To describe the clinical characteristics, as well as the maternal and perinatal outcomes in pregnant women with the antiphospholipid syndrome in a reference institution.
Materials and methodsA retrospective descriptive study was carried out in order to evaluate pregnant women with antiphospholipid syndrome according to Sydney criteria or rheumatologist criteria between 2010 and 2016. Cases with hereditary thrombophilia, cervical incompetence, history of hepatitis B, hepatitis C, and human immunodeficiency virus were excluded. The information on sociodemographic and clinical variables was collected from the review of medical records.
ResultsSixteen pregnant women were included; seven (43.8%) patients had a history of abortion, five (71.4%) on more than one occasion; these losses occurred after week ten. Nine (56.3) had antiphospholipid syndrome associated with systemic lupus erythematosus. The main serological marker was lupus anticoagulant in 12 (75%) pregnant women. Two (12.5%) patients had triple positivity and six (37.5%) double positivity of antiphospholipid antibodies. The most frequent obstetric complications were premature rupture of membranes (28.6%), pre-eclampsia (13.3%), and placental insufficiency (11.8%), which were more common in patients with systemic lupus erythematosus, as well as in those with triple and double antiphospholipid antibody positivity.
ConclusionsObstetric antiphospholipid syndrome was associated with systemic lupus erythematosus in the majority of cases; however, the latter was in remission. The worst obstetric outcomes were observed in patients with this association or in cases with triple or double antiphospholipid antibody positivity.
Describir las características clínicas, así como los desenlaces maternos y perinatales en gestantes con síndrome antifosfolípido en una institución de alta complejidad.
Materiales y métodosEstudio descriptivo retrospectivo que evaluó gestantes con síndrome antifosfolípido según criterios de Sidney o según criterio de reumatólogo entre 2010 y 2016. Se excluyeron aquellos casos con trombofilias hereditarias, incompetencia cervical o con antecedente de hepatitis B, hepatitis C y virus de inmunodeficiencia humana. La información se recolectó a partir de la revisión de historias clínicas.
ResultadosSe incluyeron 16 gestantes; 7 (43,8%) pacientes tenían antecedente de aborto, 5 (71,4%) en más de una ocasión; estas pérdidas ocurrieron después de la semana 10. Nueve (56,3) tenían síndrome antifosfolípido asociado a lupus eritematoso sistémico; el principal marcador serológico fue el anticoagulante lúpico en 12 (75%) gestantes. Dos (12,5%) pacientes tenían triple positividad y 6 (37,5%) doble positividad de anticuerpos antifosfolípidos. Las complicaciones obstétricas más frecuentes fueron: rompimiento prematuro de membranas (28,6%), preeclampsia (13,3%) e insuficiencia placentaria (11,8%), las cuales se presentaron más en pacientes con lupus eritematoso sistémico, así como en aquellas con triple y doble positividad de anticuerpos antifosfolípidos.
ConclusionesEn esta cohorte, el síndrome antifosfolípido obstétrico estuvo asociado a lupus eritematoso sistémico en la mayoría de los casos; sin embargo, éste último estaba en remisión. Los peores desenlaces obstétricos se observaron en pacientes con síndrome antifosfolípido y lupus, o en los casos que tenían triple o doble positividad de anticuerpos antifosfolípidos.
The antiphospholipid syndrome (APS) is an autoimmune, non-inflammatory disease associated with the persistent production of antiphospholipid antibodies (aPL) such as: anticardiolipin antibodies (ACA), or anti-β2 glycoprotein-I (aβ2GPI) antibodies, with evidence of functional activity determined by the presence of lupus anticoagulant (LA). This entity may result in venous or arterial thrombosis (thrombotic APS), or obstetric morbidity (preeclampsia, preterm delivery, intrauterine growth restriction or fetal loss).1,2
Being a syndrome, it tends to be classified as one single disease, but the pathophysiological mechanisms of the thrombotic and obstetric manifestations differ, since whilst in thrombotic APS the double “hit” theory applies – meaning that the aPLs generate the first “hit” by causing a prothrombotic state, but a second “hit” is needed to drive thrombi formation (pregnancy, prolonged immobilization, surgery or discontinuation of anticoagulation) –, in obstetric APS, the aPLs generate directly an inflammatory process and complement activation, as a result of the characteristic clinical outcomes during pregnancy.2–6
The prevalence of aPL among the general population ranges between 1–5%, while the prevalence of APS ranges between 40–50 cases per 100,000 people, being more frequent in women.5,7 However, its presence in certain specific populations is higher, such as for instance in patients with thrombotic events or recurrent fetal losses, when the frequency of these antibodies may rise to 40%.1 APS may be present in patients with no clinical or laboratory evidence of some other condition (primary APS) or may be associated to other diseases, mainly systemic lupus erythematous (SLE), malignant tumors, infections or certain substances.5,7
It has been reported that up to 40% of the patients with SLE may present aPL, but a smaller percentage may develop clinical manifestations of thrombotic APS. Additionally, both diseases may cause clinical manifestations during pregnancy, causing changes in the evolution of pregnancy in the presence of APS or SLE, or both combined.8–10
The most representative study so far published worldwide, assessing obstetric APS is a cohort of 247 European women, mostly white, called EUROAPS. The most common obstetric manifestations in this cohort were recurrent miscarriage and fetal loss. Furthermore, thrombosis and progression to SLE in patients with obstetric APS was less common than in the classical APS, suggesting different pathophysiological mechanisms.1 Other international studies have addressed their research toward establishing causal relationships between the type of antibodies and the obstetric outcomes.11,12
In our setting, 2 studies have been conducted describing the characteristics of patients with APS, and among them the subgroup of obstetric APS, with fetal demise being the most frequent obstetric manifestation.13,14 These studies have provided relevant data about APS, but further knowledge is required about the characteristics and maternal and perinatal outcomes. This is the reason for conducting this study, with the objective of assessing the epidemiological, clinical and therapeutic characteristics, as well as obstetric, thrombotic and postpartum morbidity in pregnant patients diagnosed with obstetric APS, at a high complexity level institution.
Materials and methodsDesign and populationA retrospective, descriptive study was conducted, with a population of patients of any age, with obstetric APS, monitored between 2010 and 2016, at the maternofetal and rheumatology medical service of a high complexity level institution. During the study period, the patients included met the Sidney 2016 criteria.15 Patients with other conditions such as hereditary thrombophilia, incompetent cervix or a history of hepatitis B and hepatitis C virus, and human immunodeficiency virus were excluded.
An e-form was developed to collect the information using MAGPI™, with fields with restricted input of data to reduce typing errors. Upon receiving ethical endorsement, the participating institution was required to submit a list of the medical records completed at the outpatient clinic, hospitalization and emergency department, that were identified with the International Classification of Diseases code (ICD-10) associated with APS: postpartum coagulation defect (O733), superficial thrombophlebitis in pregnancy (O222), deep phlebothrombosis during pregnancy (O223), cerebral venous thrombosis during pregnancy (O225), other venous complications during pregnancy (O228), non-specific venous complication during pregnancy (O229), superficial thrombophlebitis during puerperium (0870), deep phlebothrombosis during puerperium (O871), cerebral venous thrombosis during puerperium (O873), other unspecified venous complications during puerperium (O879), other venous complications after miscarriage, ectopic pregnancy (O087), recurrent miscarriage (O262), other types of thrombophilia (D686), other specific coagulation disorders (D688) and unspecified coagulation defect (D689).
Afterwards, the medical records of the patients that met the eligibility criteria for the trial were reviewed and the socio-demographic, clinical and therapeutic information was collected from the time of admission to the institution until the end of pregnancy.
VariablesSocio-demographic, clinical: concomitant chronic diseases; history of SLE and visceral organic involvement, SLEDAI activity index; obstetric history: number of pregnancies, gestational age at the time of delivery, stillbirths, occurrence and number of miscarriages before or after 10 weeks of gestation, number of live children, preterm delivery (before week 34), preeclampsia and eclampsia, placental insufficiency, premature rupture of membrane, intrauterine growth restriction, gestational age at birth and intra/post-partum complications.
APS-associated variables: age at diagnosis, serum markers, pharmacological treatment, history or development of thrombosis and type of thrombosis; non-obstetric (infection, thrombocytopenia, kidney failure) and obstetric complications.
Statistical analysisThe qualitative variables were expressed as absolute and relative frequencies, and the qualitative using the means and the standard deviation (SD) or the median and the interquartile range (IQR), depending on the data distribution. All of the analyses were done using the IBM SPSS® software, version 22.
Ethical considerationsAccording to the current national legislation, this research was classified as a no risk investigation, given its retrospective design that required reviewing medical records. Endorsement of the Health Research Committee, and the authorization by the participating institution were obtained.
Results700 medical records of patients with APS-associated diagnoses ICD-10 were reviewed, of which 16 pregnant women (17 pregnancies) met the eligibility criteria. The average age of the mothers was 30.5 years (SD: 3.9); 12 (75%) women came from the urban area and 15 (94%) had tax-payer insurance. In terms of schooling, 5 (31%) had been to college.
The mean age at the time of the APS diagnosis was 28 years (SD: 5.7) and the average gestational age was 20.2 weeks (SD: 11.2; range: 4–37.2). Two patients (12.5%) met the criteria for thrombotic APS (one patient with SLE associated with a history of arterial thrombosis and one patient had a history of venous thrombosis) and 14 (87.5%) patients were classified as obstetric APS, with no thrombotic complications.
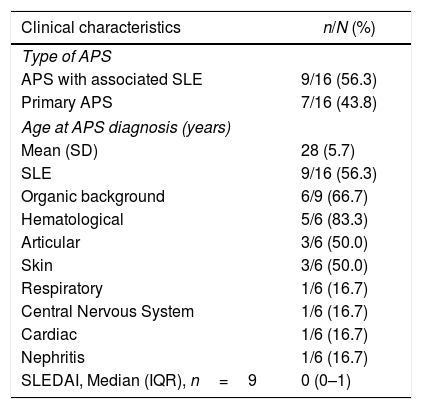
Of the 16 patients, 9 (56.3%) had a history of associated SLE; of these, 6 (66.7%) joined the study with a history of organic involvement, primarily hematological; however, the mean SLEDAI at admission was 0 points (Table 1). In addition to SLE, other underlying conditions were identified, including: sickle cell anemia (n=1), primary biliary cirrhosis (n=1), obesity (n=1), autoimmune hepatitis (n=1) and thrombocytopenic idiopathic purpura (n=1).
Clinical characteristics of pregnant women with obstetric antiphospholipid syndrome in an institution in Medellín, 2010–2016.
| Clinical characteristics | n/N (%) |
|---|---|
| Type of APS | |
| APS with associated SLE | 9/16 (56.3) |
| Primary APS | 7/16 (43.8) |
| Age at APS diagnosis (years) | |
| Mean (SD) | 28 (5.7) |
| SLE | 9/16 (56.3) |
| Organic background | 6/9 (66.7) |
| Hematological | 5/6 (83.3) |
| Articular | 3/6 (50.0) |
| Skin | 3/6 (50.0) |
| Respiratory | 1/6 (16.7) |
| Central Nervous System | 1/6 (16.7) |
| Cardiac | 1/6 (16.7) |
| Nephritis | 1/6 (16.7) |
| SLEDAI, Median (IQR), n=9 | 0 (0–1) |
In terms of the gyneco-obstetric history, most patients had multiple pregnancies (62.5%). Seven patients (43.8%) had a history of miscarriages, 5 of them (71.4%) had experienced 3 miscarriages and all the losses occurred after week 10. None of them met the criterion for recurrent fetal demise as an obstetric APS criterion.
Stillbirths occurred in 3 patients (18.8%) and these happened between weeks 24 to 28. Six (37.5%) patients had children born alive; of these, only one (16.7%) had been born prior to week 37. One patient had a history of placental insufficiency and another one of preeclampsia; none had a history of eclampsia.
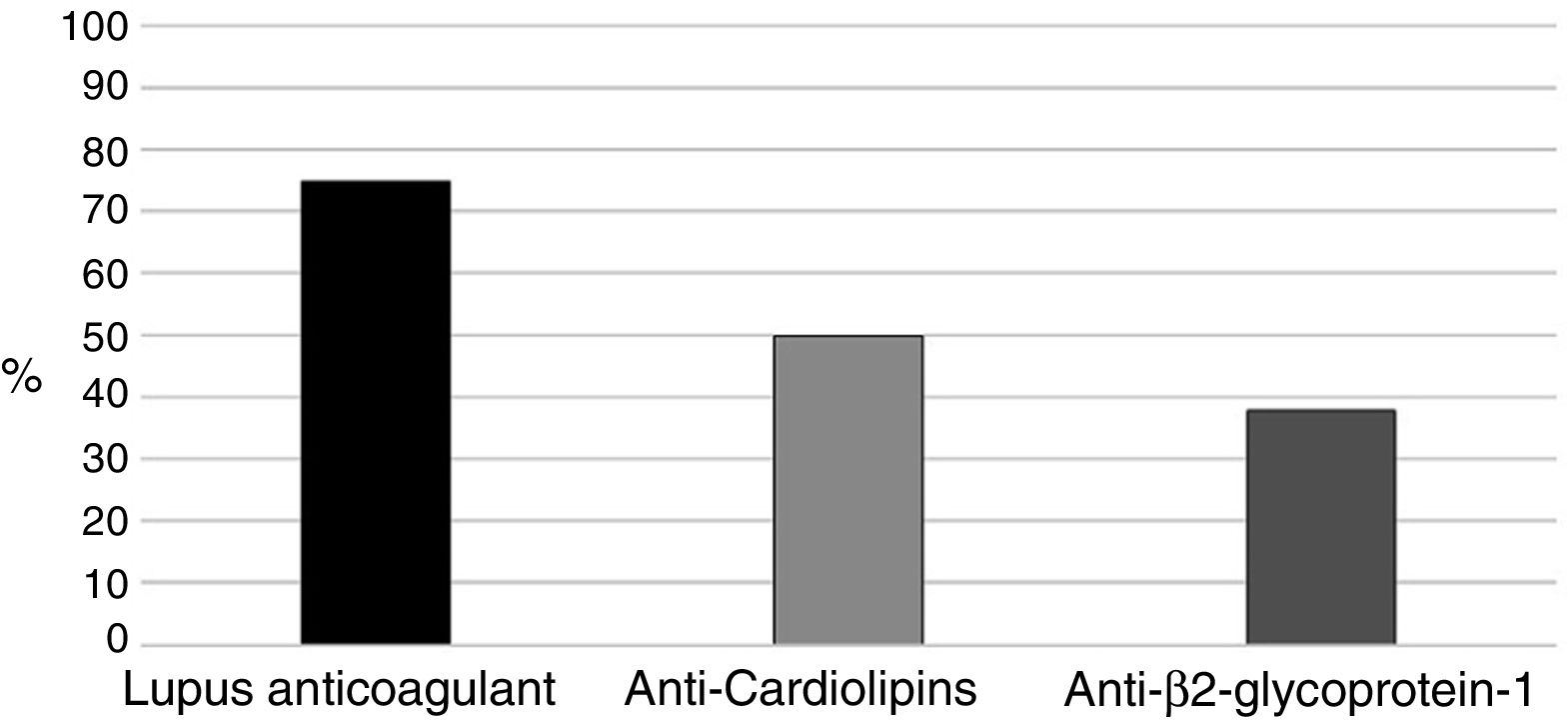
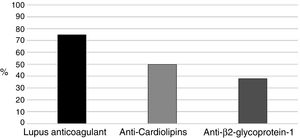
With regards to aPL (positive in all 16 patients), the primary marker was LA (Russell's viper venom confirmatory test) (75%), followed by aCL in 8 pregnant women (50%) (Fig. 1). Two (12.5%) patients were triple positive and 6 (37.5%) double positive including LA, 2 in the presence of anti-β2glucoprotein I antibodies (aβ2GPI) and the remaining 4 had ACA.
Of the 17 pregnancies, 6 (35.3%) experienced complications (2 cases of gestational intrahepatic cholestasis, 2 serious events of thrombocytopenia (platelet count below 20,000mm–3), one patient with rheumatoid arthritis and another one with undifferentiated connective tissue disease). Five of these patients with complications had LA, of which 2 had triple aPL positivity and experienced a lupus relapse that required hospital admission due to severe thrombocytopenia in one patient, and development of serositis and rheumatoid arthritis in the other one.
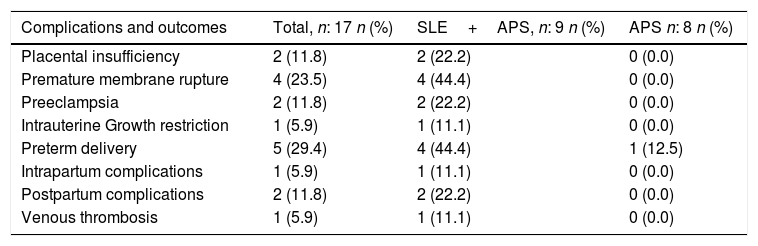
Moreover, the obstetric complications were more frequent in patients with SLE and associated APS (Table 2). The placental insufficiency evidenced with ultrasound, regardless of the diagnosis of IUGR and preeclampsia, was present in 2 pregnant women with SLE and positive LA; One of them had a double positivity in the presence of aCL. Likewise, the 4 cases of premature membrane rupture in patients with SLE, developed in 3 pregnant patients with LA (one case of double positivity with aCL and the other with triple positivity).
Complications and obstetric outcomes in patients with obstetric antiphospholipid syndrome in an institution in Medellín, 2010–2016.
| Complications and outcomes | Total, n: 17 n (%) | SLE+APS, n: 9 n (%) | APS n: 8 n (%) |
|---|---|---|---|
| Placental insufficiency | 2 (11.8) | 2 (22.2) | 0 (0.0) |
| Premature membrane rupture | 4 (23.5) | 4 (44.4) | 0 (0.0) |
| Preeclampsia | 2 (11.8) | 2 (22.2) | 0 (0.0) |
| Intrauterine Growth restriction | 1 (5.9) | 1 (11.1) | 0 (0.0) |
| Preterm delivery | 5 (29.4) | 4 (44.4) | 1 (12.5) |
| Intrapartum complications | 1 (5.9) | 1 (11.1) | 0 (0.0) |
| Postpartum complications | 2 (11.8) | 2 (22.2) | 0 (0.0) |
| Venous thrombosis | 1 (5.9) | 1 (11.1) | 0 (0.0) |
The two cases of preeclampsia in patients with APS and associated SLE also occurred in patients with LA, one in the presence of double positivity with aCL; The only case of IUGR presented in one of the patients with double positivity (LA+aCL), that also experienced placental insufficiency and preterm delivery. Furthermore, 3 patients presented thrombocytopenia over the gestation period, one had triple positivity of aPL and one had double positivity of aPL (AL+aβ2GPI); of these, one had preterm delivery and the other one premature membrane rupture and preterm delivery.
There was just one complication during delivery (hemorrhage due to uterine atony) in one patient with double positivity of aCL (LA+aCL). During the postpartum, there were 2 complications due to infections: one patient with endometritis and a second one with surgical site infection; both complications were found in patients with APS and associated SLE (Table 2).
In terms of other obstetric outcomes, none of the patients developed gestational diabetes, eclampsia, arterial thrombosis or fetal demise; likewise, there were no maternal deaths.
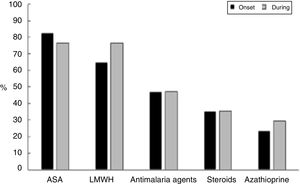
Fig. 2 shows the therapeutic management given to these patients, who received acetylsalicylic acid (ASA) in 14/17 pregnancies (82.4%) and low molecular weight heparin (HBPM) at prophylactic doses in 11/17 cases (64.7%) (indicated based on the obstetric manifestations of APS); this treatment remained constant in most of the patients throughout pregnancy. Only one patient did not receive ASA or LMWH in the course of her pregnancy, though she was treated with immunosuppressors. This latter patient developed venous thrombosis, lupus relapse with thrombocytopenia requiring steroid pulses and presented preterm delivery as an obstetric complication.
DiscussionThis trial assessed the principal characteristics of pregnant women with primary APS or associated with SLE, as well as the maternal and perinatal outcomes in a high complexity level institution. The key findings included: (1) The patients with obstetric APS associated with SLE were more numerous that the patients with primary APS; (2) the main clinical criterion for obstetric APS was miscarriage after 10 weeks; (3) the triple positivity for aPL was observed in pregnant mothers with SLE, who developed both clinical and obstetric adverse outcomes; and (4) the obstetric complications and outcomes associated with the presence of aPL were more frequently observed in patients with APS and associated SLE.
The results achieved show some differences as compared to other published series; one of these differences was the average age of the patients in this cohort, which was 10 years younger1,11–14,16; this may be explained by the younger age at which Colombian women become pregnant as compared to the Europeans,17,18 as well as by the nature of the Colombian cohorts in which thrombotic manifestations were assessed, that tend to present a later onset.19,20
In contrast to previous studies where patients more often exhibited primary APS,1,21,22 most of the patients had APS associated with SLE. This fact may be attributed to the type of institution and the population studied, in which there was a higher probability to recruit patients with severe comorbidities because of the level of complexity.
Moreover, the gestational age at admission to the trial was in average 20.2 weeks, which is already late, considering that the primary manifestations of the Anti-Phospholipid Syndrome develop over the course of the first trimester of pregnancy.23,24 The main clinical criterion for obstetric APS was miscarriage after week 10, different from other cohorts in which the criterion was recurrent fetal losses.11 This may reflect administrative difficulties to access high-level complex medical care (rheumatology or maternofetal medicine) or it could be due to a lack of awareness of the pathology among the medical community, and so cases of recurrent fetal losses may have been overlooked.
In terms of the serum profile, triple positivity of aPL was present in two pregnant women with obstetric APS associated with SLE; these patients had both clinical and obstetric adverse outcomes, similar to the report by Latino et al. and Pons Estel et al.,21,25 reflecting a profile of higher obstetric risk associated with this serologic group. Outcomes such as placental insufficiency, restricted intrauterine growth and preterm delivery, were only seen in patients with LA or double positivity including LA. This is important since LA is a functional test associated with poorer obstetric outcomes.1,26
In addition to the complications and obstetric outcomes, these were more frequent in patients with associated SLE, which could be an indication of a more aggressive behavior of the disease, in the presence of aPL. Several studies have reported an increased frequency of obstetric morbidity in patients with SLE and positive aPL (up to 47%).27 In this study, the patients with SLE that experienced unfavorable obstetric outcomes had aPL, particularly LA or aCL and, although due to the descriptive design of this research, it is not possible to determine any type of relationship, the findings somehow evidence the aPL involvement in the obstetric outcomes of mothers with SLE, and are consistent with the publications.28
With regards to pharmacological management, all patients admitted in the trial were being treated for APS or SLE; this is so because these were patients arriving at a referral center and also due to the advanced gestational age at the time of admission. Most patients received antiplatelet or antithrombotic therapy indicated for APS, and in most of the cases, this treatment was continued throughout pregnancy. The only patient who did not receive ASA or LMWH developed venous thrombosis, thrombocytopenia and had preterm delivery, which evidenced the importance of antithrombotic management for this condition.8 Furthermore, none of the patients experienced pregnancy loss, in contrast to other cohorts with obstetric APS, that reported up to 16.9% of losses.25 The probable explanation may be the small number of patients included in this study and ethnic differences, since the treatment administered of the Argentinian cohort by Latino et al.25 was the same treatment given to the patients in this cohort.
One of the weaknesses of this study is the small number of pregnant women assessed, in addition to its retrospective nature, probably leading to under-registration of potentially eligible patients. However, this number of patients was the result of recruiting over 6 years, and all the potential ICD-10 codes for this disease were comprehensively reviewed by 2 investigators, as well as the databases of both services (Rheumatology and Maternofetal Medicine) in order to reduce any potential selection biases of the population, in addition to striving to identify any undetected cases using the diagnostic codes.
Another bias that could have existed was the hospital bias. Since these were patients receiving care at a high level of complexity institution, probably this fails to reflect the conditions of pregnant women receiving lower levels of care, which could limit the applicability of the results. Nevertheless, the patients assessed in this study reflect the population with the opportunity to receive care at a rheumatology service that often deals with patients with obstetric APS and associated SLE.
The major strength of this research effort is that – as far as we know – this is the first attempt in our country to assess in detail the characteristics of obstetric APS, its co-existence with SLE, the immunological profile and the clinical outcomes. Finally, whilst the descriptive design of the study prevents us from establishing any type of association, the results allow us to formulate hypotheses for new studies and expand our knowledge in this particular area.
ConclusionsA series of homogeneous baseline characteristics were observed in this series of patients with APS, with an average age 10 years younger than other cohorts and in which there were poorer obstetric and non-obstetric outcomes in patients with associated SLE. In addition, those patients with triple positivity of antiphospholipid antibodies or double positivity including LA, experienced more aggressive obstetric complications.
FinancingThe authors’ resources.
Conflict of interestsThe authors have no conflict of interests to disclose.
Please cite this article as: Restrepo Ocampo C, Arango Gutiérrez L, Rodríguez Padilla LM, Mesa Navas MA, Velásquez Franco CJ, Gutiérrez Marín JH, et al. Manifestaciones clínicas y desenlaces maternos y perinatales en gestantes con síndrome antifosfolípido obstétrico de una institución de alta complejidad: estudio descriptivo. Rev Colomb Reumatol. 2020;27:73–79.