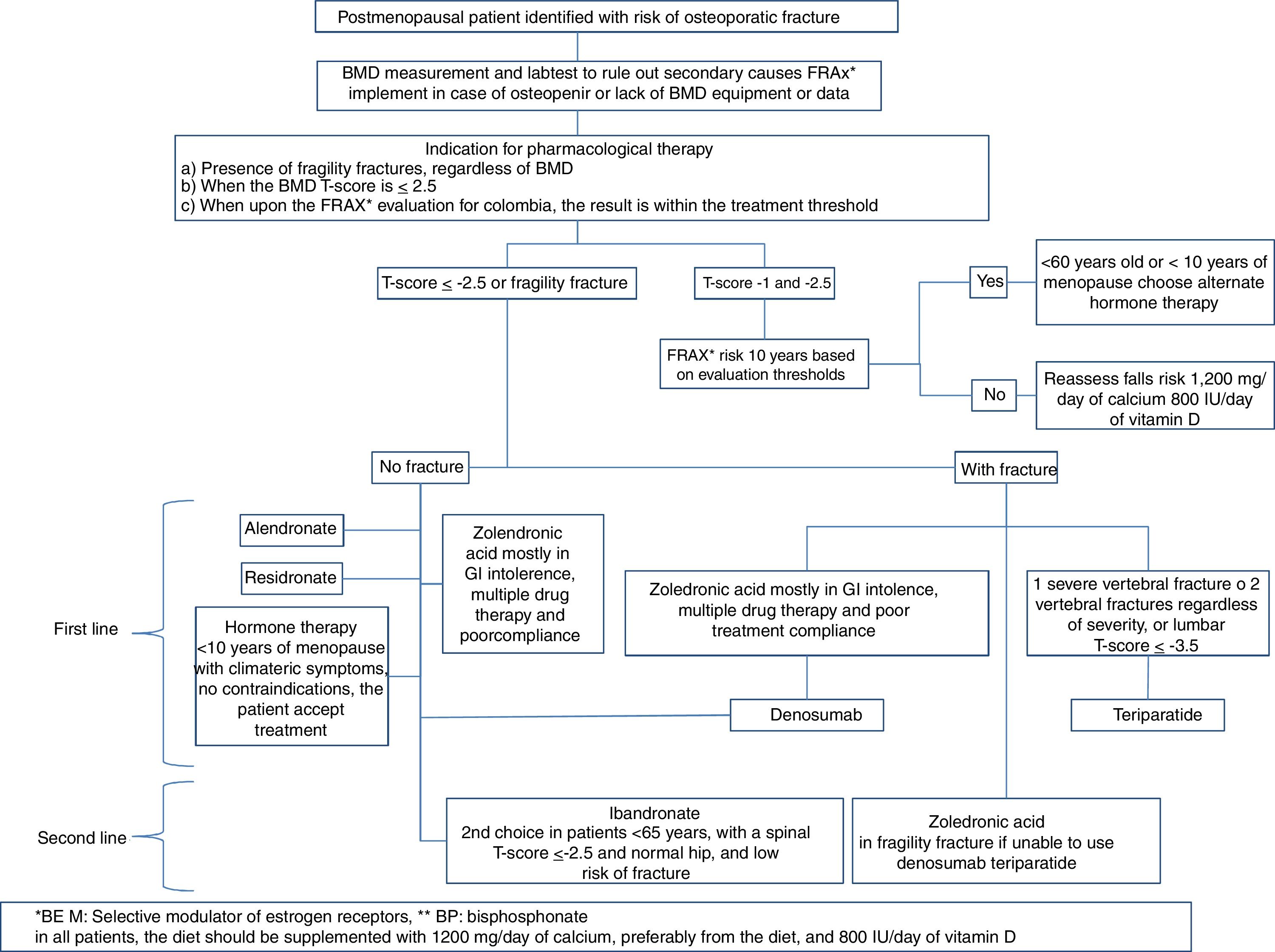
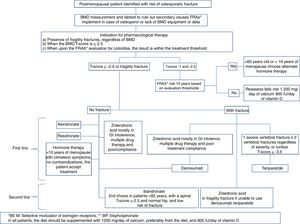
In which patients should anti-osteoporosis therapy be initiated?Therapeutic decision:
Our consensus advices treating under any of the following circumstances:
- •
In the presence of fragility fractures, regardless of the BMD
- •
When the BMD T-score is equal or less than −2.5
- •
When, upon completing the FRAX® evaluation for Colombia, the result is within the treatment threshold
70% of all fragility fractures develop without having a densitometry-based diagnosis of osteoporosis.63,64 Usually osteopenia should be associated either with minimum trauma fractures or with a very high risk of fracture, evaluated through risk assessment tools such as FRAX®, in order to deserve treatment with anti-osteoporosis drugs.65
In the United States setting, treating postmenopausal women (45 years and older) with osteopenia (T-score between −1.0 and −2.5) is considered to be cost-effective, if in 10 years the probability of hip fracture is 3% or more, or if the risk of major fragility fracture (vertebral body, hip, forearm, or shoulder) is higher or equal to 20%, according to the FRAX®66 calculator. In Colombia, these percentages for risk are not applicable.
Position: this consensus recommends to start osteoporosis drug therapy in patients with no fractures, with a low bone mass or osteopenia, if the FRAX® stratified risk at 10 years is within the intervention threshold. First, you should rule out the presence of vertebral fractures.
The order recommended in this consensus for choosing therapy for patients with low bone mass or osteopenia without any fragility fractures is as follows:
First line67–71- •
Less than 10 years after the onset of menopause: estrogen therapy, particularly if menopause-related symptoms are present, if there are no contraindications for the use of estrogen therapy, and if the patient accepts treatment.
- •
Selective Estrogen Receptor Modulator (SERM) in patients without any history or risk factors for thromboembolic disease, or vasomotor symptoms, regardless of age, and with risk of breast cancer.
- •
Whenever there is a contraindication for the use of estrogens and SERMs, or if the patient refuses to accept any of the first-line therapies, bisphosphonates may be used, ideally in patients over 60 years old, due to the risk/benefit ratio of receiving therapy for more than 10 continuous years.
- ∘
Alendronate: oral dose of 70mg/week for 3–5 years
- ∘
Zoledronic Acid: this agent may be considered in case of low compliance with oral bisphosphonate or gastric intolerance. Intravenous dose of 5mg every 2 years, 2 doses.
- ∘
Risedronate: 35mg/week or 150mg/month, oral administration, for 3 years.
Estrogens: Hormone replacement therapy (HRT) is an excellent option to consider when the intervention for preventing osteoporosis and reducing the risk of fractures is indicated in symptomatic and asymptomatic postmenopausal women less than 60 years old.67 It should be noted that the estrogens+progestins arm of the Women's Health Initiative (WHI) trial – particularly bone health issues – in 2002, was the first randomized trial showing the value of HRT to prevent hip fractures, vertebral fractures, and fractures at other locations. This was also established among the estrogen-only group of the WHI in 2004. The estrogens+progestins branch of the WHI included 16,608 postmenopausal, asymptomatic non-hysterectomized women aged 50 through 79 years old (average age: 63 years). The patients were randomized to receive 0.625mg of conjugated equine estrogens plus 2.5mg of medroxyprogesterone acetate or placebo. The reports of hip fractures, vertebral fractures, and other fragility fractures were routinely recorded. The hazard ratio (HR) estimates for hip fracture was 0.66 (95% CI: 0.45–0.98). This study measured the BMD in a subgroup of patients (n=1024) at baseline, and 1 and 3 years later. In this subgroup of women with known BMD, the average baseline BMD of the total hip had a T-score of −0.94 and in the lumbar spine the average T-score was −1.3. After three years of estrogen+progestin therapy, the BMD percentage differences between the patients receiving therapy versus the placebo group was 4.5 and 3.6% in the lumbar spine and in the total hip, respectively.72–75
Raloxifene: raloxifene is a selective estrogen receptor modulator and is currently being used both for prevention and treatment of postmenopausal osteoporosis. The data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial assessed the health-related quality of life, the clinical reduction of vertebral fractures over one year, and the relationship between the BMD and the bone turnover biochemical markers in terms of the reduction of vertebral fractures.76
The findings support the use of raloxifene to lower the risk of vertebral fractures through improved BMD, but this is not the case in the hip region.77
Positive estrogen receptor breast cancer dropped by 90%, without increasing the incidence of endometrial cancer with raloxifene. The most severe side effect of raloxifene was a higher incidence of deep venous thrombosis and pulmonary embolism.78
Since there is no therapeutic effect in terms of relieving climacteric symptoms, and there is a potential risk of embolism with the use of raloxifene, this drug is prescribed for clear indications such as for the management of osteoporosis, prevention of fractures, and lower incidence of invasive breast cancer, with a careful follow-up for the risk of embolism. It is reasonable to use raloxifene as an adequate medication for postmenopausal women without climacteric symptoms, due to its risk/benefit profile and safety, using the global health index proposed by the WHI.79
It has been shown that raloxifene prevents osteoporosis in postmenopausal women with low bone mass and prevents vertebral fractures in women with osteoporosis or low bone mass, but it has not been proven to reduce the risk of non-vertebral fractures. Raloxifene reduces the risk of invasive breast cancer in postmenopausal women with osteoporosis or at high risk of breast cancer. It has been shown that the risk of embolism increases with raloxifene, and therefore it should not be used in women at high risk of embolism. Although raloxifene does not increase or decrease the risk of coronary events or cerebrovascular accidents in general, in the trial with raloxifene in postmenopausal women with a higher risk of coronary events, the incidence of fatal stroke was higher among the group of women receiving raloxifene versus placebo.80
Alendronate: in women with a low bone mass who fail to meet the densitometry criterion for osteoporosis, alendronate has proven to be effective at reducing the risk of vertebral fractures. The absolute benefit of this therapy in women with a T-score between −1.6 and −2.5 is higher in women with a prevalent vertebral fracture, or in women with additional risk factors.81 Alendronate therapy at a dose of 10mg/day did not show statistically significant results for the primary prevention of fractures, except for vertebral fractures, for which the reduction was clinically significant.82
In women with a low BMD but without vertebral fractures, 4 years of alendronate increased the BMD and decreased the risk of the first fracture. Alendronate significantly reduced the risk of clinical fractures in women with osteoporosis, but not in women with a higher BMD.83
Zoledronic Acid: the study for the prevention of osteoporosis in postmenopausal women with osteopenia (T-score <−1.0 and >−2.5) showed that both, two annual 5mg IV infusions during 24 months, and one single 5mg IV infusion at the beginning and over 24 months, significantly increased de average BMD with regards to the baseline as compared with placebo in the lumbar spine, total hip, femoral neck and trochanter. Bone turnover markers were significantly reduced with both treatment regimens (annual and bi-annual), as compared to placebo. The study did not contribute with any fracture data. Based on this evidence, Zoledronic acid administered at a 5mg dose every 2 years, was approved in the United States for the prevention of postmenopausal osteoporosis in women with low bone mass.84–86
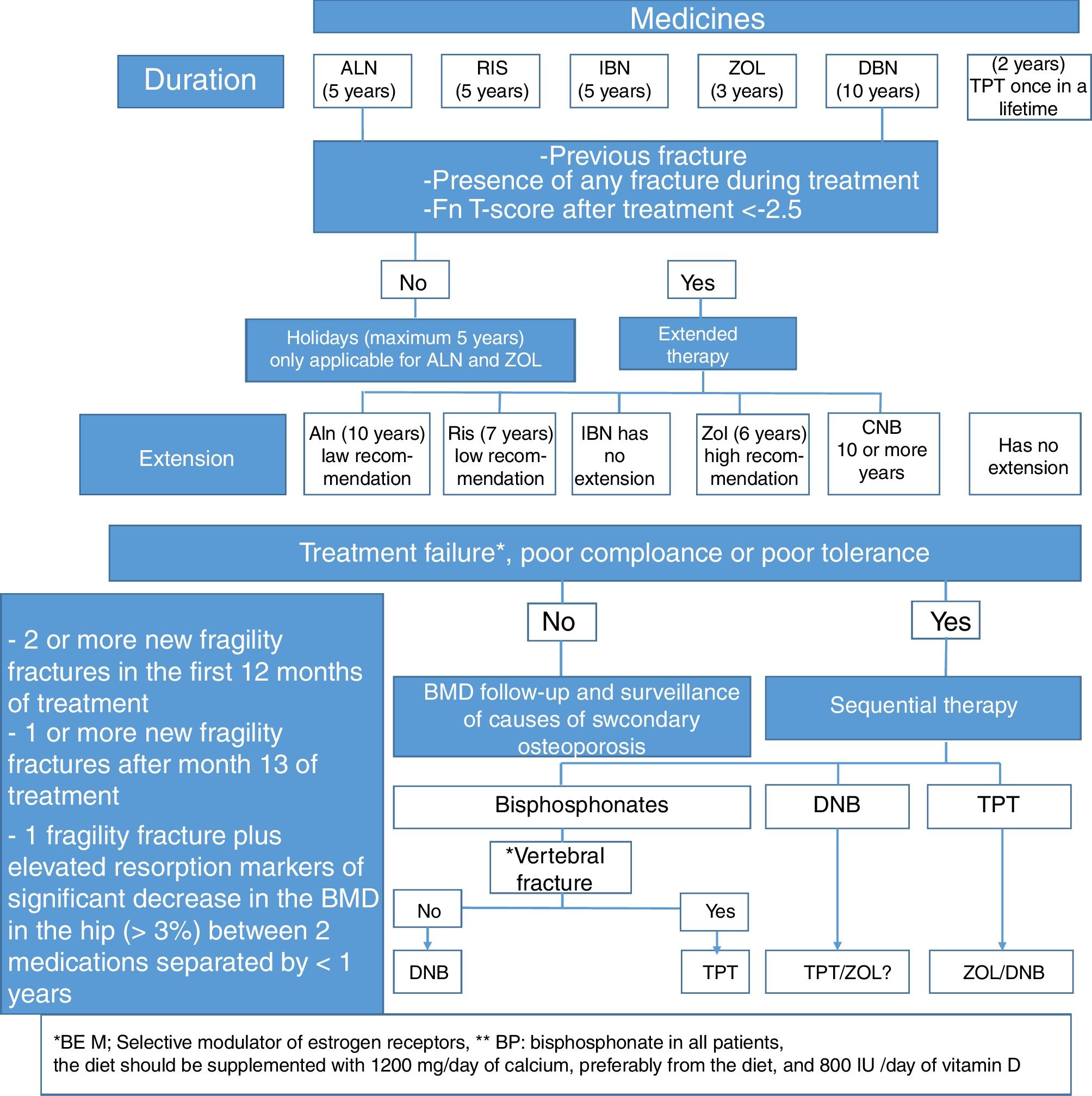
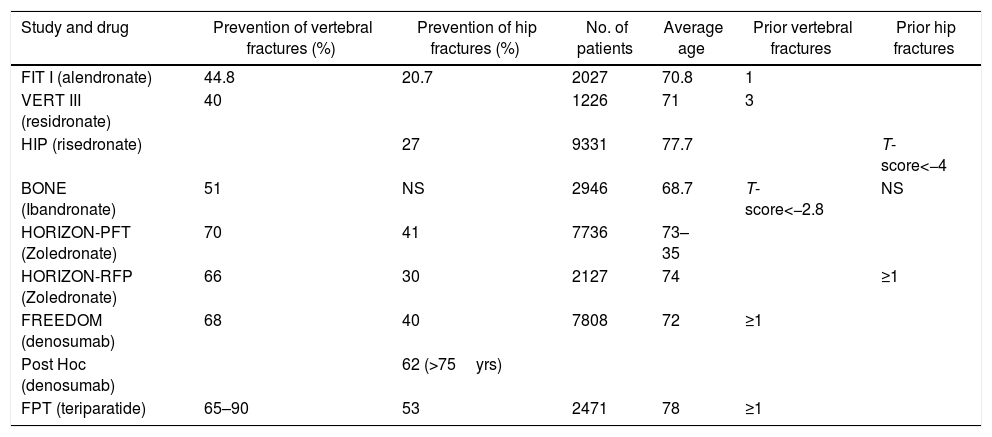
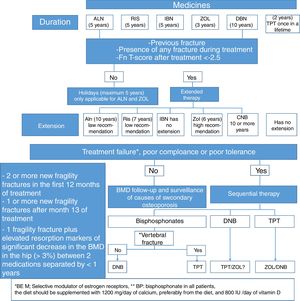
Which should be the medical treatment for postmenopausal osteoporosis in Colombia, considering the costs and our healthcare system?The treatment regimen we suggest for postmenopausal osteoporosis, based on the medicines described in Table 5, and whose algorithm is summarized in Fig. 3, is the following:
- •
Calcium and vitamin D in patients treated for osteoporosis.
Comparative table of the anti-fracture efficacy of each drug.
| Study and drug | Prevention of vertebral fractures (%) | Prevention of hip fractures (%) | No. of patients | Average age | Prior vertebral fractures | Prior hip fractures |
|---|---|---|---|---|---|---|
| FIT I (alendronate) | 44.8 | 20.7 | 2027 | 70.8 | 1 | |
| VERT III (residronate) | 40 | 1226 | 71 | 3 | ||
| HIP (risedronate) | 27 | 9331 | 77.7 | T-score<−4 | ||
| BONE (Ibandronate) | 51 | NS | 2946 | 68.7 | T-score<−2.8 | NS |
| HORIZON-PFT (Zoledronate) | 70 | 41 | 7736 | 73–35 | ||
| HORIZON-RFP (Zoledronate) | 66 | 30 | 2127 | 74 | ≥1 | |
| FREEDOM (denosumab) | 68 | 40 | 7808 | 72 | ≥1 | |
| Post Hoc (denosumab) | 62 (>75yrs) | |||||
| FPT (teriparatide) | 65–90 | 53 | 2471 | 78 | ≥1 |
The supplement of calcium and vitamin D has been widely recommended to prevent osteoporosis and subsequent fractures. However, there is a considerable controversy with regards to the association of those supplements and the risk of fractures. A meta-analysis published in January 2016, including 8 studies with 30,970 participants, showed that the supplement of calcium and vitamin D resulted in a statistically significant reduction of 15% in the risk of total fractures (SRRE: 0.85; 95% C.I.: 0.73–0.98) and a 30% reduction in the risk of hip fractures (SRRE: 0.70; 95% C.I.: 0.56–0.87). Numerous sensitivity and subgroup analyses resulted in similar associations.87
Based on the recommendations of the Colombian Consensus on Vitamin D,88 this consensus recommends the following regimens, although each case must be individualized and each regimen shall be adapted to the clinical practice setting.
- ∘
Supplementation: 1000IU/day
- ∘
Insufficiency: 1000–2000IU/day
- ∘
Deficiency: 2000–6000IU/day
The dose should be adjusted based on the response to achieve levels of 40ng/ml. The equivalent to 10,000IU/day shall not be used, except under special or individual circumstances. If the patient is obese (BMI>30), the recommended dose should be doubled.88
First line of choiceBisphosphonatesFirst choice: Alendronate, risedronate or zoledronic acid (zoledronic acid is preferred in case of poor compliance, oral intolerance, multiple drug therapy or older than 75 years with a GFR >30ml/min; it is contraindicated in renal failure with a GFR >30ml/min and in hypocalcemia).89
Alendronate is an aminobisphosphonate administered orally at a weekly dose of 70mg (it should be taken with an empty stomach, without taking any food, and the patient should not lie down for at least 30min). The efficacy data have been collected from studies conducted with a dose of 10mg/day,90 but it was subsequently91 shown that the 70mg/week dose is not inferior. A Cochrane Collaboration systematic review in 2008 concluded82 that alendronate reduces vertebral fractures by 45%, both in postmenopausal osteoporotic women with prior vertebral fracture (RR: 0.55; 95% CI: 0.43–0.69) and without vertebral fracture (RR: 0.55; 95% CI: 0.30–0.80). In non-vertebral fractures the risk reduction was only significant in patients with prior vertebral fracture (RR: 0.77; 95% CI: 0.64–0.92). Similar results were observed in terms of hip fracture (RR: 0.45; CIc95%: 0.46–0.70) and distal radius (RR: 0.50; 95% CI: 0.34–0.73). In the long term, the Fracture Intervention Trial Long-Term Extension trial (FLEX)92 randomized women treated with alendronate for 5 years into three groups: placebo, 5, and 10mg/day of alendronate. The patients were followed for another 5 years,93 and at the end of that period the patients in the placebo group had more clinical vertebral fractures than those that continued therapy (RR of the treated groups combined: 0.40; 95% CI: 024–0.86). There were no significant differences in the other types of fractures. A post hoc analysis of this study found that in women with a T score of less than −2.5 in the femoral neck and without a prior vertebral fracture, the continuation of therapy reduced the incidence of non-vertebral fractures by 50% (RR: 0.50; 95% CI: 0.26–0.96).94 A subsequent analysis concluded that both older age and lower BMD in the femoral neck at the time of removing alendronate, are associated with a 2.17 fold increased risk of fracture (95% CI: 1.38–3.41).95
Alendronate has shown to increase the BMD at the level of the lumbar spine and the hip, in both treatment and prevention trials.90 The increase in BMD in osteoporotic women after one year of therapy was around 4–5% in the spine and 3% in the hip.96
After 10 years of therapy, the lumbar BMD continued to increase, although discretely, while the hip BMD was stabilized. After removing alendronate, there is a residual BMD effect.93 In the FLEX trial, 5 years after the interruption of treatment, the BMD decreased by 2.4% (95% CI: 2.9–1.8%) in the total hip and by 3.7% (95% CI: 4.5–3.0%) in the lumbar spine, though the latter decrease did not reach the baseline values. Alendronate reduces the levels of bone remodeling markers such as alkaline phosphatase (around 30%), and osteocalcin (around 50%) and collagen derivatives (PIR and D-PIR [around 50%], NTX [70%], CTX [80%]). A residual effect on markers was also present after the interruption of alendronate. In the FLEX trial, following the suppression of alendronate, the CTX values increased by 55.6%, PINP increased by 59.5%, and bone alkaline phosphatase increased by 28.1%. Notwithstanding these results, the final values were somewhat lower than the baseline values 10 years before.93
Alendronate is usually well tolerated. The most frequent side effects involve the upper GI tract (esophagitis, esophageal ulcer). New side effects have been recently described, including esophageal cancer, atrial fibrillation, increased mandibular osteonecrosis and subtrochanteric and atypical diaphyseal femoral fractures, and rare cases of ocular inflammation.97
Residronate is another aminophosphonate administered orally at a dose of 35mg per week or 150mg per month. It has to be taken on an empty stomach as well, without food or laying down for 30min. The efficacy data have been collected from trials conducted with a dose of 5mg/day,98 after showing that the 35mg/week dose is not inferior.99
The 2008 Cochrane Collaboration review concludes100 that the reduction of vertebral fractures due to the is residronate in secondary prevention is of 39%, with no significant reduction in primary prevention.
The risk of non-vertebral fracture was reduced in one of the most relevant trials98 by 39% (95% CI: 6–61%). However, in the second major trial,101 the 33% reduction did not reach statistical reduction. A meta-analysis102 gives a figure of risk reduction of non-vertebral fractures of 27% (95% IC: 13–39%), and the Cochrane Collaboration publication100 reports a 20% reduction (RR: 0.80; 95% IC: 0.72–0.90). Once again, the reduction in primary prevention was non-significant. According to the meta-analysis of the Cochrane Collaboration, the reduction in hip fractures with the use of residronate is 26% (RR: 0.74; 95% CI: 0.59–0.94).
The effects on vertebral fractures initially observed in 3-year trials have been confirmed in a 5-year extension.103 A second 7-year extension104 showed that the incidence of fractures over the 6–7 years of treatment is similar to the 1–3-year incidence. A non-inferiority study has shown the efficacy of administering 150mg in one single day per month.105 Residronate has a positive BMD effect on osteoporotic women, which at 3 years represents around 5–6% in the lumbar spine and 2% in the femoral neck. At 7 years, the BMD continues to increase and the hip remains stable.
Residronate reduces the levels of remodeling markers,106,107 such as bone-specific alkaline phosphatase (around 25%), osteocalcin (around 40%), and collagen derivatives (PIR y D-PIR [around 30–60%], NTX [60%], and CTX [73%]), with similar results with the daily or weekly administration.
Risedronato is well tolerated.99,105 The most frequently described side effects have been gastrointestinal.
Zoledronic acid is an aminobisphosphonate for IV use. The HORIZON-PFT48,108 is a 3-year randomized, double blind, placebo controlled clinical trial, conducted in osteoporotic postmenopausal women with a BMD ≤−2.5 or ≤−1.5 plus a moderate vertebral fracture, or mild vertebral fractures. 21% of the patients were treated with anti-osteoporosis drugs other than bisphosphonates or PTH, such as sex hormones, raloxifene or calcitonin. The patients were assigned to receive placebo o 5mg of IV zoledronic acid per year. The RR of morphometric vertebral fractures after three years was 0.30 (95% CI: 0.24–0.38). The HR for hip fractures was 0.59 (95% CI: 0.42–0.83), and for non-vertebral fractures was 0.75 (95% CI: 0.64–0.87); in other words, 70% prevention of vertebral fractures, 42% of hip fractures, and 25% non-vertebral fractures. The HORIZON-RFT109 was conducted in both males and females with a previous hip fracture. The female to male ratio was 75:25. Patients were treated either with placebo or with 5mg/year of IV zoledronic acid. The mean follow-up was 1.9 years. The primary endpoint was the occurrence of new clinical fractures. The HR for the latter was 0.65 (95% CI: 0.50–0.84), the HR for non-vertebral fractures was 0.73 (95% CI: 0.55–0.98); for clinical vertebral fractures was 0.54 (95% CI: 0.32–0.92), and for hip fractures was 0.70 (95% CI: 0.41–1.19). The HORIZON-PFT trial continued with a 6-year extension,110 in which more than 1200 patients of the treated group were divided into 2 groups, one of which continued treatment and the other received placebo. The incidence of morphometric vertebral fractures in the first group was 50% lower than in the second group (OR: 0.51; 95% CI: 0.26–0.95).
A post hoc analysis conducted in women over 75 years old shows the efficacy of the drug for reducing vertebral and non-vertebral fractures in this age group. However, the reduction in the number of hip fractures did not reach statistical significance.111
In the HORIZON-PFT48,108 the BMD as compared to placebo increased by 6.7% in the lumbar spine, by 5.1% in the femoral neck, and by 6.0% in the total hip. The HORIZON-RFT109 showed an increase of 5.5% in total hip after 36 months, with a simultaneous drop of 0.9% in the placebo group. In the femoral neck, the corresponding increase was 3.6%, with a drop of 0.6% in the placebo group. With regards to remodeling markers, in the HORIZON-PFT48,108 trial, the levels of CTX, bone-specific Alkaline Phosphatase and PINP at 12 months were 59, 30 and 58% lower in the treated group versus placebo, respectively.
In terms of adverse effects, the most frequently reported was a “flu-like” condition or “acute phase reaction”, with in the HORIZON-PFT48,108 affected approximately 30% of the population after the first injection, around 6% after the 2nd injection, and 2% after the 3rd injection. Following the infusion in the HORIZON trials, there as a tendency to present lower levels of calcemia, but these were transient and asymptomatic. Another adverse effect to be mentioned is the description in the HORIZON-PFT trial of a higher incidence of the so-called “severe atrial fibrillation” in the zoledronic acid-treated group (2.5% vs. 1%, p<0.001); in contrast, this effect was not found in the HORIZON-RFT trial. However, in this latter trial, there was a higher frequency of creatinine elevation among the treated group of patients (1.3% vs. 0.4%) that regressed before one month. There were differences in the creatinine levels between the treated and the placebo group of patients at 3 years. Also in both trial, a higher incidence of inflammatory ocular problems was identified (0.4% versus 0.1% in the HORIZON-PFT trial, and 3.3% versus 2.7% in the HORIZON-RFT trial). In none of these two trials (HORIZON-PFT and HORIZON-RFT) were mandibular osteonecrosis cases spontaneously reported. A subsequent search addressed specifically to identify this condition in the first trial, pointed to the possibility of this complication presenting in one case in each group of the HORIZON-PFT. A particularly interesting beneficial effect was noted in the HORIZON-RFT trial: a 28% reduction in overall mortality (for any cause) in the group assigned to zoledronic acid (p=0.01).
Hormone therapy during menopauseMenopause hormone therapy (MHT) is a treatment option for postmenopausal osteoporosis.67–71 In general, En general, its use for the treatment of osteoporosis is restricted to women with less than 10 years after the onset of menopause and with climacteric symptoms, that do not have any contraindications for the use of estrogen therapy and accept treatment.71
MHT comprises a large number of estrogen formulations, alone or combined with progestin; some of these formulations are approved for the prevention of osteoporosis in postmenopausal women with a high risk of fracture. It has been shown that a dose of 0.625mg/day of equine estrogen+2.5mg/day of medroxiprogesterone acetate, reduced vertebral, non-vertebral and hip fractures in a population of postmenopausal women that were not discriminated by BMD or risk of fracture.74,112
Second line (or first line in selected cases)Ibandronate
This consensus feels that ibandronate may be used as a second line therapy in patients under 65 years old with a spinal T-score <−2.5 and a hip T-core ≥−1.
Ibandronate is an aminobisphosphonate that can be administered by mouth and IV. The oral administration may be monthly (150mg tablets), and the intravenous administration every 3 months (3mg vial). The low absorption of the drug requires avoiding any food intake for 60min after its administration.113
A daily administration of 2.5mg of ibandronate in women with densitometry-diagnosed osteoporosis and vertebral fracture, reduces the risk of vertebral fracture by 60%114 (RR: 0.38; 95% CI: 0.25–0.59). The post hoc analysis of a group of women with a T-score below −3.0 showed less non-vertebral fractures vs. Placebo.115
In the patients treated with 2.5mg per day of ibandronate, the BMD increases in the lumbar spine by 6.5% at 3 years 116 and in the total hip by 3.4%.117 A non-inferiority study with this dose (MOBILE trial, initially one year long) showed the efficacy of administering 100 and 150mg per month.118 The IV administration of 2mg every 3 months, to osteoporotic women, increases the lumbar BMD by 5% and the total hip BMD by 3% after one year.119 In a non-inferiority study, the IV administration of 2mg every 2 months, or 3mg every 3 months for one year (DIVA trial)120 has shown that both regimens are not only non-inferior, but are superior to the oral administration of 2.5mg per day, in terms of its effect on BDM. A 5-year extension of the DIVA trial (DIVA-LTE) showed an increase in BMD in the lumbar spine of 8.1% with 3mg intravenously every 3 months.121 Both extension trials has shown that the safety of the medication is preserved when administered for these time periods.
Remodeling markers decrease with the recommended guidelines.122,123 Serum CTX dropped from 60 to 70%, the urine NTX from 50 to 60%, osteocalcin from 3 to 50%, and bone-specific alkaline phosphatase from 30 to 40%.
Ibandronate is well tolerated. In the various clinical trials, the incidence of adverse reactions has been similar to placebo, including GI manifestations. Flu-like manifestations are an exception, which are observed with the higher intermittent doses (1–3%) and with IV administration (5–8%), mostly after the first injections. The 5-year extension of the MOBILE trial has not delivered any new information in terms of safety and tolerability.117
- ∘
Denosumab:
This consensus recommends using denosumab as the first line therapy in any of the following circumstances:
- •
In Chronic renal disease with GFR less than 30ml/min, having ruled out or corrected hypocalcemia.
- •
In patients older than 75 years with a T-score below −2.5 in the hip.
- •
In any case in which the above-mentioned therapies are contraindicated.
- •
In cases of osteoporosis with a high risk of fracture because of old age, a history of non-vertebral fragility fracture, or multiple risk factors.
Denosumab is a humanized monoclonal antibody that binds to RANK ligand, and has shown to be effective in reducing the risk of new morphometric vertebral fractures in women with postmenopausal osteoporosis, at a dose of 60mg administered subcutaneously, every 6 months. In the crucial FREEDOM trial (which excluded women with a T-score <4, more than one vertebral fracture or a severe vertebral fracture) the relative risk reduction of vertebral fractures at 35 months was 68% versus placebo (absolute risk 2.3% with denosumab vs. 7.2% with placebo). The protective effect was observed after the first year of therapy. The reduction in the risk of clinical vertebral fractures was of 69%, and in the risk of multiple vertebral fractures was of 61%.124,125
In the extension trial with denosumab up to 8 years (without a comparator group) the incidence of new vertebral fractures remained low, while the patients received treatment (1.5, 1.3, and 1.3% during the years 4/5, 6 and 7/8).126 In the extension trial, 4450 women treated with denosumab in the primary trial were enrolled, 2207 from the placebo group).
A protective effect of denosumbab against non-vertebral fractures was also identified, with a relative reduction of 20% (p=0.01) and of hip of 40% (p=0.04)124 which was maintained for the following years until completing 8 years with the drug; the rate of non-vertebral fractures was 1.5, 1.2, 1.8, 1.6 and 0.7% (during years 4, 5, 6, 7 and 8, respectively). The accumulated incidence of hip fractures during the 5 extension years was of 0.7%.126 In the primary trial, the subgroup of women with a femoral neck T-score of less than or equal to −2.5 the relative risk reduction of distal radius fracture was 40% (p=0.03).127
After 8 years of continuous use, an increase in BMD in the lumbar spine of 18.4% was observed, of 8.3% in the total hip, of 7.8% in the femoral neck and of 3.5% in the radius (all: p<0.05). In comparative trials with various bisphosphonates (at 12 months) in patients with no previous treatment or managed oral bisphosphonates, larger improvements in the BMD were observed with denosumab.128–130 It is not possible to establish a comparison in terms of the protective effect against fractures.
The 10-year extension trial with denosumab showed a 21% gain in bone density in the lumbar spine in the group receiving denosumab uninterruptedly since the FREEDOM trial, and a 16.5% improvement in the group that crossed over from placebo to denosumab. The improvement in bone density of the hip was of 9.7 and 7.4%, respectively.131,132
In the primary trial, a higher incidence of eczema, cellulitis (excluding erysipelas) and flatulence was observed. There was no difference with regards to placebo in terms of serious adverse events such as infections, cardiovascular, cerebrovascular events, or cancer. No cases of maxillary osteonecrosis were reported.
A systematic review of 15 trials that reported adverse events associated with the use of denosumab included 10 comparative studies with placebo (5951 patients with denosumab, 5671 with placebo) and 8 studies with active comparator (3407 with denosumab, 2894 with comparator). Serious adverse vents were reported in 23.8% of the patients receiving denosumab vs. 24.9% of the patients receiving placebo. No significant differences were found in terms of adverse events associates with cancer or infections.133
In the FREEDOM open extension study, 13 cases of maxillary osteonecrosis were reported: 7 in the group of patients that completed 10 continuous years with denosumab and 6 in the crossover group (placebo for 3 years, followed by denosumab for 7 años). The incidence of osteonecrosis was of 5.23 per every 10,000 patient years. Until may 2014, the estimate of denosumab exposure was 1,960,405 patient years and 47 cases of maxillary osteonecrosis were reported; 38 of these patients had previously received bisphosphonates.134
Denosumab is contraindicated in patients with a history of hypocalcemia, patients with renal failure have a higher risk of hypocalcemia and should be monitored.125 However, since denosumab is a monoclonal antibody, it may be used in patients with a GFR <30vml/min with a contraindication for the use of bisphosphonates.
A retrospective study of Medicare patients in the United States, assessed the rate of hospital admissions of patients with rheumatoid arthritis treated with biologics (infliximab or abatacept) that initiated denosumab (n=1354) or zoledronic acid (n=4460). There was no increase in the rate of infections requiring hospitalization in both groups (denosumab: 14.9 per every 100 patient years; zoledronic acid: 13.9 per every 100 patient years).135
The antiresorption of denosumab is reversible. Following its discontinuation, there may be a rebound effect with vertebral fragility fractures as a result of the over-activation of bone resorption.136–138
- ∘
Teriparatide:
This consensus recommends the use of teriparatide as first line therapy in any of the following circumstances:
- •
In patients with vertebral fragility fractures (one severe or 3 or more with any level of severity), ruling out all secondary causes of vertebral fracture.
- •
In patients with a T-score of the lumbar spine ≤−3.5.
Teriparatide is approved as first line therapy in women with postmenopausal osteoporosis with a high risk of fracture. It is indicated in case of treatment failure with the antiresorptive agents.139 Teriparatide reduced the incidence of new and non-vertebral vertebral fractures in the Fracture Prevention Trial. After a mean administration of the medication of 21 months, 5% of the patients receiving 20μg of teriparatide experienced at least one new vertebral fracture, in contrast with 14% in the placebo group (relative risk reduction of 65%).140
In the case of multiple vertebral fractures, the risk reduction was 77%, and of moderate to severe vertebral fractures, 90%.140 When reassessing the x-rays of the primary trial with the quantitative morphometric technique, the risk reduction of new vertebral fractures was 84%.141 The pharmaco-economics studies conducted from the perspective of the United Kingdom and the United States payers, have shown that teriparatide therapy is cost-effective in women over 65 years old, with a very high risk of fracture (T-score <−3 and prevalent fragility fracture), but not in women with a lower risk of fracture, due to the high cost of the medication and the small increases in quality of life adjusted life years (QALY), as compared against other therapies.142
Non-vertebral fragility fractures occurred in 3% of the patients with teriparatide and in 6% of the patients receiving placebo in the primary study, for a relative risk of 0.47 with 20μg of teriparatide (95% CI: 0.25–0.88).140
Significant BMD improvements were achieved with teriparatide at the level of the lumbar spine (9.7% vs. 1.1% in the patients receiving placebo); in the same study, there was also an increase in BMD at the level of the femoral neck, though it was less significant.140
Vertebral fractures are the most frequent fragility fractures and may cause back pain with functional limitation and impaired quality of life. A systematic review included 5 controlled clinical trials. The rates of back pain in general, moderate to severe back pain, and svere back pain per every 100 patient years were lower in the teriparatide-treated group of patients (relative risk reduction [RR] for any back pain: 0.66 [95% CI: 0.55–0.80]; moderate to severe back pain: 0.60 [95% CI: 0.48–0.75]; severe back pain: 0.44 [95% CI: 0.28–0.68]).143 This effect persists after discontinuation of treatment and has been observed for up to 30 months.144
When is the use of combined therapy for the treatment of patients with postmenopausal osteoporosis considered?Until now, there is no evidence of risk reduction of fracture with the combination of an antiresorptive and an anabolic agent; however, there is evidence of an increase in BMD with the combination, versus individual therapy. This consensus dos not recommend the use of this combination for now.145
Which are the most frequent adverse events of treatments for osteoporosis?Adverse effects exclusive of bisphosphonatesUpper GI tract adverse effectsOral bisphosphonates may cause esophagitis, esophageal ulcers, and even bleeding. Up to 20–25% of the patients treated with daily formulations may complain about dyspeptic disorders. All of these upper GI effects are less frequent with the weekly or monthly formulations, and may be significantly avoided if the drug is properly taken (with a glass of water and not lying down for the next 30–60min). There is no evidence to conclude an association with esophageal cancer.
Acute phase reactionAcute phase reactions have been basically described following the administration of IV bisphosphonates. This comprises flue-like manifestations, with fever, headache, myalgia and arthralgia. These manifestations usually present between 24–36h following the administration of the medication and usually regress in 3 days. They occur in 25–35% of patients receiving zoledronic acid for the first time. The incidence and intensity of the manifestations declines in subsequent administrations. The use of acetaminophen prior to injecting the drug and for 24–48h after the administration,146 as well as adequate previous hydration, reduce the occurrence of these episodes by 50%.
Atrial fibrillationOriginally described with zoledronic acid in the HORIZON trial147 and apparently reaffirmed as well for alendronate in a case control trial.148 However, a second clinical trial with zoledronic acid and the cancer trials in which the patients received much higher doses of bisphosphonates failed to confirm these findings. Neither have they been confirmed in post hoc analyses of the key trials with alendronate, risedronate or ibandronate. Nevertheless, two recent meta-analyses do suggest an increased incidence in atrial fibrillation, though not accompanied by an increase in the frequency of stroke or mortality.149,150
Renal failureThe nephrotoxicity of bisphosphonates is mostly related to the serum levels reached, hence it is only a problem with IV bisphosphonates.151 The simultaneous use of other potentially nephrotoxic agents (NSAIDs, diuretics) encourage the development of nephrotoxicity; the presence of pre-existing renal failure (hence the need to measure GFR before their administration), the presence of dehydration at the time of the injection and the fact that the dehydration developed excessively fast (the recommendation for zoledronic acid is an infusion time >15min, since shorter times have shown to increase the incidence of elevated serum creatinine levels). Some authors have suggested evaluating the renal function at some point during the days following the administration of the agent. Bisphosphonates are not recommended in patients with glomerular filtration rates below 30ml/min. None of the trials with oral bisphosphonates have included patients with filtration rates below 15ml/min (stage 5 of chronic renal failure).
Ocular adverse effectsDifferent types of ocular inflammatory reactions have been described, including conjunctivitis, uveitis, iritis, episcleritis, scleritis or keratitis. These are rare complications reported in about 0.05 and 1% of the cases.152–154
They may develop with either oral or IV bisphosphonates, and the time of occurrence ranges from hours to years.
One trial155 has indicated that the incidence of visual inflammatory processes is similar in patients with osteoporosis treated with bisphosphonates or treated with other drugs (raloxifene, strontium ranelate). In any case, upon the occurrence of these inflammatory processes, treatment discontinuation is mandatory.
Shared adverse effects of bisphosphonates and denosumabHypocalcemiaIn general, bisphosphonates and denosumab may cause a slight drop in calcemia, with no clinical impact.156,157 There are several circumstances in which they may cause clinically significant hypocalcemia: IV administration, drop in GFR, vitamin D deficit, previous tendency to low levels of calcemia (hypomagnesemia) and extremely high turnover (Paget's disease). In the particular case of denosumab, chronic renal failure.
Prior to the administration of bisphosphonates, it is wise to make sure that the patient receives vitamin D supplementation, that calcemia is normal, and that the GFR is >30ml/min/m2. These precautions are particularly important in the case of IV bisphosphonates, for which additionally creatinine and serum calcium values should be measured, following the injection of the medication.
Maxillary osteonecrosisThis is defined as an area of bone exposure in the maxillofacial region that won’t heal after 8 weeks, in a patient receiving bisphosphonates or denosumab therapy.
Maxillary osteonecrosis may relapse in individuals that have not been exposed to these drugs. The lesions develop more often in the mandible rather than in the maxilla.
The risk of maxillary osteonecrosis in patients treated with antiresorptives for osteoporosis is very low (1/1500–1/100,000 patients – year, according to the trials), and its decelopment is associated with poor oral hygiene (periodontitis) and having sustained dental trauma. The bone turnover markers (CTX) are not helpful to identify individuals at risk.158
If the recommendation is to maintain antiresorptive therapy against osteoporosis, then therapy must be continued. The introduction of treatment holidays does not reduce the risk (in the case of bisphosphonates).
If the patient requires oral treatment, it should be initiated immediately. The treatment should be minimally invasive, and in case of a long treatment, it may be advisable to do it in stages.
Atypical femoral fracturesAtypical femur fractures are similar to maxillary osteonecrosis in terms of basically presenting in patients treated for an extended period of time with bisphosphonates or denosumab. The ASBMR establishes the following requirements159:
- •
Localization between the lesser trochanter and the suprachondylar ridge
- •
Presence of at least 4 of the 5 major criteria listed hereunder; none of the minor criteria are required.
The major criteria include:
- •
Minimal or absent trauma
- •
Fracture line originating at the outer lateral cortex with transverse orientation, though it may become oblique as it progresses through the femur
- •
Minimal or absent comminution
- •
Localized thickening of the endosteum or periosteum in the outer cortex.
- •
Outer cortex affected (incomplete fracture) or both cortices affected (complete fracture)
The following are considered minor criteria:
- •
Generalized increase in cortical thickness of the femoral diaphysis
- •
Presence of prodromal unilateral or bilateral symptoms (such as dull pain in the groin or the thigh)
- •
Bilateral fracture
- •
Delayed fracture healing
The incidence increases with the use of these drugs and with exposure time (the mean exposure to bisphosphonates when an atypical femur fracture develops is 5–7 years). The relative risk may be extremely high (the figures reported range between 2.1 and 128), but the absolute risk is small (around 5 and 100 per 100,000 patient years, according to the studies). The incidence drops rapidly after removing the medication. Some have mentioned an association with the use of glucocorticoids.160
If a cortical thickening area is observed in a patient, an MRI should be done in order to identify the presence of a cortical fracture and bone marrow edema, which are indicative of a stress fracture. A previous bone scan may help to show increased focal uptake in the femoral diaphysis. If the presence of any of these alterations is confirmed, discontinue the use of antiresorptive therapy; if the femur is fractured, proceed to operate on the patient.
TeriparatideA higher incidence of osteosarcoma has not been reported in patients treated with teriparatide versus the general population, since the launch of these medication. The most frequent adverse events are nausea, cramps, and orthostatic hypotension, which are all usually mild and transient. Hypercalcemia has been observed, but it is usually mild, transient, and asymptomatic.161
Great care should be exercised with teriparatide in patients at high risk of developing osteosarcoma.
Teriparatide is contraindicated in children, adolescents, and young adults in whom the epiphyseal closure has not been completed; in bone Paget's disease, in cases of unexplained alkaline phosphatase elevation, patients with history of osteosarcoma or any primary or metastatic cancer that have been exposed to radiation therapy compromising the bone. Teriparatide is contraindicated during pregnancy and lactation.
Patients with GFR TFG <30ml/min present elevated plasma concentration of the drug. No efficacy trials have been conducted in patients with end-stage kidney disease. Teriparatide is contraindicated in patients with primary hyperparathyroidism or with uncorrected secondary hyperparathyroidism in patients with osteomalacia.162 Neither is the use of teriparatide recommended in patients with lytic changes associated with multiple myeloma or any other hematological malignancy. Teriparatide may cause hypercalcemia.
As soon as teriparatide is discontinued, its benefits are rapidly lost and hence immediately after the last dose, an antiresorptive agent must follow.96
Duration of treatmentOsteoporosis is a chronic condition that will require some type of continuous treatment; however, this does not mean that the same drug should be administered throughout that time. This consensus believes that the key objective of treatment shall be to reduce the risk of fragility fractures.
Along these lines, this consensus recommends:
- •
Patients treated with bisphosphonates shall be evaluated for continuity of treatment after 3 years (intravenous) or after 5 years (oral) of initiation of therapy.
- •
Under any of the following circumstances, the patient should continue receiving treatment
- •
Absence of fractures but a T-score of the femoral neck <−2.5 SD
- •
Development of any osteoporotic fracture during treatment
- •
Presence of fragility fractures before the end of this period
If none of these circumstances are present, treatment may be removed. If the treatment is maintained, then regular re-assessments must be conducted to consider the possibility of discontinuation. The recommendation is once a year.
The consensus recommends a time limit of 10 years with alendronate (low recommendation),93 up to 7 years with risedronate,104 and up to 6 years with zoledronic acid,110 since there are no studies available on the efficacy of the medications beyond thaat time and after that period, the possibility of atypical femoral fracture may rise.
If the decision is made to discontinue antiresorptive therapy but the patient is still at risk, the antiresorptive agent should be replaced by another drug with a different mechanism of action, such as teriparatide.
When removing bisphosphonate therapy, discontinuation shall only be temporary (holidays) and never beyond 5 years.
The recommendation to take a “holiday” is not applicable to risedronate or ibandronate, since these agents have not been studied in this context and their affect fades out fairly quickly.
It has been suggested that BD control may help in making the decision: if the BMD continues above the “target” value (i.e., T-score −2.5), may be patient can be maintained without treatment.
In the case of denosumab, treatment holidays are not strictly recommended, since upon discontinuation, there is no residual effect and additionally the bone turnover rises above the baseline values (“rebound effect”).136,163 If the decision is made to discontinue denosumab, starting an alternative treatment is recommended.
What is therapeutic failure?In patients receiving treatment and when no new fragility fractures have developed, the BMD has increased, and the remodeling markers have dropped, the assumption is that the response has been adequate. Nevertheless, in patients with good compliance, the failure rate may be of up to 32%.164
In the absence of compliance issues, and upon ruling out any secondary causes, the following circumstances are considered therapeutic failure:
- •
Two or more new fragility fractures during the first 12 months of treatment
- •
One or more new fragility fractures since month 13 of treatment
- •
One fragility fracture plus elevated resorption markers or significant decrease (>3% between two serial measurements, ideally at the same institution, with the same technician, and less than one year apart) of the BMD of the hip.
In case of therapeutic failure, the first step is to check for compliance, any secondary causes of osteoporosis and external risk factors such as smoking, glucocorticoids use, etc., which are the main causes of unsatisfactory treatment response.164
This consensus makes the following recommendations for change:
- •
A bisphosphonate shall be replaced by denosumab is the failure to treatment presented as non-vertebral fracture, or by teriparatide if a vertebral fracture develops.
- •
In case of failure with denosumab, the patient shall be referred for evaluation by a specialist, since she could continue with teriparatide.
- •
Any treatment starting with teriparatide shall continues with a potent antiresorptive such as zoledronic acid or denosumab.
The recommendations on this point are summarized in Fig. 4.
Non-pharmacological measuresIs exercise effective to reduce the risk of falls?Yes. Exercise, considered as the time devoted to planned physical, structured, and repetitive physical activity, has shown a strong correlation with the primary prevention of osteoporosis and the secondary prevention of the consequences thereof.165
In order to reduce the risk of falls in the presence of osteoporosis and osteopenia, it is necessary to develop bone mass, both in terms of quantity and quality. In the case of women, the best choice is to develop that bone mass before the onset of menopause. Indirectly, while doing physical exercise to improve bone quality, other aspects tend to improve, such as the quality of the muscle mass and proprioception, hence avoiding the risk of falls.166–168
The objectives of physical activity and exercise in osteoporosis are166,169:
- •
Increasing the bone mass and the muscle mass
- •
Reducing bone loss
- •
Reducing the risk of falls
- •
Ensuring patient safety
The risk factors to experience falls are:
- •
Visual, neurological, and musculoskeletal disorders
- •
Disability
- •
Muscle weakness
- •
Certain psychotropic drugs
- •
Postural hypotension
- •
Poor proprioception
- •
Environmental risks
- •
Cognitive dysfunction
- •
Gait disorders
Falls are a significantly important risk factor for fractures which increases with aging: 33.3% of individuals aged 65 or above, experience one fall/year. It has been proven that physical fitness prevents the prevalence of falls. An adequate exercise prescription shall include proprioception training and muscle strengthening; Tai Chi166 has been reported as one of the highly effective sport modalities.
Therapeutic exercise shall meet one objective based on a previous and comprehensive evaluation of the patient, and most comply with the following parameters:
- •
Be safe for the patient
- •
Prevent falls and fractures
- •
Arrest of delay the progression of muscle mass loss
Yes, the objectives of exercise in primary and secondary prevention of falls are:
Increased bone massThe more advantage an individual takes of his/her bone mass gain peak, the higher the reserves for the future; this highlights the years of adolescence and young adulthood (before age 30), when the objective is bone mass gain, in contrast with the postmenopausal woman in whom the objective is to decrease the loss of bone mass. While genetics is a key variable in determining bone mass, 30% is independent from genetic factors.
It has been found in trials with school children that the mere inclusion of plyometric exercises (exercises involving fast and explosive jumping, including coordination, strength, and aerobics) has beneficial bone health results. In general, the recommendation is to practice any kind of sport during childhood and adolescence. The benefits of exercise on the bone mass as best achieved with an adequate nutritional intake. It has been shown in adolescents that the impact of exercise on the bone improved when combined with an intake of 1200mg/day of calcium. Increasing the bone mass by 10% during the peak of young adulthood, could delay the development of osteoporosis for 13 years, and reduce the risk of fractures by 50%.166,169,170
Reduce bone lossUpon reaching menopause, it becomes critical to implement strategies to reduce the rate of bone mass loss, adopting exercise routines; however, walking alone is not effective to prevent bone loss or to encourage bone gain. This physical activity contributes with other types of cardiovascular benefits. Exercise during menopause shall ideally be prescribed by a doctor specialized in physical activity and sports medicine, who not only prescribes exercise objectively, but individualizes the prescription assessing cardiovascular, metabolic, osteomuscular, and congenital risks.166
Together with exercising, adequate intake of 1200mg/day of calcium, as well as 800IU/day of vitamin D and protein in the diet, are necessary to prevent fragility fractures.
What type of exercise if recommended for the primary and secondary prevention of osteoporosis- •
Exercise test, ergometry, or stress test
There is no specific modality in a setting of osteoporosis. Doing these tests prior to prescribing exercise depends on the opinion of the sports medicine practitioner or exercise physiology doctor. To this end, the American College of Sport Medicine (ACSM) establishes the following special considerations167,169,171:
- •
The cycle ergometer is recommended as an alternative to the treadmill in patients with severe vertebral osteoporosis, particularly if they experience painful ambulation.
- •
Vertebral fractures may result in spinal deformity with displacement of the center of gravity. This affects the stability on the treadmill, and it may be necessary to consider a parapet or other options.
- •
Maximum strength tests may be contraindicated in severe osteoporosis; however, there are no guidelines to support this contraindication.
- •
Exercise prescription (FITT principle: frequency intensity, time, and type)
- •
Patients in whom we want to prevent osteoporosis:
- ∘
Frequency: weight-nearing aerobic exercise 3-5 times/week; strength exercise (muscular endurance) 2–3 times/week.
- ∘
Intensity: moderate-vigorous aerobics (≥60% VO2 or MHR reserve); moderate strength exercise (60–80% 1 maximum repetition) with 8–12 repetitions, or vigorous strength exercise (80–90% 1 maximum repetition) with 5–6 repetitions that always involve major muscle groups.
- ∘
Time: the recommended duration is 30–60min between aerobics and strength exercise.
- ∘
Type: the recommended modes of training are: aerobics, tennis, walking up and down the stairs, walking combined with jogging, and exercises involving jumping such as volleyball, basketball, and plyometrics; strength exercises such as weight lifting, machines, suspension exercise, self-loading, functional, inter alia.
- ∘
- •
Patients with osteoporosis:
- ∘
Frequency: weight-bearing aerobics 3–5 times/week; strength exercise (muscle endurance) 2–3 weeks/week.
- ∘
Intensity: moderate aerobics (40–60% VO2 or MHR reserve); moderate strength exercise (60–80% 1-MR) with 8–12 repetitions; however, some individuals may be able to tolerate higher intensities.
- ∘
Time: the recommended duration is 30–60min between the aerobics and strength components.
- ∘
Type: easy to control impact aerobics such as walking, jogging, pilates, Tai Chi, inter alea.
- ∘
- •
Sports that involve risk of falls or contact sports are not recommended; for example: cycling, skiing, horseback riding, soccer, handball, judo, karate, or boxing, inter alia. Swimming has shown any benefits in bone mass gain.171
- ∘
Recommendation: warm-up and cool-down
- ∘
Coordination and balance exercises
- ∘
Exercises focusing on the trunk and abdomen muscles
- ∘
Avoid a high level of activity
- ∘
Self-loading exercises (with your own weight).
Special considerations in patients with osteoporosis u osteopenia:
Progressive intensity
- ∘
Avoid explosive movements or high impact loading
- ∘
Avoid twisting, flexing or compressing the spine
- ∘
Balance and flexibility activities
- ∘
Level of experience with the exercise
- ∘
Self-monitoring ability
- ∘
- •
Proposal of a general exercise plan in osteoporosis:
- •
15min warm-up, including stretching, coordination and balance exercises
- •
Basic workout:
- •
20–40min of aerobics and team sports; or:
- •
30min of weight-bearing exercise of all muscle groups and back and abdomen exercises
- •
5–15min of relaxing exercises and stretching
- •
3–5 days per week
- •
Rotate aerobics with weight-bearing sessions
- •
Individualized progression based on the evaluation by physical activity and sports medicine
It is recommended to refer the patient to a sports medicine doctor to issue an objective and individualized prescription, in order to obtain the best benefits. For rehabilitation of patients with osteoporotic fractures, refer the patient to a physiatrist.
Does discontinuing alcohol use reduce the risk of osteoporosis?The results of the studies on the effects of alcohol consumption on bone health are varied; however, most conclude that mild to moderate alcohol use (less than 3 glasses per week or 14g or less of ethanol in women and 28g or less in men) is beneficial, and increases BMD, while reducing the risk of fracture.172–178 Heavy alcohol consumption (more than 70g per day or most days, or both) is associated with a decrease in BMD, bone quality disruption and increased risk of fractures.179
Alcohol discontinuation has shown changes in bone markers and in BMD.179
Are braces helpful for the treatment of vertebral fractures from postmenopausal osteoporosis?Conservative therapy for spinal fractures due to osteoporosis, including braces, is the most widely used. However, the use of corsets is not free of complications,180 such as pressure ulcers and subsequent infection, brace intolerance, reduced pulmonary capacity and axial muscular atrophy. It is indicated for a period of 2–3 months180,181 and regular clinical radiological controls should be conducted during its use to assess progress of pain and of the fracture.
The use of braces for the treatment of thoracolumbar spine fractures is not clear at present, and further trials are required.180–183
Conflict of interestNo conflict of interest to disclose
General coordinators
Adriana Medina Orjuela
Service of Endocrinology – Hospital de San José, School of Medicine, Fundación Universitaria de Ciencias de La Salud, Bogotá, Colombia
Óscar Rosero Olarte
Osteoporosis Research Center, Osteollanos, Villavicencio, Colombia
Theme coordinators
Pedro Nel Rueda Plata
Definition and Epidemiology Chapter
Instituto Endocrinológico de Colombia, Bogotá, Colombia
Fabio Sánchez Escobar
Diagnostic chapter
Gynecology, Universidad de Antioquia, Medellín, Colombia
Monique Chalem Choueka
Pharmacological therapy chapter
Rheumatology section, Hospital Universitario Fundación Santa Fe de Bogotá, Instituto de Reumatología, Bogotá, Colombia
Miguel Ángel González Reyes
Chapter on non-pharmacologic measures
Geriatrics orthopedics and traumatology, Unidad Médica Cecimin, School of Medicine, Fundación Universitaria Sanitas, Bogotá, Colombia
Special collaborators
Patricia Clark
Clinical Epidemiology Unit, Hospital Infantil de México, Clinical Epidemiology, School of Medicine, Universidad Nacional Autónoma de México, México DF, México
Orlando Angulo Ceballos
School of Medicine, Fundación Universitaria de Ciencias de la Salud, Bogotá, Colombia, Universidad del Norte de Barranquilla, Barranquilla, Colombia
Carlos Federico Molina Castaño
School of Medicine, Universidad CES y Universidad de Antioquia, Medellín, Colombia
Please cite this article as: Medina Orjuela A, Rosero Olarte Ó, Rueda Plata PN, Sánchez Escobar F, Chalem Choueka M, González Reyes MÁ, et al. II Consenso Colombiano para el Manejo de la Osteoporosis Posmenopáusica. Rev Colomb Reumatol. 2018;25:184–210.