Systemic lupus erythematosus is the prototype of non-organ specific autoimmune diseases, with a fluctuating course between remission and crisis. The complexity of pathophysiological mechanisms opens up the possibility to develop multiple research topics to facilitate their understanding and generate potential therapeutic targets. The Wnt signaling pathway and its main inhibitor, Dickkopf-1 protein, have a major role in biological phenomena, such as bone homeostasis. However, recent studies have enabled other extra-osseous processes regulated by Dickkopf-1 to be recognized. These include: preserving the integrity of kidney glomerular membranes, senescence reversal characteristics of mesenchymal cells of interest in optimizing transplantation plans as a therapeutic measure, and joint homeostasis. Some of these results have led to further research into Dickkopf-1 and systemic lupus erythematosus, in order to consolidate the information obtained given the great clinical and therapeutic potential involved.
El lupus eritematoso sistémico es el prototipo de las enfermedades autoinmunes no órgano-específicas, con un curso fluctuante entre periodos de remisión y crisis. La complejidad de sus mecanismos fisiopatológicos mantiene la necesidad de desarrollar nuevos tópicos de investigación que faciliten su entendimiento y generen potenciales blancos terapéuticos. La vía de señalización Wnt y su principal inhibidor la proteína Dickkopf-1 tienen un rol trascendental en fenómenos biológicos como la homeostasis ósea. Sin embargo, estudios recientes en lupus eritematoso sistémico han permitido reconocer otros procesos extraóseos regulados por la proteína Dickkopf-1. Entre ellos: la preservación de la integridad de las membranas glomerulares a nivel renal, reversión de rasgos de senescencia de células mesenquimales de interés en la optimización de los planes de trasplante como medida terapéutica; y la homeostasis articular. Alrededor de estos resultados han de suscitarse nuevas investigaciones sobre la proteína Dickkopf-1 y lupus eritematoso sistémico, que consoliden la información obtenida dado el gran potencial clínico y terapéutico que implica.
Systemic lupus erythematosus (SLE) is the prototype of non-organ specific autoimmune diseases, characterized by a chronic inflammatory process, mainly due to deposition of autoimmune complexes and activation of the complement system; being reflected in a variable clinical course between periods of exacerbation and remission.1,2
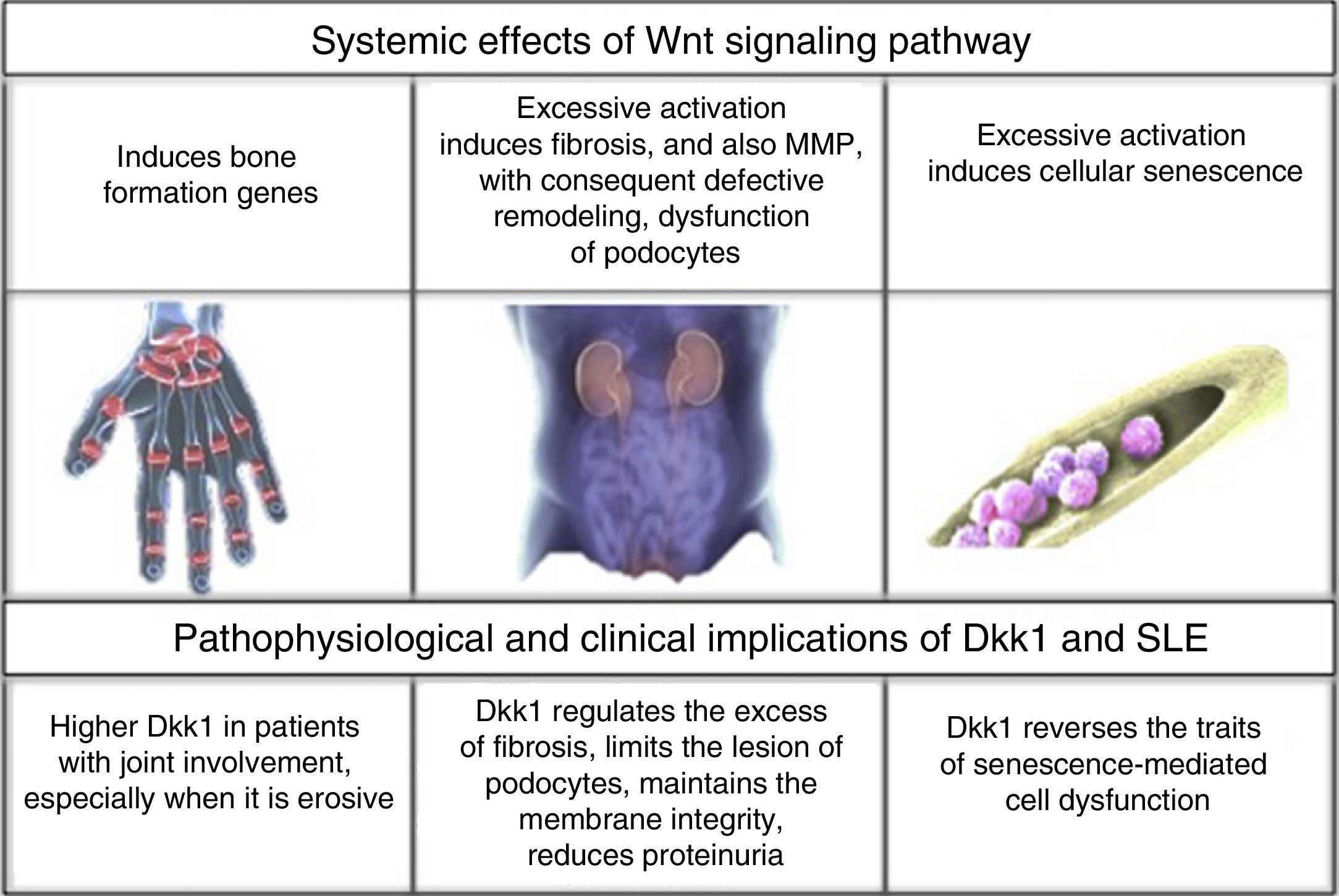
Joint involvement affects 50%–70% of patients with SLE, with a clinical spectrum of manifestations that includes synovitis, tenosynovitis, synovial hypertrophy, capsulitis and erosive arthritis.3 The latter is present in 2% of patients with arthritis due to SLE.4 In parallel to this, the bone involvement, a consequence of the chronic inflammatory context of the disease, is directly related with an increased risk of osteoporosis, independently of the effects that the treatment may have.5,6 According to the above, it becomes interesting the characterization of biomarkers that facilitate the physiological identification of the pathways,7 that even though they do not allow a damage of the bones and joints as severe as in rheumatoid arthritis (RA), are related with an imbalance in the process of bone remodeling.1,8
Renal commitment is other of the most frequent involvements,9 especially in Afro-descendants and Hispano-Americans, among them it is described that 53% of the Colombian patients with SLE could have it,10 especially with class III and IV glomerular lesion, being one of the major causes of morbidity and mortality.11 Although the advent of steroids in 1950 and later of cyclophosphamide and other pharmacological elements changed the history of the disease,12 the search for controlling and even curative tools still remains; new molecular targets and the bone marrow transplantation are some of the proposals;13,14 however, they require a deeper understanding of the pathophysiology of this entity.
The Wingless signaling pathway (Wnt), and one of its main inhibitors, the Dickkopf-1 protein (DKK1), have recently become the object of multiple investigations, given their important implications in different physiological and pathological processes. As a consequence of their systemic actions, especially on organogenesis, angiogenesis, bone biology and immune regulation;15,16 have been generated proposals for therapeutic targets that seek to regulate the excessive activation of this signaling pathway in different types of cancer, osteoporosis, neurological diseases and autoimmune disorders.17,18 Regarding the latter, attention has been focused on pulmonary fibrosis due to systemic sclerosis,19 RA,20,21 ankylosing spondylitis22 and osteoarticular and renal involvement in SLE.23
This topic review aims to compile state-of-the-art information on the expression of DKK1 in relation with the clinical presentation of SLE in murine and human models.
Search strategyFor this topic review were evaluated clinical trials, observational studies, original works and topic reviews, conducted in humans and animal models, published in English and in Spanish, without limit in the time of publication. A primary search in Pubmed was conducted using the MeSH terms: lupus eritematoso sistémico, DKK1, dkk1, proteína dickkopf-1, vía de señalización Wnt (systemic erythematosus lupus, DKK1, dkk1, dickkopf-1 protein, Wnt signaling pathway). In addition, a secondary literature search with free terms in relation with the topic of interest of the project was carried out.
Wnt signaling pathwayStudies in murine models with cancer and oncogenic retroviruses, allowed the discovery of the great number of soluble glycoprotein ligands that make up the family of Wnt molecules, which share the presence of multiple cysteine residues.24 They activate many signaling pathways, all of them evolutionarily preserved, that depend on the coupling to one of their more than 10 receptors known until now of the Frizzled (fz) family.25 The abbreviation Wnt comes from the unification of Wg (Wingless) ant Int1 (integration) which were the names assigned to the genes encoding the Wnt molecules in the Drosophila fly and in the human correspondingly, but that made reference to the same gene.26
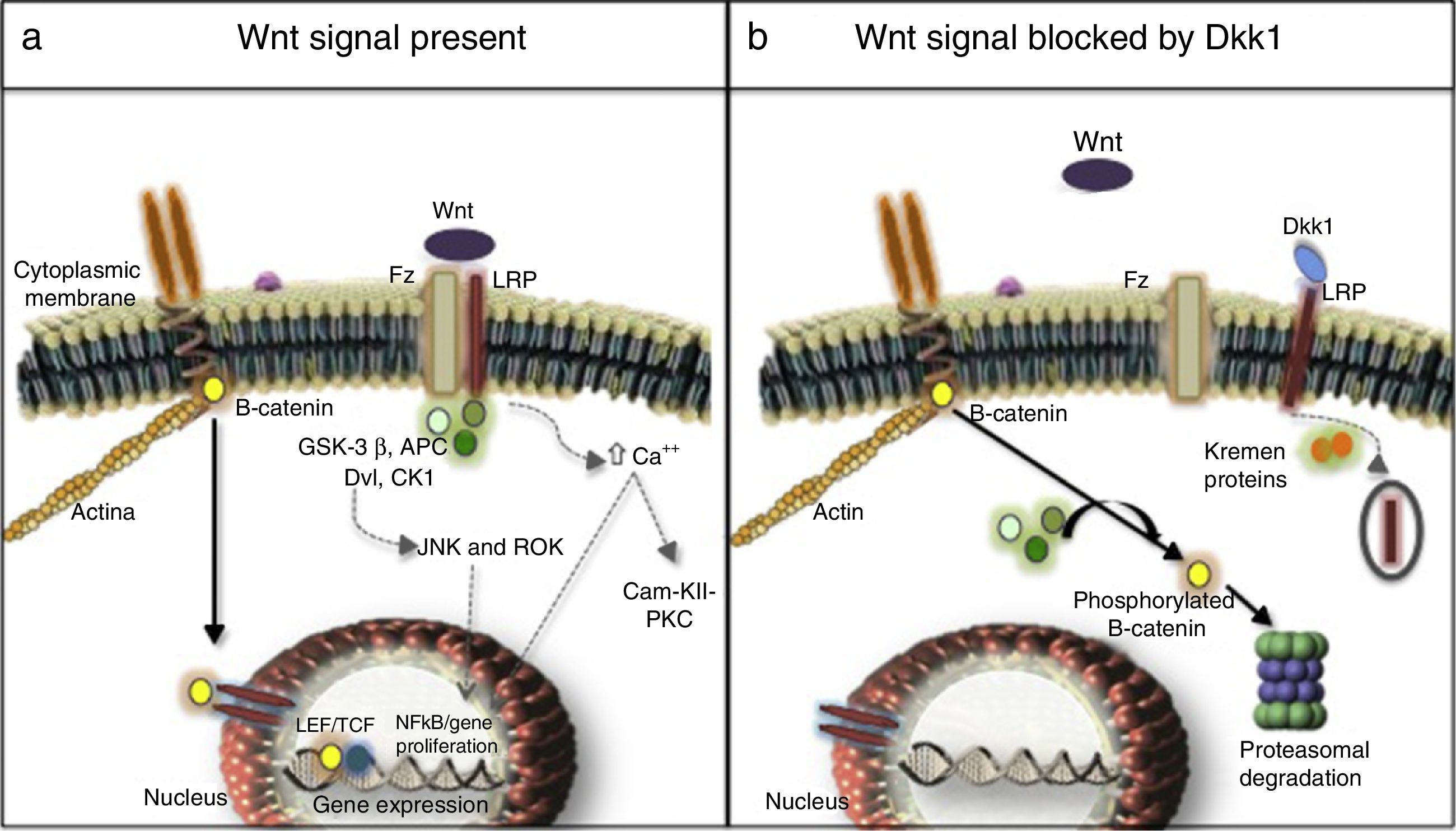
Three signaling pathways activated by Wnt molecules have been described: the canonical or β-catenin-dependent pathway, the non-canonical calcium-dependent pathway and the non-canonical Disheveled (DVL) protein-dependent pathway27,28 (Fig. 1). Results of a research in murine models (Yu et al., 2015) evidenced that some Wnt molecules have a predilection for the activation of one or several signaling pathways. For example, it was found in this research that Wnt4 activated only the non-canonical pathway and prevented the translocation of NFκB to the nucleus with very beneficial effects in the bone and control of inflammation. In contrast, Wnt5 with a dual effect, was an activator of the canonical pathway by interacting with the Ror2 receptor, being determined as a proinflammatory agent, and it was also an activator of the non-canonical pathway with induction of osteoclastogenesis.29,30
(a) Canonical (continuous arrows) and non-canonical (dashed arrows) signaling pathways. (b) Dkk1-mediated blockade. Frizzled receptor (Fz), low density lipoproteins or LDL receptor protein (LRP), Disheveled (Dvl), glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis coli (APC), and casein kinase 1 (CK1), transcription factors such as the lymphoid enhancer factor and (LEF) and T-cell factor (TCF), protein kinase C (PKC), calmodulin kinase II (Cam-KII), kinases (JNK, ROK) Dickopff-1 (Dkk1).
After being secreted, the Wnt molecules bind to the Frizzled receptor (Fz), allowing the heterodimerization of the receptor with the low-density lipoproteins or LDL receptor protein (LRP), triggering the signaling cascade, in which are involved elements such as DVL, glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis coli (APC), and casein kinase 1 (CK1). In resting state, β-catenin is phosphorylated by GSK-3β, which allows the formation of a complex with APC, CK1 and annexin, favoring the labeling by ubiquitination to be led to proteolysis. In contrast, in the activation state, when Wnt and the Fz/lRP complex are coupled, the DVL protein is activated to inhibit GSK-3β; favoring the accumulation of β-catenin in the cytoplasm and its translocation to the nucleus to interact with specific response genes, in collaboration with transcription factors such as the lymphoid and T-cell stimulating factor (TCF).23–26 In the bone, the target cells are osteoblasts and osteocytes and the group of genes that are activated are those which participate in bone formation such as c-myc, twist, fibronectin, c-jun, axin 2, ctgf, among others. An excessive negative regulation of the signaling of Wnt pathway due to excess of DKK1 has been linked to the erosive course of RA.27 Recently, it was found in the kidney hyperactivation of the pathway in the cells of the tubular epithelium with an increase in the production of extracellular matrix and fibrosis, along with an increase in the activity of metalloproteinases, and therefore, an increase in the remodeling of the extracellular matrix with a deleterious effect in the integrity of the membrane.31–33
Non-canonical pathwaysThey are β-catenin-independent pathways, one of them is the calcium-dependent: on one hand the anchoring of the Wnt molecules causes the release of the cation into the cytoplasm, with the consequent activation of protein kinase C and calmodulin kinase II (Cam-KII), and on the other hand, it facilitates the activation of NFkB with its proinflammatory effect. Another pathway is the DVL-dependent, which when it is activated mobilizes GTPases that stimulates kinases such as JNK and ROK responsible for regulating the cell growth and proliferation, due to their cytoskeletal organizational function.23,34
Regulation of the canonical pathway and DKK1The most studied mechanisms are extracellular mediated by the binding to LRP of ligands such as DKK1 and sclerostin which prevent the formation of the Wnt-Fz/LRP 5/6 complex and induce the internalization of LRP in the presence of Kremen proteins.34 DKK1, encoded by the dkk1 gene in the long arm of chromosome10,35 is encoded by the target genes of the activated Wnt pathway, i.e., it is part of a negative feedback mechanism in osteoblasts and osteocytes. On the other hand, the assembly of 2 molecules of Wnt with the soluble proteins related to Fz (sFRP) also can prevent the anchoring to its membrane surface receptor. Other inhibitors are Wnt inhibitory factor and Cerberus.36 The intracellular regulation with less information, to date, is related to the levels of DVL, GSK-3β, APC, CK1, axin, and the nuclear transcription factors.37,38
DKK1 and joint involvement by SLEThe pathophysiological implications of Wnt pathway and one of its main inhibitors, DKK1, in the joint involvement by SLE, are originated in the framework of a large amount of information generated on the interferon-induced genes (IIG), one of the main mediators of the disease that have been posed as the main responsible for the non-erosive behavior.39
For example, in the Catholic University of Louvain, in Belgium (Toukap et al., 2007), analyzed 3 groups of patients: 6 with SLE, 7 with RA and 6 with osteoarthrosis, who had in common knee synovitis and before receiving any type of treatment were submitted to the study of this joint: synovial fluid and tissue and serum markers. In this way, 40 genes related to IIG, especially those of IFI27, IFI44, IFI44L, TLR-2, and STAT 1 were found increased; and 34 diminished, related to the homeostasis in the formation of extracellular matrix such as chondroitin sulfate 2 proteoglycan, latent-transforming growth factor beta protein 2 (LTBP-2), versican and fibroblast activation protein alpha (FAP), in comparison with the other groups.40 The IIG have been labeled as a key mediator in SLE, since once the immune complexes are internalized in the dendritic cells, especially in the professional, these cells activate the transcription of type I IFN, which facilitates the differentiation of B lymphocytes into plasma cells producers of antibodies, the activation of T cells and the maturation of more dendritic cells.39 New therapies under research, such as rontalizum, sifalimumab and α-quinoid are targeted to blockage, especially of interferon-α (INF-α), with which they promise to achieve an adequate control of the disease, without increasing the risk of bone deterioration whose protection depends more on interferon-β (INF-β), because it is a 100 times more potent inhibitor of osteoclastogenesis than INF-α.41
In agreement with the foregoing, in a cohort of 90 subjects (Baker LePain et al., 2011): children and adolescents, it was evidenced a negative correlation between bone resorption and SLE activity, and INF-β was also proposed as a protective factor, by directly inhibiting c-fos, one of the second messengers involved in the differentiation of osteoclasts, which results in the decrease of the generation of tartrate-resistant acid phosphatase (TRAP) necessary for bone resorption.42
Although the foregoing would entail that SLE is a protective factor against osteoporosis, is not like that, other increased inflammatory mediators such as the tumor necrosis factor, interleukin 1 and the receptor activator of nuclear factor-kappa B ligand (RANK-L) explain the deterioration of the bone mineral density (BMD) seen in these patients.5 This is illustrated by a study (Tang et al., 2013) comparing the BMD of patients with SLE without steroid treatment in the last 10 years previous to the evaluation and the BMD of healthy patients; the first group had lower BMD values measured by high-resolution CT.43
To establish the link between interferon induced genes, the Wnt signaling pathway and its inhibitor DKK1; it would be required the generation of new research that allow to expand knowledge about the predominantly non-erosive course of lupus arthritis. Until now, the existing information has been oriented to determine the specific association between DKK1 and SLE.23 (Fig. 2).
In one study (Li Long et al., 2010), 3 groups were configured: 130 patients with SLE, 100 patients with RA and 30 healthy individuals; and it was found that DKK1 was 2–3 times higher in the patients with SLE and joint involvement vs. in those who did not have such involvement or who did not have SLE; and it was especially elevated in the few patients with erosive behavior; in the latter case, with values comparable to those of the patients with RA. In addition the anti-citrullinated peptide antibodies (anti-CCP) where higher in patients with lupus arthritis than in those with the disease but without arthritis. In those in whom these antibodies were negative, DKK1 was significantly elevated; with which it is postulated that it might be more sensitive that the former as a prognostic marker, a novel proposal that requires further studies. In this publication, there was not relationship between the disease activity measured by SELENA SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment – Systemic Lupus Erythematosus Disease Activity Index) and DKK1 levels.44
DKK1 and renal involvement in SLEThe influence of DKK1 has been evaluated not only in the joint involvement, but also in renal commitment. In 97 patients with SLE referred to a Hospital in Shanghai (Wang X-d et al., 2014) for renal biopsy, it was found hyperactivation of the Wnt pathway in comparison with healthy individuals and patients with some type of renal tumor. The above was demonstrated by an increase in the intensity of β-catenin staining by immunohistochemistry of the glomeruli in the biopsies, increased levels by Western blot and by reverse transcription polymerase chain reaction (RT-PCR); as well as an increase in AXIN-2 and DKK1, that are part of the elements of response at the end of the signaling pathway; and finally, increase in serum DKK1 measured by Enzyme-Linked ImmunoSorbent Assay (ELISA). However, the relationship was inversely proportional to the degree of interstitial fibrosis, the levels of complement C3 and anti-deoxyribonucleic acid antibodies (anti-ADN); without correlation with the activity measured by SLEDAI, probably due to the non-specificity of its overall score. This allowed to deduce that Wnt hyperactivation induces an exponential synthesis of collagen and extracellular matrix proteins that promote fibrosis, while increased levels of DKK1 may have, in this context, a protective role against kidney deterioration by dissolving the excess of matrix deposit.45
Similarly, another study (Dai et al., 2009) in murine model demonstrated that excessive activation of the Wnt pathway promoted the podocyte dysfunction by inhibition in the production of nephrin, with which increased the presence of proteinuria, while the increase of DKK1 prevented the glomerular injury in murine models without lupus.46
In contrast to the above, one study (Tveita et al., 2011) in murine models of lupus nephritis (NZB/NZW) found something different, a negative correlation between the presence of DKK1 in mesangial and tubular cells, and the cellular integrity. DKK1 was related to an increase in pro-apoptotic factor such as caspase 3, increased generation of antigenic residues derived from apoptosis and, therefore, a successive increase in the levels of anti-DNA antibodies.47 One of the postulations that would explain the discrepancies in the findings, could be that the opposite effects were consequence of different types of Wnt molecules; however, further studies are required to clarify the information. Knowing the role that DKK1 plays in lupus nephritis can open the doors to new therapeutic targets for this organ involvement that stills claims the highest morbidity and mortality in SLE.48
DKK1 and bone marrow in SLEAnother role of DKK1 in SLE was elucidated in a hospital affiliated with the Nantong University, China (Gu et al., 2014) in relation to the bone marrow mesenchymal cells. The decrease in the proliferation and, thereby, in the cell division, increase in the size, and cytoskeletal disorders are traits of senescence of these cells; hence, in patients with SLE these senescence traits were marked in contrast with the findings in healthy individuals. This is explained by a greater activation in the Wnt signaling pathway, increase in β-catenin in the cytoplasm and the nucleus, decrease in GSK-3β, and increase in the activity of β-galactosidase (marker of senescence) and in the p53/p21 pathway suppressor of the cell cycle, which is proposed to be the main mediator of aging induced by such hyperactivation. The application of 100ng/ml of DKK1 in the culture plates during 48h suppressed all these traits, and especially decreased the levels of elements of p53/p21 pathway.49 Since the bone marrow transplant is one of the tools that is under ongoing exploration in SLE, is crucial to understand the cellular conditions in this regard, because it offers an explanation of why the allogenic donor is the choice and which factors should be monitored to have success; one of them: the adequate functionality of DKK1 responsible, in this case, for the integrity of the bone marrow mesenchymal cells.50
ConclusionsCurrently, SLE continues being a challenge for the clinician, therefore it is important in research, due to the diversity of its manifestations y and to its fluctuant course; all of this as a consequence of the extensive scope of immunological, genetic and biological pathways that may be altered. In order to find new therapeutic targets for the disease that will improve the quality of life of the patients and markedly reduce the morbidity and mortality derived from it; and with the purpose of postulating more efficient and accurate diagnostic and prognostic biomarkers, it is necessary to deepen the study of these pathophysiological pathways. In a novel way, findings on the role of the Wnt signaling pathway and its main inhibitor DKK1 make an incursion in the mist of the information generated in relation with different molecular aspects of SLE, giving indications of a notorious role in the imbalance of bone remodeling with effects on the integrity of the synovial joint surfaces; and a prominent role in renal homeostasis. Increased levels of DKK1 have been found to be deleterious for osteoarticular preservation; and in contrast, protective at the renal level by limitation of the process of fibrosis and lesion of the podocytes, the latter with some ambiguous results. In the bone marrow also with a protective effect, DKK1 reverses the senescence traits of the mesenchymal cells, with positive implications in the transplantation as a therapeutic plan. Around these results new works about DKK1 should arise, which would consolidate the information obtained, because of the potential benefits as a biomarker and even as a therapeutic target.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Ospina Caicedo AI, Ballesteros DA, Romero Sánchez MC, Munevar Niño JC. La proteína Dickkopf-1 y el lupus eritematoso sistémico: nuevos campos en investigación, México. Rev Colomb Reumatol. 2016;23:259–265.