Brain stem gliomas are a heterogeneous group of tumors regarding both clinical presentation and prognosis. They can be classified on the basis of their biological behaviour, anatomical location and radiographic appearance on MRI. The choice of treatment depends largely on whether the tumor is a diffuse intrinsic pontine glioma or not. A better understanding of the biology of these tumors can be the key for progress in treatment. The purpose of this article is provide updated information to enable a detailed understanding of this group of tumors and thus help to optimize the management of this condition in the pediatric population.
Los gliomas del tronco encefálico son un grupo heterogéneo de tumores tanto en la presentación clínica como en el pronóstico. Se pueden clasificar en función de su comportamiento biológico, localización anatómica y la apariencia radiográfica en la RM. La elección del tratamiento depende en gran medida de si el tumor es un glioma intrínseco difuso del puente o no. Una mejor comprensión de la biología de estos tumores puede ser la clave para el progreso en el tratamiento. El propósito de este artículo es proporcionar información actualizada que permita una comprensión detallada de este grupo de tumores y así ayudar a optimizar el tratamiento de esta condición en la población pediátrica.
The term “brain stem gliomas” is an inaccurate unifying classification- suggesting that these tumors share the same behaviour when in fact their biology is very heterogeneous, comprising low grade and high grade tumours, with significant differences in clinical picture, treatment and prognosis. Treatment and prognosis depends not only on the histological features, but also its location within the brain stem. In recent decades, the treatment has progressed significantly, a consequence of advances in imaging technology and microsurgical techniques. Surgery is the initial treatment of choice in the focal gliomas and chemotherapy is used only in persistent, recurrent or inoperable cases. Furthermore, the conformal external beam radiation therapy is the only known effective treatment for diffuse intrinsic pontine glioma (DIPG), but thanks to recognition by the scientific and medical community of the importance of biopsy in the past years, there has been an explosion in biological studies that keep opening the possibility of new treatments.1 In this article, the most recent literature on brain stem gliomas, including epidemiology, pathology, classification, symptoms, diagnosis, treatment and prognosis is reviewed; with focus on possible future therapy and in order to help optimize the management of this condition in children and adolescents and be a support for future studies that let us improve the survival of patients with these tumors.
The authors conducted a search of references of recent articles addressing the subject of interest in the database MEDLINE, through PubMed search engine using keywords, combining various operators and search filters. Subsequently the selected articles were downloaded using the database of the university's library.
EpidemiologyBrain stem gliomas account for 10–20% of all primary CNS tumors in childhood and adolescence1,2 accounting for ∼200–300 cases per year in the US. It is one of the most difficult to treat in pediatrics.3
The average age of onset is from 5 to 10 years old and the ratio is 1:1 among boys and girls. There are no recognized premalignant lesions; however, a number of familial cancer syndromes have been associated with it, including neurofibromatosis type 1, Li-Fraumeni syndrome, and tuberous sclerosis, among others.4
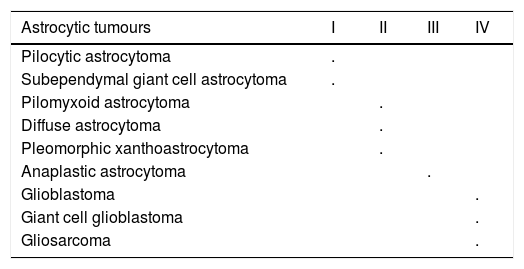
PathologyThe latest WHO classification of CNS tumors maintains the grading system for tumors in general and is based on four histological criteria to determine the degree of malignancy: degree of nuclear pleomorphism, degree of mitosis, vascular proliferation and necrosis. The criteria for each type of tumour apply only to untreated tumors, because the therapy can alter tumor morphology.5,6 Therefore, the WHO system recognizes four degrees of malignancy for astrocytic tumors, providing an estimate of their biological behavior and covers grade I astrocytomas, the least aggressive that can be cured by surgery alone, up to grade IV astrocytomas; highly aggressive tumors, infiltrating the surrounding brain tissue and fatal within an average of one year.6 See Table 1.
WHO Grades of CNS tumours.5
| Astrocytic tumours | I | II | III | IV |
|---|---|---|---|---|
| Pilocytic astrocytoma | . | |||
| Subependymal giant cell astrocytoma | . | |||
| Pilomyxoid astrocytoma | . | |||
| Diffuse astrocytoma | . | |||
| Pleomorphic xanthoastrocytoma | . | |||
| Anaplastic astrocytoma | . | |||
| Glioblastoma | . | |||
| Giant cell glioblastoma | . | |||
| Gliosarcoma | . |
Unfortunately, 85% of brain stem gliomas are high-grade gliomas and most of these correspond to diffuse intrinsic pontine glioma (DIPG).7
Diagnosis, clinical picture and imagesToday, many authors believe that you can make a reliable diagnosis without a histopathological diagnosis. In fact, in 2007 Schumacher and colleagues carried out a blind review of the clinical, radiological and histological data of 142 pediatric cases with brain stem involvement, including 78 cases diagnosed with gliomas. The three observers were able to correctly identify lesions on magnetic resonance imaging (MRI) in 96.5% of cases on average; concluding that biopsy can be reserved for atypical cases.8,9
Other studies have evaluated the correlation between MRI characteristics and histopathological diagnosis of diffuse high-grade glioma in patients with intrinsic brainstem lesions, finding accuracy of diagnosis in 90% of enhancing lesions on MRI.10 But in turn, in a series of 44 patients with suspected DIPG, 10% had a different histopathological diagnosis from glioma11 and in a cohort of adults, the diagnosis was different from the original in 9 of 13 cases.12 Additionally, in 2011, Hankinson et al. reported the results of a survey of pediatric neurosurgeons on MRI findings in selected cases of DIPG (including typical and atypical cases): 75% or greater agreement about whether a tumor was typical or atypical was found only in 43.8% of cases and it was concluded that in clinical practice, diagnosing DIPG based only on imaging characteristics and medical history, does not reach the appropriate threshold to be considered a standard management.13
However, the Second Consensus Conference of Pediatric Neurosurgery has endorsed the conclusion recorded by Schumacher14 and therefore beginning treatment without pathological confirmation is an accepted practice. However, several authors emphasize the importance of an histopathological diagnosis and highlight that although many experimental agents have been tested in recent decades, there is currently no evidence of improved overall survival in this group of patients, and only detailed knowledge of these tumors can allow the development of new agents and improve results, a process that undoubtedly requires the availability of tumor tissue.11
For these reasons, some studies have sought to define the morbidity of surgical procedures, when performed on tumors in this location. The results suggest that stereotactic biopsy can be performed safely in children, with minimal morbidity, the same rate of postoperative complications when compared to biopsy procedures in other locations of the brain, 5% lower mortality and diagnostic yield of 90–100%.11,15,16
In this context, conventional MRI (along with other advanced techniques such as diffusion tensor imaging and tractography), are relevant for assessing the optimal biopsy site, and identifying tumor regions with high level features.4,17,18 Additionally, some studies have identified certain image biomarkers at diagnosis that allow differentiation between early versus later stages of the disease, discern biological properties of the tumor and correlate these features with the results.17,19 One of these biomarkers is tumor contrast material enhancement on T1-weighted images. Studies suggest that enhancement (manifest or “hidden”) occurs in about 90.2% of cases of DIPG diagnosis.17 Additionally, enhancement at baseline and over time has been associated with shorter survival; while greater decreases in tumor volume and ADC map measures (apparent diffusion coefficient) have been associated with longer SLP and SG (p=0.006).19
Consequently, MRI has become the method of primary diagnosis of brain stem gliomas and based on the characteristics of MRI and other factors such as size and location there have been many classification systems.20 Epstein and McCleary grouped nonexophytic brainstem gliomas into focal medullary, dorsal exophytic, diffuse and cervicomedullary junction tumors. Choux, et al. described four tumor types: type I, diffuse brain stem gliomas; type II, focal intrinsic tumors (solid or cystic); type III, exophytic; and type IV, cervicomedullary. Mehta et al. in 2009, proposed a specific classification for the intrinsic gliomas, indicating with this term that the tumor is still contained within the limits of the brainstem, classifying as expansive, diffuse infiltrative and ventral location varieties.20–22
Others have considered all brain stem gliomas should be categorized simply as diffuse or focal, or DIPG and all others, based on the opportunity for surgical treatment, an important prognostic factor.21,23 We can then make a description of the clinical picture and imaging of brain stem gliomas according to this classification.
Diffuse intrinsic pontine glioma (DIPG)DIPG are the most frequently occurring brain stem gliomas (60–85% of cases) and have the worst prognosis of all brain tumors in children. The onset is usually subacute, with symptoms of less than 3 months of evolution and the majority are high-grade gliomas.1
The classic clinical presentation includes the triad of multiple cranial nerve involvement, ataxia and pyramidal signs. Because of its origin in the pontine region of the brain, the commitment of the sixth and seventh cranial nerves is especially common.1 You can have an atypical presentation with psychiatric signs (changes in mood, behavior, obsessive-compulsive symptoms), or acquired torticollis.8
Overall, the classic MRI of brain stem gliomas is hypointensity on T1-weighted images and hyperintensity on T2, and necrosis in about 22% of cases. In addition to these common findings, DIPG is characterized by marked pons hypertrophy and infiltration (involvement greater than 50% of its volume), usually larger than 2cm in length at debut, with cefalocaudal extension to the midbrain, brain peduncles, cerebellum or medulla oblongata, and ill-defined margins, all reflections of their invasive nature.1,3,24
It is characteristic of DIPG to have a ventral exophytic component of the tumour with basilar artery entrapment and minimal or no contrast enhancement, allowing it to be distinguished between pilocytic astrocytomas and all other tumors found in the brain stem.3,24 Finally, leptomeningeal spread at diagnosis is rare, but can occur and if there are signs suggestive of disseminated disease it is advised to take pictures of the neuraxis.1
In imaging spectroscopy, there are choline/N-acetylaspartate (NAA) and creatine/choline ratios significantly higher, with strong peak in lipids.7,25 Magnetic resonance spectroscopy is a useful tool to guide the biopsy procedure and monitoring. After radiation therapy (RT), conventional MRI shows contrast enhancement, finding that this can be attributed to the effect of radiation necrosis or progression. Radionecrosis is not easily differentiated from tumor progression compared only to contrast enhancement, but the increase in the ratio cholin/NAA and creatine/choline, associated with a significant reduction in “apparent” levels of citrate in the enhancement areas, are metabolic changes suggestive of malignant transformation.18,25
Focal brainstem gliomasIn general, the focal gliomas are tumours of favourable prognosis, whose histology is almost always pilocytic or other low-grade gliomas. They are characterized by slow growth rate and limited infiltrating capacity, causing long-lasting symptoms.4,23 They are subdivided into:
Focal pontine glioma or focal medulla oblongata gliomaThese comprise 10–20% of brain stem gliomas in children and are often exophytic on dorsal location.4 These arise on subependymal glia and therefore, most of the tumor resides within the fourth ventricle, sometimes rubbing it out. Typical clinical picture is insidious, with long standing nonspecific headache and vomiting.4,18
Standard MRI usually shows well defined lesions, non infiltrating, without edema, with the typical hypointensity on T1 and hyperintensity on T2 and a dorsal exophytic expansion toward the fourth ventricle. It is common to see an associated cystic component.4,18 After gadolinium administration, the solid part of the tumor shows homogeneous and intense enhancement, which makes it difficult to distinguish from ependymoma and choroid plexus papilloma. Recent evidence suggests that tumors which show a more lateral and ventral exophytic pattern, are usually of greater grade than those projecting into the fourth ventricle.4
Cervicomedullary junction gliomasThese contribute 5–10% of brain stem gliomas in children.4,18 The epicenter of these gliomas can be in the medulla oblongata or upper cervical spinal cord, causing brain stem dysfunction and myelopathy signs. Therefore, patients whose tumor site is within the medulla oblongata first develop nausea, vomiting, obstructive hydrocephalus, growth retardation, difficulty speaking and swallowing, chronic aspiration, sleep apnea and head tilted positions. When the focus of the tumor is in the upper cervical spine, symptoms are characterised by neck pain, progressive quadriparesis with changes in gait, hyper- or hyporeflexia, pathological plantar reflex and sensory symptoms; or early establishment of handedness and motor regression in infants. However, it is common to find a mixture of signs and symptoms.2,3
On MRI these tumours are well circumscribed and show mixed intensity signal within the solid tumour and uniform contrast enhancement. MRI is also useful to identify any cyst or syringomyelia associated.4,18
Midbrain gliomasThese include focal tumours and some with diffuse extension towards the interpeduncular cistern or third ventricle, and tectal plate gliomas.22 The tectal gliomas are a subgroup with unique characteristics and represent less than 5% of brain stem gliomas. Usually they are low-grade astrocytomas, located in the midbrain retroacuaductal region in proximity to the aqueduct of Sylvius, causing obstructive hydrocephalus.26 Because of the indolent biological behavior, the usual clinical presentation is intracranial hypertension caused by hydrocephalus. Sometimes, they present with Parinaud syndrome and signs of endocrine dysfunction (precocious puberty, short stature).26,27
The typical initial MRI shows a lesion inside the tectal plate, sometimes with a subsequent exophytic component and the typical findings of hydrocephalus. These tumours usually show isointensity on T1-weighted images, hyperintensity on T2 and proton density sequences, often without contrast enhancement.26,27
Gliomas in other locationsThese are quite rare and represent less than 5% of brain stem gliomas. Most are low-grade tumors, but there have been reports of glioblastomas. The clinical presentation depends on location, but typically is long standing. MRI appearance varies; It can be a solid image that enhances the contrast and cystic.4,18
Differential diagnosisIt is important to differentiate between brain stem gliomas, other tumours, infectious processes, vascular malformations, storage diseases, neurodegenerative and demyelinating processes.1 Although less common in children, tumours of other histologic types can be found in the brainstem, such as atypical teratoid/rhabdoid tumor and embryonal tumours; tumours that usually present leptomeningeal spread at diagnosis.25
TreatmentThe choice of treatment depends not only on histological features, but also on location. The images help identify tumors amenable to surgical resection and candidates for conservative medical management (e.g., tectal gliomas and brainstem gliomas in patients with NF-1).4
SurgeryIn recent years, there have been great advances in the field of neurosurgery and to date there are twelve possible areas of “safe” access to brain stem gliomas described and therefore it is no longer considered an inoperable region.20,22 This progress has been driven by recognition that biological behavior is quite heterogeneous in this broad category of tumors and demands individualized treatment strategies 14,28
Regarding the potential surgical treatment, brain stem gliomas retain the division into two groups noted previously.23 With regard to DIPG, (tumors not amenable to resection due to its invasive nature), the Consensus on Pediatric Neurosurgery of 1996 had concluded that biopsy was not indicated in this tumor when there are suggestive images, because the histological classification did not change therapy or results. This view has prevailed in the second Consensus Conference on Pediatric Neurosurgery (CPN 2011), where it was concluded:
Statement 9: Biopsy of a “typical” DIPG (defined by clinical history and typical imaging findings) is justified when the patient is part of an ethically approved clinical study in which the tissue obtained will be used to investigate or inform the role of biological markers after treatment selection or molecular tumor grading.14
Statement 10: Biopsy of “atypical” pontine region tumors:
- (a)
performed by an experienced pediatric neurosurgeon, it is indicated to confirm the diagnosis and guide therapy.
- (b)
could be considered for therapy or research purposes. Other types of brain stem gliomas may be candidates for biopsy after a multidisciplinary discussion by the team of pediatric neuro-oncology.14
At the other extreme, it is now possible to identify that focal lesions are amenable to resection. These lesions, although of benign histology and biology, commonly show steady growth over time.4,18 So, focal dorsal exophytic gliomas are usually treated with surgery as the primary treatment modality. If a wide resection is achieved, adjuvant therapy is often deferred and treatment involves continuous observation only.18 Surgery is the treatment of choice for cervicomedullary junction gliomas, although total resection is not posible in approximately 75% of cases, due to poorly demarcated tumor margins or the unacceptably high risk of causing neurological deficit. Despite the subtotal resection, long-term tumor control is usually excellent.23
Deserving special comment are the tectal plate gliomas: benign lesions, which are properly treated with only shunt by endoscopic third ventriculostomy22 electively at presentation, because it seems to reduce mortality and morbidity associated with VP (ventriculoperitoneal) shunts.8,28 Tumor volume greater than 2cm in diameter and contrast enhancement can be radiological predictors of the need for additional treatment.26
In the other low-grade brainstem gliomas of deeper or ventral location, that are not susceptible to resection given the difficulty of surgical access, radiotherapy or chemotherapy is used, depending on the patient's age.24,28
RadiotherapyIn DIPG, involved-field RT (radiation therapy), administered at 30–33 fractions over 6 weeks to 54–60Gy total dose, is the only effective treatment but only temporarily, to delay tumor progression without change OS.30,31 Some studies suggest that hypofractionation (39 or 44.8Gy in fractions 4 times a week for 3 to 4 weeks respectively), may be a reasonable alternative to conventional fractionation in DIPG treatment, with similar results and the advantage of reducing the burden of treatment on children, their families and the health system; reducing by more than 50% the number of fractions and total treatment period.31,32
Furthermore, the role of hyperfractionation has been studied, but it has shown increased toxicity.33 Gamma Knife radiosurgery or stereotactic brachytherapy with iodine-125 (125I), are alternative minimally invasive, effective and safe for long term treatment of symptomatic low grade gliomas, non resectable or incompletely resected.34,35
ChemotherapyThe only indication of chemotherapy in brain stem gliomas justified to date, is in patients with recurrent low grade gliomas, symptomatic and non resectable, whom because their age, RT must be deferred as much as possible.23,36
In DIPG results have been disappointing and what role chemotherapy plays in the management of these patients has still not been established.1 Multiple clinical trials have evaluated the usefulness of different systems with strategies of single agent or multiple, including radiotherapy prior chemotherapy, radiotherapy with concomitant chemotherapy and adjuvant chemotherapy, but none have shown benefit in children with DIPG compared to RT alone.8,28,29
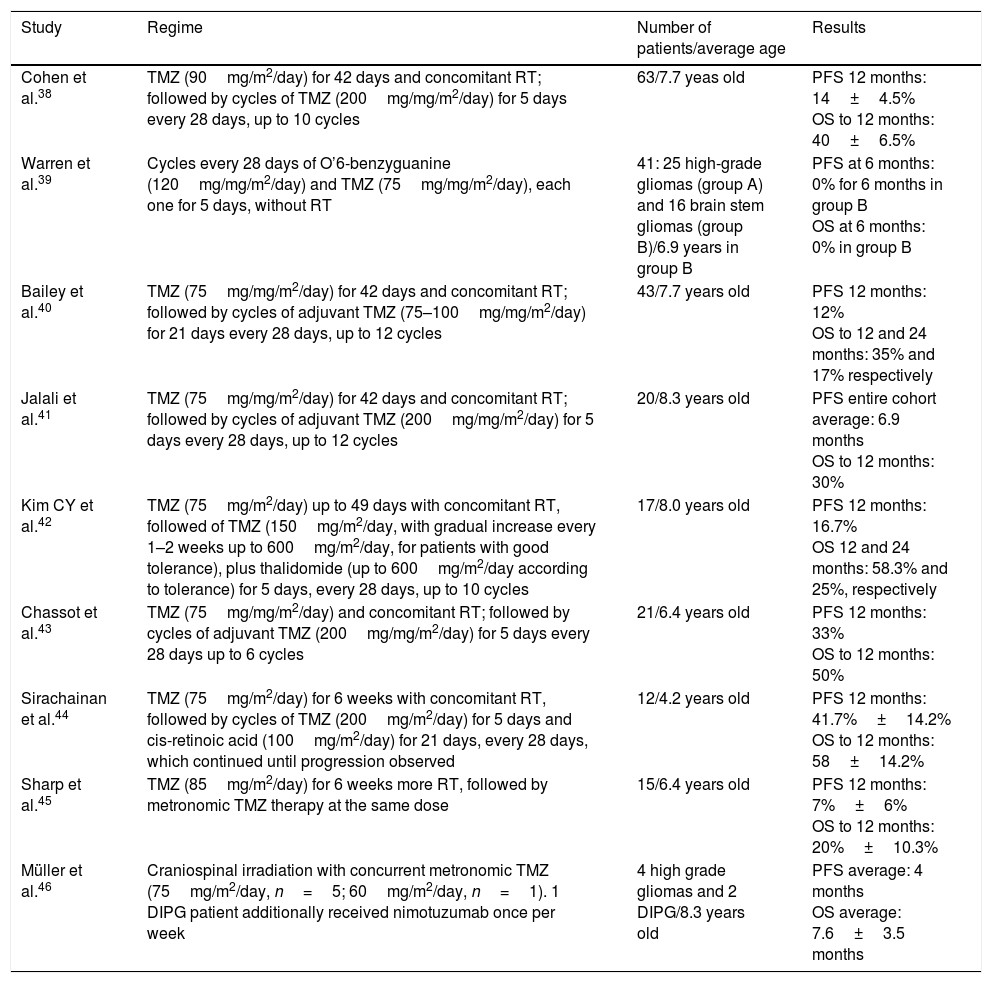
TMZ (Temozolomide) has been studied in multiple clinical trials, given their proven benefit in adults with high-grade gliomas37 but has failed to show significant improvement in results of pediatric patients with DIPG.28 The lack of effectiveness has been attributed, at least in part, to overexpression of DNA repair proteins in tumor cells. The enzyme is called O6-methylguanine-DNA methyltransferase (MGMT) and has been associated with this drug resistance.28,29 For these reasons, its routine use is not recommended in DIPG.28,38 See Table 2.
Summary of studies with temozolomide (TMZ) in DIPG.
| Study | Regime | Number of patients/average age | Results |
|---|---|---|---|
| Cohen et al.38 | TMZ (90mg/m2/day) for 42 days and concomitant RT; followed by cycles of TMZ (200mg/mg/m2/day) for 5 days every 28 days, up to 10 cycles | 63/7.7 yeas old | PFS 12 months: 14±4.5% OS to 12 months: 40±6.5% |
| Warren et al.39 | Cycles every 28 days of O’6-benzyguanine (120mg/mg/m2/day) and TMZ (75mg/mg/m2/day), each one for 5 days, without RT | 41: 25 high-grade gliomas (group A) and 16 brain stem gliomas (group B)/6.9 years in group B | PFS at 6 months: 0% for 6 months in group B OS at 6 months: 0% in group B |
| Bailey et al.40 | TMZ (75mg/mg/m2/day) for 42 days and concomitant RT; followed by cycles of adjuvant TMZ (75–100mg/mg/m2/day) for 21 days every 28 days, up to 12 cycles | 43/7.7 years old | PFS 12 months: 12% OS to 12 and 24 months: 35% and 17% respectively |
| Jalali et al.41 | TMZ (75mg/mg/m2/day) for 42 days and concomitant RT; followed by cycles of adjuvant TMZ (200mg/mg/m2/day) for 5 days every 28 days, up to 12 cycles | 20/8.3 years old | PFS entire cohort average: 6.9 months OS to 12 months: 30% |
| Kim CY et al.42 | TMZ (75mg/m2/day) up to 49 days with concomitant RT, followed of TMZ (150mg/m2/day, with gradual increase every 1–2 weeks up to 600mg/m2/day, for patients with good tolerance), plus thalidomide (up to 600mg/m2/day according to tolerance) for 5 days, every 28 days, up to 10 cycles | 17/8.0 years old | PFS 12 months: 16.7% OS 12 and 24 months: 58.3% and 25%, respectively |
| Chassot et al.43 | TMZ (75mg/mg/m2/day) and concomitant RT; followed by cycles of adjuvant TMZ (200mg/mg/m2/day) for 5 days every 28 days up to 6 cycles | 21/6.4 years old | PFS 12 months: 33% OS to 12 months: 50% |
| Sirachainan et al.44 | TMZ (75mg/m2/day) for 6 weeks with concomitant RT, followed by cycles of TMZ (200mg/m2/day) for 5 days and cis-retinoic acid (100mg/m2/day) for 21 days, every 28 days, which continued until progression observed | 12/4.2 years old | PFS 12 months: 41.7%±14.2% OS to 12 months: 58±14.2% |
| Sharp et al.45 | TMZ (85mg/m2/day) for 6 weeks more RT, followed by metronomic TMZ therapy at the same dose | 15/6.4 years old | PFS 12 months: 7%±6% OS to 12 months: 20%±10.3% |
| Müller et al.46 | Craniospinal irradiation with concurrent metronomic TMZ (75mg/m2/day, n=5; 60mg/m2/day, n=1). 1 DIPG patient additionally received nimotuzumab once per week | 4 high grade gliomas and 2 DIPG/8.3 years old | PFS average: 4 months OS average: 7.6±3.5 months |
Abbreviations: TMZ=temozolomide, RT=radiotherapy, PFS=progression-free survival, OS=overall survival.
Hoping to improve the response to conventional radiotherapy, various radiosensitizers have been investigated in the treatment of DIPG. The COG (Children's Oncology Group) conducted a Phase 2 study combining gadolinium-motexafin to RT, finding no benefit in overall survival.47 In Helsinki, due gliomas are highly vascularized tumors, the effect of RT with concomitant topotecan as a radiosensitizer was evaluated in DIPG, followed by antiangiogenic metronomic combination (thalidomide, etoposide and celecoxib) but significant difference in OS were not found compared to controls treated only with RT (median OS of 12 months vs. 10.5 months, respectively; log rank 0.127). However, there was a particular subgroup of patients (17%) who reported OS greater than 2 and 5 years, suggesting that anti-angiogenic therapy for patients with DIPG deserves further study in the future.48
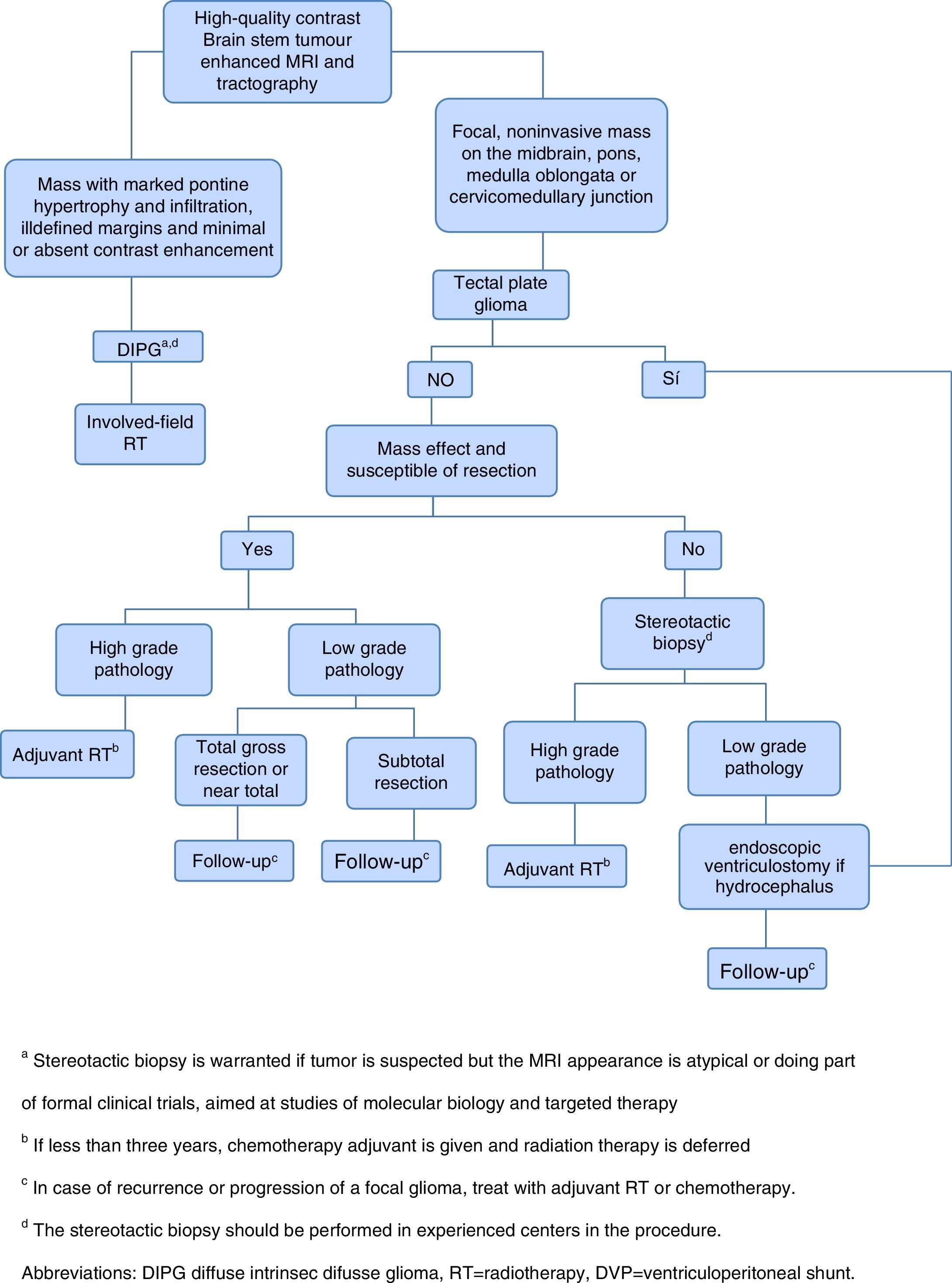
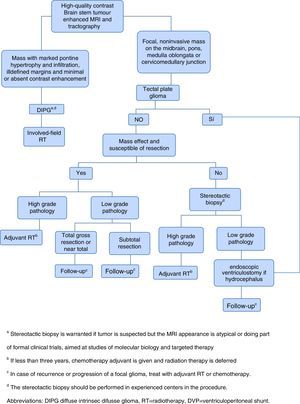
Given the frustrating lack of efficacy of modern chemotherapy in the treatment of DIPG, it has started the tireless search for new therapeutic alternatives.1 The current approach of treatment in children with newly diagnosed brain stem gliomas is summarized in Figure 1.
The future: targeted therapyThe biology of DIPG remained unknown until recently when the neurosurgical expertise along with the recognition by the scientific and clinic community of the importance of tissue sampling at diagnosis. We know now that pediatric DIPG is a heterogenous disease with variable molecular phenotypes, different from adult high-grade gliomas and even other pediatric high-grade gliomas.49
Identical mutations on two histone H3 genes, histone H3 family 3A (H3F3A) and histone H3b (HIST1H3B), have been identified in almost 80% of DIPG. These mutually exclusive mutations that result in the substitution of methionine for lysine at position 27 (K27M) of the histone, have drawn attention as potential targets for targeted therapy.50,51 Therefore some studies suggest that DIPG treatment should include an epigenetic modifier which inhibits JMJD3 demethylase (H3K27 histone demethylase) allowing physiological recovery in histone methylation, with reports of greater than 50% reduction in tumor size.49
In the last 5 years multiple agents of target therapy in the treatment of DIPG have been introduced, most of which are in phase I and II; and therefore survival rates should be interpreted with caution. A phase I trial of tipifarnib, an farnesyl transferase inhibitor (and thus Ras inhibitor), with concomitant radiotherapy, reported a 1 year OS rate of 36%.53 Also, they have been studied in phase I and II trials, the imatinib, an TKC inhibitor, reporting 1 year OS of 46%;54 and gefitinib, an epidermal growth factor receptor (EGFR) inhibitor, which has reported OS rates at 1 year between 48 and 56.4%.55,56 There is interest in identifying molecular markers to predict response to therapy, in similar way as the status of the MGMT,7,38 and there are signals that the presence of EGFR overexpression and mutations may predict response to EGFR inhibitors.52 In DIPG patients treated with erlotinib (another EGFR inhibitor) and concomitant RT, EGFR expression showed marginal association with better PFS, with a median of 10 months in EGFR positive (n=6), versus 6 months in EGFR negative patients (n=11) (HR: 0.35, p=0.058), but without improvement in OS (HR: 0.47; p=0.20).57
Other recent strategies have focused on the impact of proliferative signals mediated by the complex interaction of receptor tyrosine kinase/RAS/phosphatidylinositol 3-kinase pathway, p53 and RB; proangiogenic and Hedgehog pathways,49,52 overexpression of c-Met pathway, Aurora kinase B and IL-13α2 receptor; PDGFR and MYCN amplification, and several other vital intracellular signaling networks, mechanisms that are emerging as potential therapeutic targets in DIPG.1 In the “focal” brain stem gliomas, increased understanding of the biology of these tumors is leading the development of inhibitors of mutated BRAF proteins that activate the MAPK pathway, which are common mutations in this group of tumors.52
Factors such as molecular intratumoral heterogeneity underlying gliomatogenesis, difficulties in selecting patients, the CNS drug penetration and evaluation of response to therapy, and therapeutic resistance of glioma stem cells have limited the effectiveness of multiple agents; and other undiscovered key routes cannot be ruled out.52 However, a recent study suggests that DIPG per se is not resistant to chemotherapy and it observed that treatment failure may be caused by the expression of efflux proteins such as P-glycoprotein (P-gp), BCRP (breast -cancer-resistance protein) and MRP (multidrug-resistance-associated proteins), which mediate transmembrane active efflux of chemotherapeutic agents in gliom cells or tumor vasculature, resulting in the decrease of intracellular drug levels and cytotoxic activity, thus constituting a first line of treatment resistance in DIPG.58
Many questions remain unanswered, including the glioma stem cells, mutations and epigenetic alterations that contribute to tumor progression and maintenance, among others. New studies have to take into account the heterogeneity of DIPG between different patients and into the tumor, the need to penetrate the blood brain barrier and the tumor per se.49 Several lines of evidence suggest that targeted therapy requires effective combinations of inhibitors with different mechanisms of action, blocking glial tumor cells intercommunication and allowing the suppression of initial and acquired tumor resistance.52
Although target therapy has failed to improve results up to date, there is optimism because of reports of patients with PFS surprisingly long.8 The development of new drugs must continue exploring these factors in depth, search that will probably indicate the right direction for the development of targeted therapy in malignant brain stem gliomas.52
PrognosisFocal brain stem gliomas generally have an excellent prognosis if the tumor is surgically accessible, with 5-year median PFS and OS of 60% and greater than 90%, respectively.21,23 The median PFS and OS in tectal plate gliomas, extends beyond the follow-up period in most series (4–10 years follow-up) only with endoscopic ventriculostomy, but there are some reports of progression up to 25% of patients after 5 years. Griesenauer et al., recently did a retrospective review of a cohort of forty-four patients diagnosed with tectal plate gliomas by MRI, whom underwent clinical and radiological follow-up for an average of 7.6 years and 6.5 years, respectively; and they found that more than 80% required only CSF shunt, confirming that most patients can be followed with MRI and clinical evaluation.26,27
On the other hand, the OS drops dramatically in cooperative studies that excluded the focal lesions. In most series, the median OS is less than 1 year and at 5 years is about 3%.30 Multiple authors have found that shorter duration of symptoms to diagnosis, nature of symptoms per se and MRI findings, reflect the degree of malignancy and can be used as predictors of outcome, helping to identify the few patients whom could benefit from a surgical procedure.59 The development of innovative treatment strategies and effective targeted therapy will be critical in order to improve the prognosis of these infiltrating tumours.3
ConclusionBrain stem gliomas are a heterogeneous group of tumors in terms of clinical presentation and prognosis. Focal gliomas generally have an excellent prognosis, due to the progress made in recent decades in surgical management linked with the development of neuroradiology. However, the main challenge remains in paediatric DIPG where less progress has been made. Currently, promising new agents are being studied in order to achieve better results, but the key is based on achieving greater understanding of the biology of this tumors.18 It is through this growing knowledge, which has recently started with the recognition of the importance of biopsy and revolution in biological studies, and through thorough preparation of new clinical trials, that finally we will achieve to change history of patients with DIPG.1,8
Human and animal rights and informed consentThis article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to thank doctor Neha Bhatnagar from the Department of Paediatric and Adolescent Oncology, John Radcliffe Hospital, Oxford University Hospitals, and doctor Victory Howard, pediatrician from the Department of Paediatric and Adolescent Oncology, John Radcliffe Hospital, Oxford University Hospitals, for their help in review the grammar of this manuscript.
This work was supported on resources provided by the National Cancer Institute from Colombia.