Periodontal disease (PD) and rheumatoid arthritis (RA) are multifactorial chronic inflammatory diseases that share similar aetiopathogenic mechanisms that lead to the destruction of both dental-alveolar tissues and synovial joints, in such way that antibodies against periodontal pathogens have been identified in the crevicular fluid and in the synovial fluid and membranes.
ObjectiveTo identify, recover, critically analyze and synthesize the available literature on the prevalence of periodontal microorganisms in synovial fluid of patients with RA.
Materials and methodsA systematic search was performed in MEDLINE, ScienceDirect, SciELO and Google Scholar using the medical subject headings “Rheumatoid arthritis”, “periodontal microorganisms” and “synovial fluid”. Articles were included that described the presence of isolated periodontal pathogens in synovial fluid of patients diagnosed with RA. The search was closed in February 2017 and was performed using PRISMA methodology. The OSTEBA critical reading sheets were used to assess the external validity and level of evidence of each article in terms of methodological rigor.
ResultsA total of 14 publications were included that described the presence of periodontal pathogens in synovial fluid of patients with PD and RA. Seven publications detected periodontal pathogens in synovial fluid, with Porphyromonas gingivalis being positive in all of them.
ConclusionsThe included studies provided evidence of the presence of periodontal microorganisms in the synovial fluid in subjects with PD and RA, associating the prevalence of P. gingivalis with increased levels of anti-cyclic citrullinated peptide (anti-CCP) antibodies, which could exacerbate inflammatory processes and produce autoimmune reactions in RA.
La enfermedad periodontal (EP) y la artritis reumatoide (AR) son enfermedades inflamatorias crónicas multifactoriales que tienen en común algunos factores etiopatogénicos y la destrucción de los tejidos dentoalveolares y de las articulaciones sinoviales, de tal forma que se han identificado anticuerpos contra microorganismos periodontales en el fluido crevicular, líquido sinovial y en la membrana sinovial.
ObjetivoIdentificar, recuperar, analizar críticamente y sintetizar la literatura disponible acerca de la prevalencia de microorganismos periodontales en el líquido sinovial de pacientes con AR.
Materiales y métodosSe realizó búsqueda sistemática en Medline, ScienceDirect, SciELO y Google Scholar a través de los descriptores en salud Rheumatoid arthritis, periodontal microorganisms y synovial fluid. Se incluyeron artículos que describieron la presencia de microorganismos periodontales aislados en líquido sinovial de pacientes diagnosticados con AR. La búsqueda se cerró en febrero de 2017 y fue realizada con metodología PRISMA. Se emplearon las fichas de lectura crítica OSTEBA para valorar la validez externa y el nivel de evidencia de cada artículo en función del rigor metodológico.
ResultadosCatorce publicaciones describieron la presencia de microorganismos periodontales en líquido sinovial de pacientes con EP y AR. Seis publicaciones realizaron detección de microorganismos periodontales en muestras de líquido sinovial, identificando en todas a P. gingivalis.
ConclusionesLos estudios incluidos evidenciaron la presencia de microorganismos periodontales en el líquido sinovial en sujetos con EP y AR, asociando la prevalencia de P. gingivalis con el aumento de los niveles de anticuerpos anti-CCP, lo que podría exacerbar los procesos inflamatorios y producir reacciones autoinmunes en AR.
Periodontal disease (PD) is a multifactorial chronic infection that leads to the destruction of the teeth supporting tissues.1 This disease starts with the conformation of a dental biofilm or bacterial plaque, which stimulates the immune system and activates diverse inflammatory mechanisms in an attempt to stop the infection, in such a way that the majority of the associated destructive processes are due to an excessive host response to the bacterial challenge.2,3
The etiopathogenesis of PD implies an increase in facultative and anaerobic bacteria in the gingival sulcus around the teeth, in what we call the subgingival microbiome, constituted by pathogenic species such as Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus, Treponema denticola, Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum, among others, and some opportunistic pathogens such as enterobacteria and non-fermenting Gram-negative bacilli, that act together to initiate an immune-inflammatory process.2,3 This subgingival dental plaque or subgingival microbiome causes destruction of the periodontal supporting and protective tissues starting from the phases described by Page and Schroeder in 1971, and reviewed by Page and Kornman in 1994: (1) an initial lesion that marks the etiopathogenic course; (2) the progression towards an established lesion with clinical signs of gingivitis; and (3) the progress of a lesion that evidences the formation of the periodontal pocket or the gingival recession due to insertion loss, in addition to the loss of bone.4–8 In Colombia, the prevalence of PD is 73%, but if we include the cases of gingivitis the prevalence is 100% in the toothed adult population.9
Since the end of the last century the study of the etiopathogenesis of the PD and its interactions with the host has allowed to know that there is an association between PD and systemic diseases, focusing mainly in the relationship of the local and systemic inflammatory response based on markers such as proinflammatory cytokines—tumor necrosis factor alpha (TNF-α) and interleukines 1 (IL-1) and 6 (IL-6)—and the C-reactive protein (CRP), in pathological processes such as cardiovascular diseases, diabetes and its complications, adverse outcomes of pregnancy, respiratory tract infections and some rheumatologic diseases.10–14 Rheumatoid arthritis (RA) is included within the latter.14,15
RA is a systemic autoimmune polyarthritis, chronic and inflammatory, of multifactorial etiology, characterized by inflammation of the synovial membrane of the tendon sheaths and the synovial gliding bursae,15 causing the generalized destruction of the diarthrodial synovial joints, usually accompanied by joint pain and edema, which leads to physical disability, decrease in the quality of life of the individual, depression and premature death.14 Its prevalence varies between 1% and 5%, being in Colombia of 0.9% with a growing tendency, according with a study conducted in 2005 from the System of Information on the Provision of Health Services.16 Although the cause of RA is still discussed, environmental, genetic and endocrine risk factors involved in the pathogenesis of the disease have been identified.17,18 However, the available evidence suggests that there is a cause–effect relationship between the magnitude and type of immune response and RA, where the genetic predisposition is also important, and acute and chronic infections possibly have influence.15,19
Given that PD and RA are chronic inflammatory disorders characterized by bone destruction and production of proinflammatory cytokines, a pathophysiological link is plausible,20–23 moreover when levels of antibodies against oral anaerobic bacteria, specifically against P. gingivalis have been detected in the synovial fluid and the synovial membrane of patients with RA,24–26 and this microorganism has the ability to citrullinate the proteins and in this case to aggravate the RA. P. gingivalis produces citrullinated antigens in the tissues that are infected by it and the citrullination of the proteins is a characteristic of RA, which is exacerbated it in those patients with uncontrolled PD.27–29 Citrullination is a post-translational intracellular physiologic process in which an arginine is changed by a citrulline, in proteins with structural function such as filaggrin, different keratins (vimentin), some collagens, myelin basic protein, fibrin, (fibrinogen), α-enolase and histones, during processes such as keratinization, inflammation and apoptosis.30–32 This process of citrullination is carried out by the family of peptydil arginine deiminase (PAD) enzymes, of which 5 isoforms have been identified so far.33,34 However, since the PAD is not expressed in the thymus, the T-lymphocytes reactive to the citrullinated antigens can survive and generate a possible autoimmune reaction against citrullinated antigens in the rest of the body. However, under normal conditions the immune system does not react against self-antigens due to the process of central and peripheral immunological tolerance. In the case of individuals with RA, anti-cyclic citrullinated peptide antibodies (anti-CCP) are produced, which mediate the inflammatory processes by favoring the activation of T-lymphocytes.30,31
This inflammatory response, accentuated by the migration of leukocytes from the synovial capillaries to the compartment of synovial joints, causes the synovitis and activates the adaptive immune response, in which the T lymphocytes infiltrate the lesion.34 Although RA has been considered as a disease with a profile of Th1 cytokines, the attention has been focused currently on a Th17 profile and its ability to produce IL-17A, IL-17F, IL-21, IL-22 and TNF-α in the presence of a proinflammatory environment generated by the production of IL-1β, IL-6, IL-21, IL-23 and TGF-β by macrophages and dendritic cells of the synovial membrane, in which the differentiation and activity of regulatory T lymphocytes is reduced, and where by synergy of IL-17 and TNF-α, the synovial fibroblasts are activated.35 In this way, a Th1 and Th17 profile triggers the increase in IL-22 or Th22, which are observed increased in the synovial membrane of patients with RA as a consequence of the proliferation of synovial fibroblasts and of the osteoclastic hyperactivity due to overexpression of the receptor activator of nuclear factor Kappa-B ligand, which eventually increases the severity of the disease.36,37 The effector cells that are found in the synovial membrane (macrophages, mast cells and NK lymphocytes) and in the synovial fluid (neutrophils) activate the innate immune response. In the particular case of the macrophages, they constitute the central effector cells of the synovitis through the secretion of cytokines, reactive oxygen intermediates, prostanoids and extracellular matrix metalloproteinases (MMPs), in addition of the classical functions of phagocytosis and antigen presentation. This expression pattern activates mainly the M1 macrophages through the Toll-like receptors TLR2/6, TLR3, TLR4 and TLR8 by cytokines, interactions with T lymphocytes and immune complexes.38,39
In this way, the cells of the synovial membrane react by altering the structure through a hyperplastic response, where the type A synoviocytes (resident macrophages) and the type B synoviocytes (fibroblasts) develop a semiautonomous phenotype characterized by disassembling intercellular unions and increasing the proliferation capacity; in addition of expressing heat shock proteins and high levels of cytokines, chemokines, MMP and tissue inhibitors of metalloproteinases (TIMP), with which they contribute to the promotion of a microenvironment favorable for the effector T-lymphocytes, the survival of B-lymphocytes and the organization of the adaptive immune response.35 With the hyperplasia, the synovial membrane losses is protective effects and the degradation of the Articular cartilage begins, because the new phenotype of synovial fibroblast synthesizes a large amount of MMP that degrade the fibrillar and non-fibrillar components of the cartilaginous extracellular matrix, in such a way that the structural damage and the biomechanical dysfunction ensues in view of the physiological regenerative incapacity of the cartilage and the inefficiency of the TIMPs.40
In view of that, the most plausible model of the etiopathogenesis of RA suggests that during the apoptosis—promoted by the damage of the joint tissues—the integrity of the cell membrane is altered, allowing an increase in the concentration of intracellular calcium and the subsequent activation of the PAD enzymes, which initiate the citrullination of different proteins in the nucleus, the cytoplasm and the extracellular matrix.30,31 From the citrullinated proteins are generated the anti-CCP targeting neoantigens that form immune complexes and activate the complement system to recruit polymorphonuclear cells, monocytes, macrophages and mast cells in the synovial tissues, which promote the inflammation of the joints and lead to a “vicious circle” where the immune response causes the inflammatory process to become chronic and makes evident the damage to the joint tissues.41–44 In addition to this, it has been possible to identify that P. gingivalis expresses a series of virulence factors involved in the tissue colonization, invasion, establishment and persistence, which allow it to evade the mechanisms of the immune system generating periodontal damage.45,46 One of these factors are the gingipains, citrullinated proteases implied in the pathogenesis of PD given their ability to degrade host proteins (fibrinogen and α-enolase in periodontal tissues) to be used by the bacteria for its growth and metabolism, altering the cell morphology and function.47 In this way, P. gingivalis is the only prokaryote—until now—in which it has been identified a bacterial PAD enzyme activity similar in function to human PAD (but non-calcium dependent and at a high inflammatory pH), reason why this periodontal microorganism constitutes one of the direct candidates to associate PD and RA.47–52 Although recent studies have found that A. actinomycetemcomitans induces the citrullination of enzymes in the neutrophils through the secretion of leukotoxin A—cytotoxin that causes degeneration and necrosis of the leukocytes—, which generates pores on the cell membrane to mobilize calcium, hyperactivate the PAD action and produce neoantigens that exacerbate the immune response.53,54
While the PAD enzyme activity and the citrullination occur physiologically in human beings, in individuals with RA susceptible to HLA-DRB (major histocompatibility complex [MHC] class II) or PTPN22 (non-specific receptor for tyrosine phosphatase), environmental factors such as cigarette smoking and PD induce the pathological production of neoantigens that lead to the formation of anti-CCP and, therefore, loss of immune tolerance.41 However, the association between PD and RA is interesting because they have common immunopathological pathways. PD has an infectious origin and RA is autoimmune; however, in both conditions there is an increase in proinflammatory cytokines (TNF-α, IL-1 and IL-6) and of MMP. The citrullinated proteins, then, either by human PAD of bacterial PAD enzyme activity are considered as neoantigens because they do not make their selection in the thymus. T-lymphocytes have MHC class ii restriction to differentiate between non-citrullinated and citrullinated peptides presented by professional antigen-presenting cells.55–59
However, one of the least studied aspects of the relationship between PD and RA is the site where P. gingivalis acts, since the anti-CCPs that contribute to the loss of immune tolerance can disseminate in the blood perpetuating, in this way, the autoimmune process of the RA, no matter that their pathogenic ecological niche (periodontal tissues) is distant from the synovial joints.58,59 Nevertheless, different studies have been able to detect P. gingivalis in synovial tissues and synovial fluid, due to its potential to generate gingival bleeding and bacteriemia,59 or to induce phagocytosis by macrophages and dendritic cells, and thus disseminate through the bloodstream.60–64 In this sense, if RA is the most common inflammatory joint disease worldwide and its etiopathogenic course leads to deformities of the joints, functional disability and decrease in life expectancy, the treatment plans should include, from a biopsychosocial approach, not only the identification of the genetic predisposition and the control of the typical environmental factors (for example, cigarette smoking), but also the control of the inflammatory states in other body sites.65 In this way, the protocols of the guidelines of clinical practice for patients with RA should include the control of periodontitis, since by treating the latter, the anti-CCP antibodies specific for P. gingivalis66–69 are reduced and the quality of life of the patients with RA is improved.70
Therefore, the objective of this systematic review is to identify, recover, critically analyze and synthetize the available literature on the prevalence of periodontal microorganisms in the synovial fluid of patients with RA.
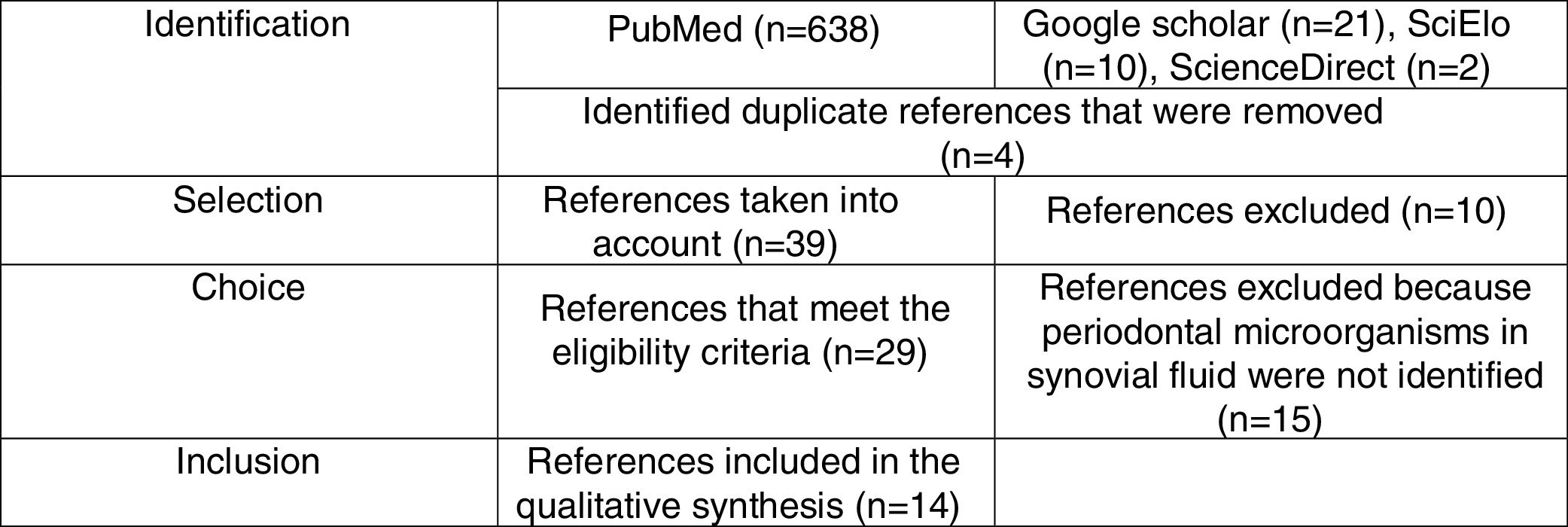
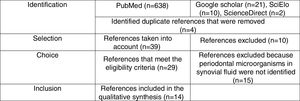
Materials and methodsA systematic literature review was conducted in Medline, Science Direct, SciELO and Google Scholar through the MeSH health descriptors Rheumatoid arthritis, periodontal microorganisms and synovial fluid combined with the Boolean connector and as well as with the filter humans. The health descriptors used were verified in the MeSH on Demand through the question Are periodontal microorganisms present in synovial fluid of patients with rheumatoid arthritis? The search was based on clinical assays, descriptive studies, systematic reviews and literature review. We included articles that identified the presence of periodontal microorganisms classified into complexes according with the order of appearance (colonization) in the biofilm,71 isolated from the synovial fluid of adult patients diagnosed with RA—without considering the evolution—, of both sexes and which did not take into account comorbidities. The systematic search closed in February, 2017 and was performed using the PRISMA methodology.72 (Fig. 1). For each of the articles, demographic (authors, year of publication and country of origin of the sample), methodological (type of study, sample, diagnoses of PD and RA, methods for the detection of periodontal pathogens and conclusions) variables and periodontal microorganisms (identified species and their prevalence) were taken into account. (Table 1).
Articles obtained through the systematic search using the PRISMA methodology.
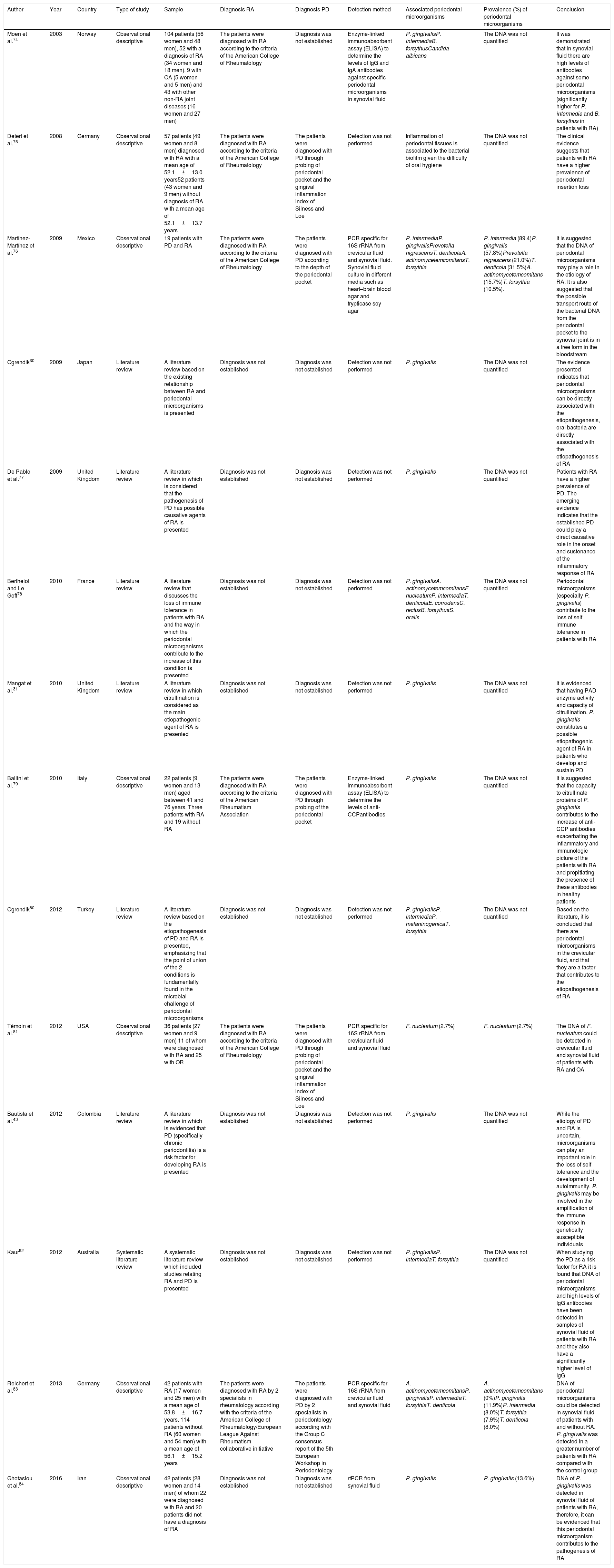
| Author | Year | Country | Type of study | Sample | Diagnosis RA | Diagnosis PD | Detection method | Associated periodontal microorganisms | Prevalence (%) of periodontal microorganisms | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Moen et al.74 | 2003 | Norway | Observational descriptive | 104 patients (56 women and 48 men), 52 with a diagnosis of RA (34 women and 18 men), 9 with OA (5 women and 5 men) and 43 with other non-RA joint diseases (16 women and 27 men) | The patients were diagnosed with RA according to the criteria of the American College of Rheumatology | Diagnosis was not established | Enzyme-linked immunoabsorbent assay (ELISA) to determine the levels of IgG and IgA antibodies against specific periodontal microorganisms in synovial fluid | P. gingivalisP. intermediaB. forsythusCandida albicans | The DNA was not quantified | It was demonstrated that in synovial fluid there are high levels of antibodies against some periodontal microorganisms (significantly higher for P. intermedia and B. forsythus in patients with RA) |
| Detert et al.75 | 2008 | Germany | Observational descriptive | 57 patients (49 women and 8 men) diagnosed with RA with a mean age of 52.1±13.0 years52 patients (43 women and 9 men) without diagnosis of RA with a mean age of 52.1±13.7 years | The patients were diagnosed with RA according to the criteria of the American College of Rheumatology | The patients were diagnosed with PD through probing of periodontal pocket and the gingival inflammation index of Silness and Loe | Detection was not performed | Inflammation of periodontal tissues is associated to the bacterial biofilm given the difficulty of oral hygiene | The DNA was not quantified | The clinical evidence suggests that patients with RA have a higher prevalence of periodontal insertion loss |
| Martínez-Martínez et al.76 | 2009 | Mexico | Observational descriptive | 19 patients with PD and RA | The patients were diagnosed with RA according to the criteria of the American College of Rheumatology | The patients were diagnosed with PD according to the depth of the periodontal pocket | PCR specific for 16S rRNA from crevicular fluid and synovial fluid. Synovial fluid culture in different media such as heart–brain blood agar and trypticase soy agar | P. intermediaP. gingivalisPrevotella nigrescensT. denticolaA. actinomycetemcomitansT. forsythia | P. intermedia (89.4)P. gingivalis (57.8%)Prevotella nigrescens (21.0%)T. denticola (31.5%)A. actinomycetemcomitans (15.7%)T. forsythia (10.5%). | It is suggested that the DNA of periodontal microorganisms may play a role in the etiology of RA. It is also suggested that the possible transport route of the bacterial DNA from the periodontal pocket to the synovial joint is in a free form in the bloodstream |
| Ogrendik80 | 2009 | Japan | Literature review | A literature review based on the existing relationship between RA and periodontal microorganisms is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalis | The DNA was not quantified | The evidence presented indicates that periodontal microorganisms can be directly associated with the etiopathogenesis, oral bacteria are directly associated with the etiopathogenesis of RA |
| De Pablo et al.77 | 2009 | United Kingdom | Literature review | A literature review in which is considered that the pathogenesis of PD has possible causative agents of RA is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalis | The DNA was not quantified | Patients with RA have a higher prevalence of PD. The emerging evidence indicates that the established PD could play a direct causative role in the onset and sustenance of the inflammatory response of RA |
| Berthelot and Le Goff78 | 2010 | France | Literature review | A literature review that discusses the loss of immune tolerance in patients with RA and the way in which the periodontal microorganisms contribute to the increase of this condition is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalisA. actinomycetemcomitansF. nucleatumP. intermediaT. denticolaE. corrodensC. rectusB. forsythusS. oralis | The DNA was not quantified | Periodontal microorganisms (especially P. gingivalis) contribute to the loss of self immune tolerance in patients with RA |
| Mangat et al.31 | 2010 | United Kingdom | Literature review | A literature review in which citrullination is considered as the main etiopathogenic agent of RA is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalis | The DNA was not quantified | It is evidenced that having PAD enzyme activity and capacity of citrullination, P. gingivalis constitutes a possible etiopathogenic agent of RA in patients who develop and sustain PD |
| Ballini et al.79 | 2010 | Italy | Observational descriptive | 22 patients (9 women and 13 men) aged between 41 and 76 years. Three patients with RA and 19 without RA | The patients were diagnosed with RA according to the criteria of the American Rheumatism Association | The patients were diagnosed with PD through probing of the periodontal pocket | Enzyme-linked immunoabsorbent assay (ELISA) to determine the levels of anti-CCPantibodies | P. gingivalis | The DNA was not quantified | It is suggested that the capacity to citrullinate proteins of P. gingivalis contributes to the increase of anti-CCP antibodies exacerbating the inflammatory and immunologic picture of the patients with RA and propitiating the presence of these antibodies in healthy patients |
| Ogrendik80 | 2012 | Turkey | Literature review | A literature review based on the etiopathogenesis of PD and RA is presented, emphasizing that the point of union of the 2 conditions is fundamentally found in the microbial challenge of periodontal microorganisms | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalisP. intermediaP. melaninogenicaT. forsythia | The DNA was not quantified | Based on the literature, it is concluded that there are periodontal microorganisms in the crevicular fluid, and that they are a factor that contributes to the etiopathogenesis of RA |
| Témoin et al.81 | 2012 | USA | Observational descriptive | 36 patients (27 women and 9 men) 11 of whom were diagnosed with RA and 25 with OR | The patients were diagnosed with RA according to the criteria of the American College of Rheumatology | The patients were diagnosed with PD through probing of periodontal pocket and the gingival inflammation index of Silness and Loe | PCR specific for 16S rRNA from crevicular fluid and synovial fluid | F. nucleatum (2.7%) | F. nucleatum (2.7%) | The DNA of F. nucleatum could be detected in crevicular fluid and synovial fluid of patients with RA and OA |
| Bautista et al.43 | 2012 | Colombia | Literature review | A literature review in which is evidenced that PD (specifically chronic periodontitis) is a risk factor for developing RA is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalis | The DNA was not quantified | While the etiology of PD and RA is uncertain, microorganisms can play an important role in the loss of self tolerance and the development of autoimmunity. P. gingivalis may be involved in the amplification of the immune response in genetically susceptible individuals |
| Kaur82 | 2012 | Australia | Systematic literature review | A systematic literature review which included studies relating RA and PD is presented | Diagnosis was not established | Diagnosis was not established | Detection was not performed | P. gingivalisP. intermediaT. forsythia | The DNA was not quantified | When studying the PD as a risk factor for RA it is found that DNA of periodontal microorganisms and high levels of IgG antibodies have been detected in samples of synovial fluid of patients with RA and they also have a significantly higher level of IgG |
| Reichert et al.83 | 2013 | Germany | Observational descriptive | 42 patients with RA (17 women and 25 men) with a mean age of 53.8±16.7 years. 114 patients without RA (60 women and 54 men) with a mean age of 56.1±15.2 years | The patients were diagnosed with RA by 2 specialists in rheumatology according with the criteria of the American College of Rheumatology/European League Against Rheumatism collaborative initiative | The patients were diagnosed with PD by 2 specialists in periodontology according with the Group C consensus report of the 5th European Workshop in Periodontology | PCR specific for 16S rRNA from crevicular fluid and synovial fluid | A. actinomycetemcomitansP. gingivalisP. intermediaT. forsythiaT. denticola | A. actinomycetemcomitans (0%)P. gingivalis (11.9%)P. intermedia (8.0%)T. forsythia (7.9%)T. denticola (8.0%) | DNA of periodontal microorganisms could be detected in synovial fluid of patients with and without RA. P. gingivalis was detected in a greater number of patients with RA compared with the control group |
| Ghotaslou et al.84 | 2016 | Iran | Observational descriptive | 42 patients (28 women and 14 men) of whom 22 were diagnosed with RA and 20 patients did not have a diagnosis of RA | Diagnosis was not established | Diagnosis was not established | rtPCR from synovial fluid | P. gingivalis | P. gingivalis (13.6%) | DNA of P. gingivalis was detected in synovial fluid of patients with RA, therefore, it can be evidenced that this periodontal microorganism contributes to the pathogenesis of RA |
The OSTEBA critical reading sheets of the Web FLC 2.0 platform developed by the Basque Office for Health Technology Assessment of the Department of Health of the Basque Government73 were used to assess the quality or validity of the scientific evidence of each article, in function of its methodological rigor. The sheet of diagnostic tests for non-experimental comparative studies includes any procedure used to know the health condition of an individual (laboratory tests, surgical, clinical examinations, imaging studies, questionnaires, etc.) with the purpose of assessing the impact of the identification of periodontal microorganisms in the crevicular fluid and in the synovial fluid of patients diagnosed with PD and RA.
ResultsIn total were included 14 publications that met the inclusion criteria, which were organized by author, year, country, type of study, sample, diagnosis of RA, diagnosis of PD, detection method, periodontal microorganisms and conclusion (Table 1). The 14 articles were published between 2003 and 2016, 8 in European countries, 2 in Latin American countries (one in Colombia), one in North America, one in the Middle East, one in the Far East and one in Oceania. In total, there were 8 observational descriptive studies, one systematic literature review and 4 literature reviews. Of the 7 descriptive studies, 4 performed detection of periodontal pathogens in crevicular fluid and in synovial fluid, 3 with PCR specific for 16S rRNA and one with real time PCR. Two studies used enzyme linked immunoabsorbent assay (ELISA) to determine the levels of anti-CCP antibodies and of IgG and IgA antibodies. Only one study made cultures in different media such as heart–brain blood agar and trypticase soy agar. In all studies the different detection tests were positive for P. gingivalis in synovial fluid. Four studies diagnosed the PD through standardized methods (in accordance with the Group C consensus report of the 5th European Workshop in Periodontology, probing depth measurement of the periodontal pocket and the gingival inflammation index of Silness and Loe) and 6 diagnosed RA according to the criteria of the American Rheumatism Association. The studies conclude that there is evidence that allows to associate PD and RA, and also point out that P. gingivalis can play an important role in the etiopathogenesis of RA.
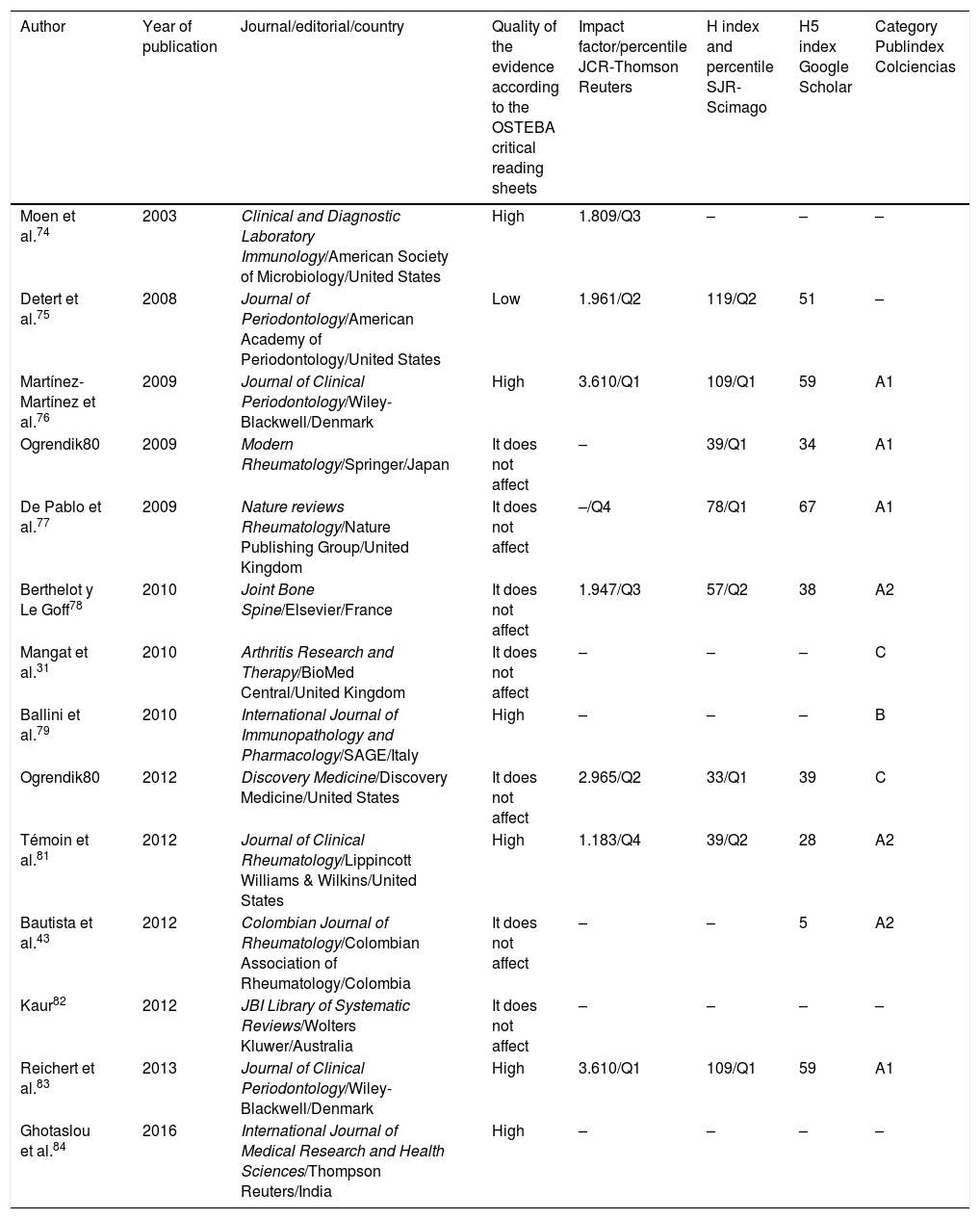
According to the OSTEBA critical reading sheets for non-experimental comparative studies, 6 publications showed a high level of evidence. Among these are the studies that used some molecular technique to detect DNA of periodontal microorganisms or specific antibodies for some species in synovial fluid. An observational study presented a low level of evidence because it only diagnosed PD in patients with RA, and the association between these 2 diseases was based on the literature cited. For 7 publications the format was not relevant. Likewise, it was identified that the studies with high level of evidence were published in scientific journals with equally high quality indicators. (Table 2).
Quality indicators of the journals in which the articles included in this study were published.a
| Author | Year of publication | Journal/editorial/country | Quality of the evidence according to the OSTEBA critical reading sheets | Impact factor/percentile JCR-Thomson Reuters | H index and percentile SJR-Scimago | H5 index Google Scholar | Category Publindex Colciencias |
|---|---|---|---|---|---|---|---|
| Moen et al.74 | 2003 | Clinical and Diagnostic Laboratory Immunology/American Society of Microbiology/United States | High | 1.809/Q3 | – | – | – |
| Detert et al.75 | 2008 | Journal of Periodontology/American Academy of Periodontology/United States | Low | 1.961/Q2 | 119/Q2 | 51 | – |
| Martínez-Martínez et al.76 | 2009 | Journal of Clinical Periodontology/Wiley-Blackwell/Denmark | High | 3.610/Q1 | 109/Q1 | 59 | A1 |
| Ogrendik80 | 2009 | Modern Rheumatology/Springer/Japan | It does not affect | – | 39/Q1 | 34 | A1 |
| De Pablo et al.77 | 2009 | Nature reviews Rheumatology/Nature Publishing Group/United Kingdom | It does not affect | –/Q4 | 78/Q1 | 67 | A1 |
| Berthelot y Le Goff78 | 2010 | Joint Bone Spine/Elsevier/France | It does not affect | 1.947/Q3 | 57/Q2 | 38 | A2 |
| Mangat et al.31 | 2010 | Arthritis Research and Therapy/BioMed Central/United Kingdom | It does not affect | – | – | – | C |
| Ballini et al.79 | 2010 | International Journal of Immunopathology and Pharmacology/SAGE/Italy | High | – | – | – | B |
| Ogrendik80 | 2012 | Discovery Medicine/Discovery Medicine/United States | It does not affect | 2.965/Q2 | 33/Q1 | 39 | C |
| Témoin et al.81 | 2012 | Journal of Clinical Rheumatology/Lippincott Williams & Wilkins/United States | High | 1.183/Q4 | 39/Q2 | 28 | A2 |
| Bautista et al.43 | 2012 | Colombian Journal of Rheumatology/Colombian Association of Rheumatology/Colombia | It does not affect | – | – | 5 | A2 |
| Kaur82 | 2012 | JBI Library of Systematic Reviews/Wolters Kluwer/Australia | It does not affect | – | – | – | – |
| Reichert et al.83 | 2013 | Journal of Clinical Periodontology/Wiley-Blackwell/Denmark | High | 3.610/Q1 | 109/Q1 | 59 | A1 |
| Ghotaslou et al.84 | 2016 | International Journal of Medical Research and Health Sciences/Thompson Reuters/India | High | – | – | – | – |
PD and RA, 2 diseases of infectious and autoimmune origin, respectively, which include chronic inflammatory processes, are a suitable model to determine if there is a two-way relationship.14,15 Both diseases are characterized by an inflammatory reaction which causes the destruction of soft and mineralized tissues (bone and cartilage) altering the morphofunction of the joints (of gomphosis type for the teeth and synovial for bone joints).20,21 Likewise, similar patterns of natural course and pathogenesis, immune response, genetic susceptibility, cellular infiltration and increased expression of enzymes and cytokines have been observed, to such an extent that the implications of the treatment both of PD and RA include the management of the clinical symptoms and the modulation of the immune response.22
It is believed that periodontal microorganisms cause a periodontal chronic inflammation and that they can exacerbate the RA in genetically susceptible individuals, associated with the detection in synovial fluid of DNA and IgG and IgA antibodies against periodontal bacteria of the red complex late colonizers such as P. gingivalis, T. forsythia and T. denticola; bacteria of the orange complex intermediate colonizers such as P. intermedia and P. nigrescens, and bacteria of the green complex early colonizers such as A. actinomycetemcomitans.24,28,71 In addition, due to the ability of P. gingivalis to citrullinate proteins, it has been proposed that this periodontal pathogen activates the humoral response after the production of anti-CCP antibodies, with which PD could be a risk factor in RA.14,50,55,57,74,85,86 It is known that the majority of anti-CCP antibodies in patients with genetic susceptibility (shared epitope) are against P. gingivalis,24,87 which results clinically relevant for the early diagnosis of RA.87
According with the results presented in 6 of the 14 studies included in this systematic literature review, the discussion will be focused on the detection of antibodies specific for periodontal microorganisms and on the prevalence of periodontal microorganisms in the synovial fluid of patients with RA.
Detection of immunoglobulin G and Immunoglobulin A antibodies against periodontal pathogens in synovial fluid of patients with rheumatoid arthritisIn the initial lesion of PD, IL-1 and TNF-α are produced by the infiltrating neutrophils and macrophages and by the activation of endothelial cells, and IL-8, a cytokine with chemotactic activity for the arrival of more polymorphonuclear neutrophils is produced in the subgingival environment—by NETosis—in the site where the bacteria are.8 Once the lesion (gingivitis) is established, the adaptive immune response is triggered with the accumulation of T CD4+ lymphocytes of Th1 profile, which produce IFNγ and IL-2 to promote the activity of macrophages and co-stimulate the B-lymphocytes which produce types IgG and IgA antibodies.88 With this, the lesion progresses to the formation of the periodontal pocket or the gingival recession due to insertion loss (periodontitis), which leads to the appearance of a more pathogenic microbiota represented by anaerobic Gram-negative bacteria.71 This lesion presents as immunological characteristics a decreased innate immune response, abundant plasma cells (50%) and T CD4+ lymphocytes of Th2 profile that produce IL-4, IL-5, IL-6 and IL-10 to favor the production of IgG4 and IgE by the B lymphocytes, in such a way that the activity of macrophages is suppressed with this profile.5,6 However, there are studies that show the overstimulation of gingival monocytes, macrophages and fibroblasts that produce more IL-1β, TNFα and MMP, generating an inflammatory environment directed by the Th1 or Th17 profile, an effect that characterizes the cyclic nature of the PD.8
Moen et al.74 performed an ELISA to determine the levels of IgG and IgA antibodies specific for 3 periodontal pathogens in samples of synovial fluid of healthy individuals and individuals diagnosed with joint disease who had PD. They found increased levels of IgG antibodies specific for P. gingivalis—with p-values without statistical significance—and of IgA specific for P. intermedia y B. forsythus—with p-values with statistical significance—in individuals with RA with respect to individuals with osteoarthritis or healthy individuals; which suggests that in individuals with RA and PD the B lymphocytes exhibit greater activation and greater production of antibodies specifically targeted against antigens of P. intermedia and B. forsythus, in such a way that there are mechanisms of capture of bacterial DNA by the synovial membrane, in addition to the deposition of antigen-antibody immune complexes in the synovial compartment and of plasma cells in the synovial tissues.74 In the specific case of P. gingivalis, although there was no difference between the levels of IgG and IgA among the different individuals included in the study sample, this periodontal pathogen appears to be a link that connects the PD and the RA, because specific IgG antibodies against P. gingivalis cross-react against neoantigens of the host (vimentin, collagen type ii, α-enolase and fibrinogen).45,46
Taking into account that P. gingivalis has PAD activity to citrullinate the gingipains as a mechanism of virulence, when the antibodies specific for these citrullinated antigens circulate through the bloodstream, they can recognize citrullinated peptides in other body tissues, including the synovial joints, where the human PAD activity is much more frequent in the proinflammatory states.56 In this way, when the IgG antibodies specific for P. gingivalis recognize the own citrullinated peptides, immune complexes are formed which trigger the effector functions of humoral immunity, which include the activation of neutrophils, monocytes and macrophages, the activation of the classical complement pathway and the activation of macrophages that favor the chronic proinflammatory status.50 These immune complexes activate a type iii hypersensitivity response, in which the inflammatory process that contributes to the loss of the immune tolerance exacerbates the clinical picture of PD and RA.26,46,47,51
In this sense, Ballini et al. determined the increased levels of anti-CCP antibodies by ELISA, in patients with PD and RA, suggesting that the ability to citrullinate of P. gingivalis contributes to the exacerbation of the immunologic and inflammatory picture of the patients with RA.79 On the other hand, it is fundamental to take into account that RA is an autoimmune disease, in which the genetic susceptibility has been proven.43 In RA the main susceptibility alleles are present mainly in HLA-DRB1 and its variants, all of them associated with the EQKRAA motif, which is located in the third hypervariable region of the DRB chain which is part of the peptide binding cleft—recognized as the shared epitope of the MHC class ii molecule—for citrullinated vimentin, type II collagen, α-enolase and fibrinogen.32,34 In this way, the β chain of the HLA-DRB1 allele constitutes the greatest genetic risk (susceptibility and progression) of the RA, because when it binds to citrullin initiates immune responses through the production of IgA and IgG by activated B lymphocytes and the stimulation of macrophages by the synthesis of IL-17, FN-γ, TNF-α and IL-6 by the T CD4+ lymphocytes. Therefore, HLA-DRB1 acts as a risk factor for the production of anti-CCP and triggers RA.43,87,89–91
Detection of DNA of periodontal pathogens in synovial fluid of patients with rheumatoid arthritisOne of the hypothesis proposed for the relationship between PD and RA is that periodontal pathogens produce bacteriemia from the gingival sulcus and colonize the synovial tissues, site where they alter the immunological tolerance and induce tissue damage by activation of the 2 complement pathways, assembly of traps for neutrophils, degradation of the extracellular matrix of the articular cartilage, and osseous resorption of the subchondral bone.48
In this way, Martínez-Martínez et al. identified bacterial DNA by PCR in 100% of the synovial fluid samples (84.2%) of 19 patients with PD and RA. The species of periodontal pathogens identified were P. intermedia (89.4%), P. gingivalis (57.8%), Prevotella nigrescens (21.0%), T. denticola (31.5%), A. actinomycetemcomitans (15.7%) and Tanerella forsythensis (10.5%). However, it was not possible to verify that there where viable bacterial cells since the culture was negative for bacterial growth. The authors suggested that the bacterial DNA could be intracellularly transported from the periodontal tissues to the synovial tissues in leukocytes and dendritic cells or by bacteriemia.76 From this dissemination route it has been evidenced that bacterial DNA contains CpG motifs able to stimulate the innate immune response after the activation of Toll-like receptors and the subsequent release of proinflammatory cytokines, which could constitute a key point for the relationship between PD and RA.92
Reichert et al.83 used a PCR test to detect bacterial DNA in samples of crevicular fluid and synovial fluid of 42 patients with RA and 114 with non-rheumatoid disease. The authors identified, in 28 patients with RA, DNA of P. intermedia (19.0%), T. forsythia (16.7%), P. gingivalis (16.7%), T. denticola (8.0%) and A. actinomycetemcomitans (2.4%). These findings allowed them to affirm that the prevalence of periodontal microorganisms in synovial fluid specifically P. gingivalis (16,7% in individuals with RA versus 3,5% in healthy individuals, p=0.045) can play a fundamental role in the etiology of RA.83 Témoin et al. also detected bacterial DNA in synovial fluid (2 of 11 patients diagnosed with RA and 3 of 25 patients with osteoarthritis), being the PCR test positive for F. nucleatum (2.7%) in crevicular fluid and synovial fluid of patients with RA.79 Finally, Ghotaslou et al. detected P. gingivalis in 3 patients with RA (13.6%) by real time PCR (rtPCR) in synovial fluid—with p-values without statistical significance with respect to the control group—, concluding that due to the increased amount of DNA in patients with RA, this periodontal microorganism could contribute to the pathogenesis of this disease.84
Level of evidenceWhile the new scientific knowledge not always modifies the behavior of clinical care, it is important to evaluate the quality of the evidence, the validity of the results and the methodological quality of the research.92–94 In this way, 6 descriptive studies that were included in this review, which used some standardized method to diagnose PD and RA, and which detected the presence of DNA of periodontal bacteria (or their IgG and IgA antibodies) in synovial fluid had a high level of evidence and were published in high-impact peer-reviewed journals, specifically the studies conducted by Martínez-Martínez et al.,76 Témoin et al.81 and Reichert et al.83 However, it should be taken into account the publication bias that has been associated, essentially, with the editorial behavior of the scientific journals and with the different strategies with which their impact is measured (for example, citation indexes), which not necessarily guarantee that the results can support clinical decision making based on the “best evidence” available.95,96
ConclusionsAccording to the results and the level of evidence of the articles included, and with the quality indicators of the journals in which they were published, it is possible to conclude—in general—that the presence of periodontal microorganisms in the synovial fluid of patients with PD and RA could explain:
- 1.
The increased levels (p<0.05) of IgG and IgA antibodies specific for P. gingivalis in synovial fluid, in addition to the increase in the levels of anti-CCP antibodies, were associated with the presence of P. gingivalis, due to their PAD enzymatic function and their ability to citrullinate proteins, which has been considered as the plausible biologic link between both diseases.
- 2.
The prevalence of DNA of P. gingivalis mainly and other periodontal microorganisms (F. nucleatum, T. forsythia, P. intermedia, A. actinomycetemcomitans, P. nigrescens and T. denticola) in synovial fluid, reported in the studies, reinforces the paradigm that both diseases can be linked, because the oral biofilm exerts a constant stimulus in the immune system, which could be associated with the local chronic inflammatory process with low-grade systemic inflammation.
However, the evidence offered by the studies included in this systematic review does not reveal that P. gingivalis is an etiologic factor of RA in humans. Although its DNA has been identified in samples of human synovial fluid, the fact that P. gingivalis stimulates the production of anti-CCP antibodies in the tissues that it infects, even in the synovial joints, as it has been proven in animal models, is still a possibility.
FundingThis original article derives from the research project Association between academic stress and C-reactive protein levels in students of the medical career of the Pontificia Universidad Javeriana Cali, which was funded by the Internal Call 2015-2016 of the Pontificia Universidad Javeriana Cali (Colombia).
Conflict of interestThe authors of the article state, that there is not any type of conflict of interest, directly or indirectly, that might jeopardize the validity of what is communicated.
Please cite this article as: Arana P, Salazar D, Amaya S, Medina M, Moreno-Correa S, Moreno F, et al. Microorganismos periodontales en el líquido sinovial de pacientes con artritis reumatoide. Revisión sistemática de la literatura - 2017. Rev Colomb Reumatol. 2018;25:271–286.