Post-anesthetic care reduces the anesthesia-related postoperative complications and mortality, shortens the length of stay at the postoperative care units and improves patient satisfaction.
ObjectiveTo establish a set of recommendations for immediate post-anesthetic care of patients that received general/regional anesthesia or profound/moderate sedation at the postoperative care units.
MethodologyThis is a process of “rapid” clinical practice guidelines adaptation, including systematic search. The illegible guidelines for adaptation were rated using AGREE II. The guideline selected to be adapted as the clinical practice handbook was Practice guidelines for post-anesthetic care of the American Society of Anesthesiologists. The manual was evaluated in terms of implementation ability, up-to-date information, relevancy, ethical considerations and patient safety by the group of anesthesiologists and epidemiologists based on Delphi.
ResultThe manual kept the recommendations on evaluation and monitoring, pharmacological management of postoperative nausea and vomiting, antagonistic actions for sedatives and analgesics and neuromuscular block agents, emergency management and anesthesia recovery, as well as the criteria for discharge from the unit. Indications about the conditions and requirements of the unit and patient admission were also included.
ConclusionsThis handbook comprises the basic guidelines for primary management of patients at the postoperative care unit. It may be amended or adapted according to the institutional requirements and for specific patient groups and is not intended to replace the existing protocols at the particular institution and does not define outcomes or prognosis.
El cuidado posanestésico disminuye las complicaciones y mortalidad posoperatorias inmediatas relacionadas con la anestesia, acorta la estancia en las unidades de cuidado posoperatoro y mejora la satisfacción de los pacientes.
ObjetivoEstablecer un conjunto de recomendaciones para el cuidado posantestésico inmediato de los pacientes que recibieron anestesia general/regional o sedación profunda/moderada en la unidades de cuidado posoperatorio.
MetodologíaUn proceso de adaptación “rápida” de guías de práctica clínica, que incluyó búsqueda sistemática. Se calificaron las guías elegibles a adaptar, mediante AGREE II. La guía seleccionada para su adaptación como manual de práctica clínica fue Practice guidelines for postanesthetic care de la American Society of Anesthesiologists. El manual fue evaluado por un grupo de anestesiólogos y epidemiólogos mediante Delphi, en términos de implementabilidad, actualización, pertinencia, consideraciones ética y seguridad del paciente.
ResultadoEl manual mantuvo las recomendaciones sobre evaluación y monitorización, manejo farmacológico de náuseas y vómito posoperatorio, antagonismo de los efectos de sedantes, analgésicos y agentes de bloqueo neuromuscular, el manejo de la emergencia y recuperación anestésica, y los criterios para egreso de la unidad. Se incluyeron indicaciones sobre condiciones y requisitos de la unidad y el ingreso del paciente a esta.
ConclusionesEste manual es una guía básica sobre el manejo primario de los pacientes en la unidad de cuidado posoperatorio, puede ser modificado o adaptado según los requerimientos institucionales y para grupos específicos de pacientes; no pretende reemplazar los protocolos existentes en cada institución ni puede definir desenlaces ni pronósticos.
The practice of anesthesiology has made considerable progress in terms of patient safety. The drop in surgery, anesthesia and perioperative care-associated mortality has been possible trough mechanisms such as improved monitoring techniques, the development and dissemination of clinical practice guidelines and other systematic approaches aimed at reducing the number of errors.1
A meta-analysis including 87 trials measuring mortality in over 3000 patients – out of 21.4 million that received general anesthesia for a surgical procedure – found that the anesthesia-related mortality has decreased from 357 per million (95% CI=324–394) from 1960 to 1969 to 52 per million during the first decade of this current century. The contribution of anesthesia to perioperative mortality prior to 1980 was 3.4% and dropped to 2.9% between 2000 and 2009. The least developed countries exhibit a 5.49 fold risk of dying from anesthesia.2 Another meta-analysis reported a reduction in perioperative mortality between 1954 and 2006 and when comparing Brazil's perioperative mortality against the developed countries, no differences were found.3
The registry trial of 1.37 million elective surgeries in Germany (ASA I and II), from 1999 to 2010, indicated that 26.2 of every million patients operated on, experienced a serious complication or died (95% CI=19.4–34.6). Of these latter patients, 7.3 of every million could be associated with anesthesia or with problems related to the anesthesiologist care (95% CI=3.9–12.3). Only one case out of eighty was due to post-anesthesia care problems.4
The most frequent complications in postoperative care units are nausea and vomiting, with an incidence rate between 10 and 30%.5 A retrospective trial including 18.473 patients detected 23% complications: 6.9% of upper respiratory tract problems; 2.7% hypotension; 1.4% dysrhythmias; 1.1% hypertension; 0.6% altered mental status and 0.6% of major cardiac events.6 Oxygen desaturation is one of the most frequent major problems.7
Postoperative complications affect the survival of both major surgery patients and the elderly.8,9 During the first days after surgery, complications such as lung failure, acute myocardial infarction, bleeding, acute heart failure and delirium may be identified.10 It has been reported than 19.3% of unplanned admissions to the ICU are related to anesthesia and that 5.4% could be prevented. However, according to the findings, 52% of those admissions may be due to anesthesia and between 74 and 92% could be prevented.11
An adequate postoperative approach results in a considerable increase in survival and reduces adverse events and unplanned ICU admissions. This handbook includes the key aspects that should be kept in mind for such adequate approach. The implementation of post-anesthesia care protocols contributes to reduce the hospital stay, the complications, the mortality and unplanned critical care admissions.12
Postoperative or post-anesthesia care was defined as the care administered at a postoperative care unit. This care must be improved so that the patient begins to recover or for an adequate transfer to more complex care units.13 The prevention of complications in this unit may lead to early discharge and availability of beds to admit patients from the ORs. Whenever complications arise, patients require timely intervention or the decision is made to transfer them to more complex care units.
A key condition for improved efficacy is the balance between the care provided to those patients that need extra care and those that do not. The handbook of Postsurgical Controls includes a set of recommendations based on the concepts of the American Society of Anesthesia,14 trough a process of adaptation of clinical care guidelines. Initially some considerations following anesthesia are discussed, and then the key aspects regarding the patient's admission to the PACU, his/her evaluation and monitoring. The second part of the handbook focuses on the prophylaxis or treatment for nausea and vomiting; treatment during emergency situations and the recovery from anesthesia, including the use of antagonists for sedatives, analgesics and neuromuscular block. Finally, the procedures for discharge of the patient from the postoperative care unit are established.
DefinitionsPost-anesthetic care. Actions undertaken to manage the patient following a surgical procedure that required anesthesia.
Anesthesia recovery. Period of time during which the effect of anesthesia slowly fades away following. The evaluation of recovery, depending on the type of anesthesia, determines the patient's discharge from the postoperative care unit.
Postoperative care unit. The area in the operating rooms with the infrastructure and necessary equipment and resources for the recovery of patients that received general or regional anesthesia, or sedation.
Post-anesthesia evaluation and monitoring. Regular evaluation and follow-up of the patient's vital signs and special conditions during the postoperative period, aimed at optimizing the patient's condition to enable his/her safe discharge from the OR environment.
MethodologyThe process included four phases. Each phase used standardized techniques and procedures for the development of evidence-based guidelines and protocols.
1. Make up of the handbook development teamA team of expert anesthesiologists and epidemiologists was organized and entrusted with the task of defining the methodological guidelines for preparing the evidence-based handbook. The team members accepted to participate in the process and had no conflicts of interest to disclose.
2. Systematic review of secondary literatureA systematic review was performed to identify the clinical practice protocols and guidelines with indications or recommendations for anesthesiology management. The analysis focused on articles published in scientific journals or technical documents – gray literature – published since 2011, both in English and Spanish.
Search strategyAn electronic search strategy sensitive to documents meeting the established criteria was designed. The initial search was completed on August 2014.
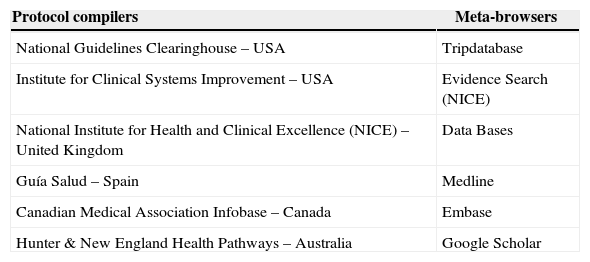
A second search included databases from protocol compilers and meta-browser agencies. Additional searchers were undertaken for guidelines in websites of anesthesiology national and international organizations and of the top ten US hospitals in 2014.15 No new clinical practice guidelines were identified in these sources. The sources of information are shown in Table 1.
Sources of information used for searching clinical practice guidelines.
| Protocol compilers | Meta-browsers |
|---|---|
| National Guidelines Clearinghouse – USA | Tripdatabase |
| Institute for Clinical Systems Improvement – USA | Evidence Search (NICE) |
| National Institute for Health and Clinical Excellence (NICE) – United Kingdom | Data Bases |
| Guía Salud – Spain | Medline |
| Canadian Medical Association Infobase – Canada | Embase |
| Hunter & New England Health Pathways – Australia | Google Scholar |
Source: Authors.
For the initial search some keywords were identified (natural language), corresponding to the health condition or area of interest (anesthesia, perioperative care, and clinical protocols). Then a baseline search strategy was developed using controlled terminology (tMeSH, Emtree and DeCS) and free language (spelling variations, plurals, synonyms, acronyms and abbreviations).
Using the baseline strategy, searchers were adapted to the various resources using extended terminology, field identifiers (title and abstract), truncation, and Boolean and proximity operators – when possible.
Searches were completed in depositories of clinical protocols, tracking keywords using the “search” tool in the Internet browser, in addition to a reproducible search in Google and Google Scholar, with no language or date of publications restrictions.
For the second search the keywords were changed (anesthesia, postoperative care, post-anesthesia care, clinical protocols, clinical care guidelines), maintaining the comprehensive first search process.
A logbook or report was generated for each search to ensure reproducibility and transparency. The references were consolidated on a Microsoft Excel database.
Trained staff did the searches audited by a Cochrane Collaboration Trials Search Coordinator.
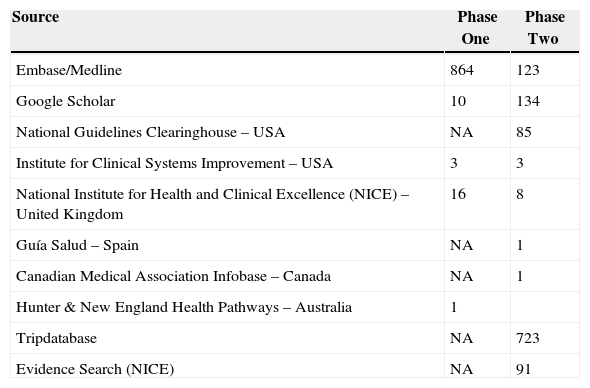
Results of the search strategiesTable 2 shows the results of the two phases of the search strategy.
Search results of clinical practice guidelines for post-anesthetic care.
| Source | Phase One | Phase Two |
|---|---|---|
| Embase/Medline | 864 | 123 |
| Google Scholar | 10 | 134 |
| National Guidelines Clearinghouse – USA | NA | 85 |
| Institute for Clinical Systems Improvement – USA | 3 | 3 |
| National Institute for Health and Clinical Excellence (NICE) – United Kingdom | 16 | 8 |
| Guía Salud – Spain | NA | 1 |
| Canadian Medical Association Infobase – Canada | NA | 1 |
| Hunter & New England Health Pathways – Australia | 1 | |
| Tripdatabase | NA | 723 |
| Evidence Search (NICE) | NA | 91 |
Source: Authors.
As of the first phase of the search, 193 references consistent with the objective of the handbook were identified, even if these were not clinical practice guidelines. Twelve documents were identified as clinical practice guidelines on postoperative care during the clearance process of the two search phases.
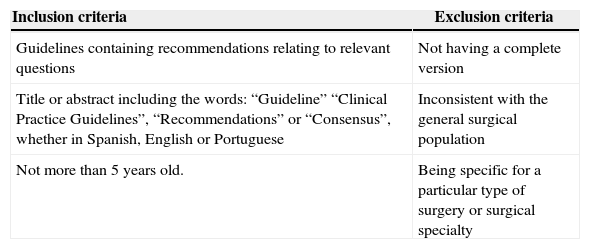
Two experts reviewed these twelve documents: one thematic (anesthesiologist) and one methodological (epidemiologist). The experts checked that the guidelines met the inclusion and exclusion criteria and were evidence-based. Four of the twelve documents met the criteria. The information about the criteria used is shown in Table 3.
Inclusion and exclusion criteria for the clinical practice guidelines found.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Guidelines containing recommendations relating to relevant questions | Not having a complete version |
| Title or abstract including the words: “Guideline” “Clinical Practice Guidelines”, “Recommendations” or “Consensus”, whether in Spanish, English or Portuguese | Inconsistent with the general surgical population |
| Not more than 5 years old. | Being specific for a particular type of surgery or surgical specialty |
Source: Authors.
The tool AGREE II (Appraisal of Guidelines for Research and Evaluation) was used to assess the quality of the evidence selected.16 This quality analysis was done in a paired mode. The documents meeting the eligibility requirements as source documents for this Handbook were identified. Appendix A summarize this process.
In accordance with the grading, the clinical practice guideline to be adopted corresponds to the American Society of Anesthesiology14 that is an update of the 2002 Guidelines.17 In the opinion of the expert anesthesiologist, the recommendations on the conditions of the post-operative care room, the patient's admission and discharge from the room, were complemented with the guidelines of The Association of Anesthetists of Great Britain and Ireland18 and the Scottish Intercollegiate Guidelines Network.19
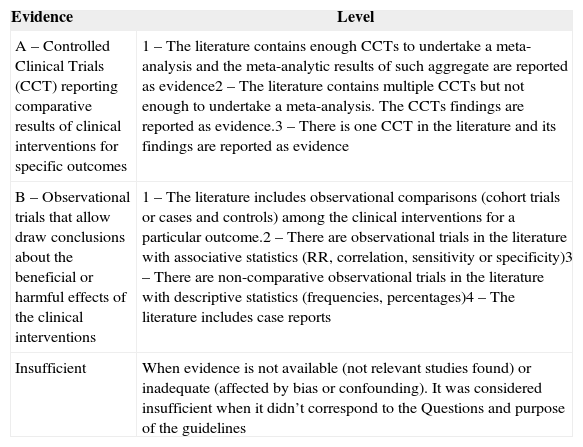
Availability and power of evidence under the baseline clinical practice guidelinesThe baseline clinical practice guidelines considered both the scientific evidence and the opinion of experts. Table 4 is a summary of the rating of scientific evidence published in journals. The category of level of evidence refers to the strength and validity of the research design. The levels refer to the strength and quality of the findings summarized in each trial (for example: statistical findings, types of data, and number of trials reporting or replicating the findings) in both categories of evidence.
Scientific evidence rating.
| Evidence | Level |
|---|---|
| A – Controlled Clinical Trials (CCT) reporting comparative results of clinical interventions for specific outcomes | 1 – The literature contains enough CCTs to undertake a meta-analysis and the meta-analytic results of such aggregate are reported as evidence2 – The literature contains multiple CCTs but not enough to undertake a meta-analysis. The CCTs findings are reported as evidence.3 – There is one CCT in the literature and its findings are reported as evidence |
| B – Observational trials that allow draw conclusions about the beneficial or harmful effects of the clinical interventions | 1 – The literature includes observational comparisons (cohort trials or cases and controls) among the clinical interventions for a particular outcome.2 – There are observational trials in the literature with associative statistics (RR, correlation, sensitivity or specificity)3 – There are non-comparative observational trials in the literature with descriptive statistics (frequencies, percentages)4 – The literature includes case reports |
| Insufficient | When evidence is not available (not relevant studies found) or inadequate (affected by bias or confounding). It was considered insufficient when it didn’t correspond to the Questions and purpose of the guidelines |
Source: Authors.
In accordance with the outcomes, the intervention was considered to be beneficial (B), harmful (H), or equivocal (E) when no statistically significant differences were identified.
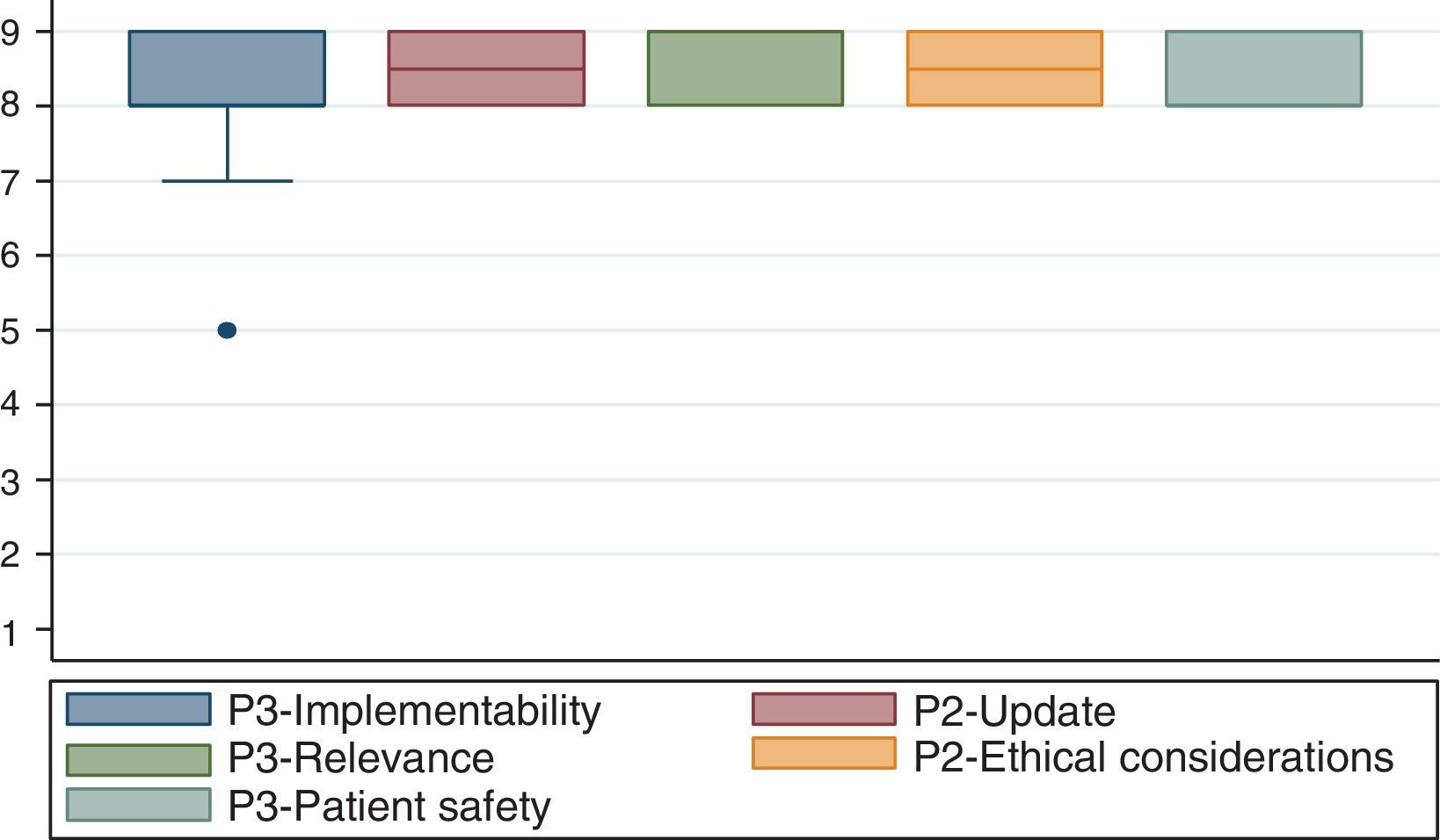
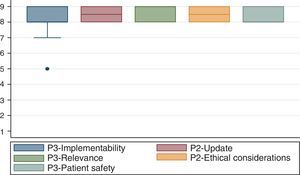
3. Participative methodA modified Delphi method was used.20 The group developer team selected the experts and convened them to a meeting held on September 18, 2014 at S.C.A.R.E.’s headquarters. Twenty-eight anesthesiologist and epidemiologists attended the meeting.
After presenting the clinical contents of the handbook and following the experts’ discussion, the following characteristics were evaluated for compliance:
- –
Ease of Implementation. Potential ease of use of the handbook by the various institutions.
- –
Up-to-Date information. Whether the indications are consistent with the current evidence.
- –
Relevancy. Whether the indications are relevant to most of the surgical environments.
- –
Ethical Considerations. Whether using this handbook was ethical.
- –
Patient Safety. Whether the patient may be exposed to a high risk when using this handbook.
A numeric nine-category scale was used to score each one of the characteristics identified. Each indication suggested was rated as recommended (appropriate), contraindicated (inappropriate), or uncertain.21
Fig. 1 exhibits the results of the agreement reached by the participants in the consensus.
Preparation and drafting of the final documentA final handbook model was designed, including the justification, the methodology, and the adaptation of the baseline clinical practice guidelines, according to the expert recommendations under the participative method. The team that prepared the handbook developed the final document.
DisclosuresAll of the participants in the working group and in the expert consensus affirmed, completed, and signed the disclosures document.
CopyrightConsultations were made and authorizations secured for using and translating part of the contents of the guidelines to prepare the handbook. The partial translation and reproduction of the material was authorized by Lippincott Williams and Wilkins/Wolters Kluwer Health, Association of Anesthetists of Great Britain & Ireland & the AAGBI Foundation y Institute of Clinical Systems Improvement. Copyright belongs to the authors of the guidelines and protocols that are duly referenced in the document.
Clinical contentsApproachThe handbook focuses on the postoperative management of the patient, emphasizing the reduction in the number of adverse events through a standardized evaluation of the recovery process, leading to improved quality of life during the post-anesthesia phase and a rationalization of postoperative care and discharge criteria.
This handbook is applicable to patients receiving general or regional anesthesia, profound or moderate sedation and may be amended (or a complementary protocol be designed) to adapt it to the needs of a particular type of patients or populations such as children and the elderly. It is not applicable to patients receiving local anesthesia without sedation, minimal sedation or patients admitted to the ICU.
This handbook is not intended to replace individualized patient care or the particular protocols of the institution. Neither is it expected to predict patient outcomes.
Fig. 2 illustrates the sequence of activities under this handbook.
Conditions or requirements of the postoperative care unitThe postoperative care unit shall preferably be located centrally to the operating rooms, allowing easy access and transit to and from the unit. Monitors, medicines, equipment and enough trained nursing staff shall all be available for managing patients during the postoperative phase and to deal with any complications.18
An anesthesiologist in charge of the patients transferred to the postoperative care unit shall be available.18 A user-friendly communications and alarms system shall be available and the staff should be trained to use it properly.18
Patient admission to the postoperative care unitThe anesthesiologist in charge of the patient shall personally hand-off the patient to the postoperative care unit staff.18
The anesthesiologist is required to give a verbal report of the patient's pre-surgical and surgical medical record, including any adverse event that may have occurred in the course of the surgical procedure.18
The anesthesiologist shall report on all the general indications for postoperative care in accordance with the medical record, the type of surgery and the anesthesia received by the patient.
Indications- •
An anesthesiologist shall be responsible for delivering the patient at the postoperative care unit, the ICU or any other unit in charge of admitting the patient for his/her immediate postoperative phase.
- •
In the event of an anesthesia-related complication during surgery, or in the course of anesthesia recovery, the anesthesiologist that administered the anesthesia, or the anesthesiologist in charge of the postoperative care unit – or else the anesthesiologist that was formally entrusted with the care of the patient – shall notify the patient or his/her representative about the type of complication and how it was managed.
- •
Any complication arising during surgery shall be reported by the surgeon responsible for the procedure.
- •
It is highly advisable that the surgical team, the anesthesiologist and the surgeon report to the patient or accompanying person the result of the surgical procedure.
- •
An anesthesiologist responsible for the patient's recovery at the postoperative care unit shall be available.
The team of practitioners and staff assistants in charge of the postoperative care unit are required to record every evaluation based on monitoring, clinical observation, reading of diagnostic follow-up tests, intervention, therapeutic or prophylactic prescription done during the emergency care and anesthesia recovery, including the prevention and treatment of complications.22
Evaluation and monitoring of the patient at the postoperative care unitRespiratory functionThe periodic evaluation and monitoring of the airway patency, the respiratory rate, and oxygen saturation (SpO2) shall be done during anesthesia recovery to reduce the number of adverse outcomes (Evidence A2-B).
Cardiovascular functionASA experts17 considered that blood pressure, pulse and EKG monitoring identify complications, reduce the number of adverse outcomes and shall be implemented during anesthesia recovery (insufficient evidence). They were of the opinion that EKG monitoring may be unnecessary in certain types of patients or depending on the anesthetic procedure.
Neuromuscular functionThe evaluation of the neuromuscular function is deemed to reduce the number of adverse events and should be carried out during post-anesthesia recovery.
The neuromuscular evaluation begins with a physical exam and may include neuromuscular block monitoring (Evidence B2-B).
Mental statusAccording to the experts’ opinion,17 every institution should have a scale to assess the mental status of the patient in the postoperative care unit. This will help to reduce the number of post-anesthetic complications (insufficient evidence).
TemperatureExperts agree that measuring the patient's temperature is associated with less postoperative complications and that temperature should be measured during the anesthesia recovery phase (insufficient evidence).
Ideally the patient shall be kept under normal temperature keeping in mind the changes in temperature self-regulation following anesthesia and surgery.
PainExperts believe that pain assessment during recovery reduces the number of postoperative adverse events (insufficient evidence).
Pain management may be started during the surgical procedure and be part of the anesthetic procedure selected for the particular patient. Pain management may be continued and evaluated during the postoperative phase.
Nausea and vomitingThe opinion of experts about the evaluation of nausea and vomiting to reduce any adverse effects is ambiguous; however, they say that such evaluation shall be performed during anesthesia recovery (insufficient evidence).
FluidsExperts agree on the benefits of monitoring hydration and fluid management. This reduces the adverse effects and improves the patient's wellbeing and satisfaction (insufficient evidence).
Urine output and micturitionThe evaluation of urine output identifies urine retention (Evidence B3-B), but the evidence is ambiguous for other complications (insufficient evidence). In the opinion of experts,17 the evaluation of urine output identifies potential complications and reduces the number of adverse effects. Depending on the particular case, such evaluation may not be on a routine basis.
There is insufficient evidence and ambiguous opinions of experts regarding the assessment of micturition for the identification of adverse events, though they consider it may be assessed during the recovery phase.
Drainage and bleedingExperts agree that the evaluation of bleeding and drainage identifies complications, reduces the number of adverse effects and may be a routine when caring for postoperative patients (insufficient evidence).
Indications- •
The periodic evaluation of the airway, the respiratory rate, oxygen saturation, pulse, heart rate and blood pressure is a requirement during anesthesia recovery.
- •
EKG monitoring should be available at the postoperative care units for patients that need to be monitored.
- •
The evaluation of the neuromuscular function shall be done during the post-anesthesia recovery phase in all patients receiving neuromuscular block with non-depolarizing agents or in patients with neuromuscular dysfunction-related medical conditions.
- •
The level of hydration should be assessed depending on the particular patient, particularly if the surgical procedure entailed a significant blood or fluids loss and required additional fluid management.
- •
Urine output and micturition shall be assessed in particular patients undergoing specific procedures.
- •
The mental status, body temperature, pain, nausea, vomiting, and drainage and bleeding may be assessed during the recovery phase.
The groups of drugs evaluated were 5-HT3 antiemetics, tranquilizers, and neuroleptics, metoclopramide and dexamethasone.
5HT3 antiemeticsA meta-analysis of the new ECCs confirmed that 5HT3 agents versus placebo were effective in the postoperative prophylaxis of nausea and vomiting and reduces the use of rescue antiemetic use (Evidence A1-B). The specific drugs are: dolasetron (reduces vomiting),23–27 granisetron (reduces vomiting)28–32 and ondansetron (reduces vomiting and the use of rescue antiemetics).28,33–45
TranquilizersThe meta-analysis of the new CCTs ratifies that droperidol reduces postoperative nausea and vomiting and the use of rescue antiemetics (Evidence A3-B).38,46–50 Several CCTs evidenced that haloperidol is also effective (Evidence A2-B).34,47,49,51
MetoclopramideThe meta-analysis of CCTs comparing metoclopramide (10mg) against placebo do not report any statistically significant differences in nausea and vomiting during the immediate postoperative period (Evidence A1-E), but show efficacy in reducing vomiting during the first twenty four hours into the postoperative period (Evidence category A1-B).35,39,44,52–55
DexamethasoneThe meta-analysis of CCTs reports that this antiemetic is effective in the prophylaxis of postoperative vomiting, reduces the use of rescue antiemetics; at higher doses dexamethasone was effective as prophylactic treatment for nausea (Evidence category A1-B).29,33,45,48,49,51,52,54–67
CombinationsThe combination of two antiemetic agents is effective for prophylaxis against postoperative nausea and vomiting (Evidence category A2-B); there were no differences in terms of side effects such as headache, dizziness, drowsiness and restlessness.24,31,32,48,53,56,68–77
UpdateA systematic review78 on the treatment of postoperative nausea and vomiting considers a similar evidence for medicines that may be effective for the prophylaxis and treatment of these events. The review favors ondansentron as the first pharmacological choice.
Indications- •
Anesthesia-related nausea and vomiting prophylaxis improves patient satisfaction and wellbeing, reducing the time to discharge of the postoperative care unit.
- •
Anesthesia-related nausea and vomiting prophylaxis improves with ondansentron, droperidol or dexamethasone, that also reduce the need for rescue antiemetics.
- •
Ondansentron is considered a first line treatment.
- •
There is no conclusive evidence regarding the use of multiple drugs for the treatment of nausea and vomiting during recovery.
A recent CCT79 reaffirmed the findings of the 2002 guidelines17 regarding the efficacy of flumazenil's antagonistic activity on the residual effects of benzodiazepines following general anesthesia versus placebo (Evidence A3-B). The 2002 Guidelines17 claimed that flumazenil reduced the time to emergence following sedation (Evidence A1-B).
Experts17 disagree on the routine use of flumazenil to reduce the number of adverse events or improving patient comfort and satisfaction.
Opioids antagonistic activityThe 2002 Guidelines17 indicated that naloxone reduced the time to emergence and recovery of spontaneous breathing (Evidence A3-B). Experts disagree about the routine use of naloxone to reduce the number of adverse events or improving the patient's comfort or satisfaction.
Reversal of neuromuscular relaxantsThe 2002 Guidelines17 stated that neostigmine is effective for antagonizing the residual effect of muscle relaxants (Evidence A1-B), although it showed increasing number of postoperative emetic episodes (Evidence A1-H).
No expert consensus has been reached about the fact that the anesthetic regimes designed to avoid the use of neuromuscular block antagonism reduce the adverse outcomes and improve patient satisfaction and wellbeing.
Indications- •
Flumazenil shall not be administered routinely, though it may be an option in the presence of respiratory depression and sedation in patients with benzodiazepines use as the underlying cause. Following the administration of the antagonistic drug, patients must be under observation for a long time to prevent the relapse of respiratory depression.
- •
Opioid antagonists (naloxone) are not recommended for routine use. However, opioid antagonists may be administered in the presence of respiratory depression attributable to opioid use. Following the administration of the antagonistic drug, patients must be under observation for a long time to prevent the relapse of respiratory depression. The acute antagonism of opioids may trigger pain, hypertension, tachycardia and pulmonary edema.
- •
Specific antagonists must be administered to revert the residual neuromuscular block if indicated.
- •
Flumazenil, naloxone, or neuromuscular block antagonists shall be available for administration as needed.
- •
Specific neuromuscular block antagonists shall be available to revert the block when appropriate.
A CCT80 showed that the administration of supplemental oxygen during transfer and at the postoperative care unit reduces the incidence of hypoxemia (Evidence A3B).
TemperatureThe 2002 Guidelines17 included evidence that active warming of the patient is associated to temperature normalization (Evidence A2-B). There is evidence that the use of normal forced air warming devices normalize the patient's temperature (Evidence category A3-B). This latter finding was reaffirmed at a recent CCT, but there is no evidence of a reduction of shivering (Evidence A3-E).81
Use of pharmacological agents to reduce postoperative shiveringThe 2002 Guidelines17 state that meperidine is effective in the management of postoperative shivering as compared to other opioid antagonists and placebo (Evidence A1-B). A recent CCT82 found that meperidine decreased shivering as compared to other drugs (Evidence A3-B).
The effects of dexmedetomidine have helped in controlling shivering in children (Evidence B3) as well as regional anesthesia-related chills (Evidence A3).83,84
Indications- •
The administration of supplemental oxygen during transfer and postoperative anesthesia care is a requirement for patients at risk of developing hypoxemia.
- •
Normal body temperature shall be one of the goals of perioperative care. When available, forced air warming devices shall be used.
- •
Meperidine may be used to control shivering during the postoperative phase, if clinically indicated and in the absence of contraindications.
This may extend the length of stay and should be required only in selected patients. It is not considered a requirement for discharge.
Requirement for the patient to drink clear fluids prior to dischargeThis may extend the length of stay of the patient in the unit. It is not considered an absolute requirement that the patient drinks clear fluids prior to discharge. The 2002 Guidelines rated this item as Evidence A2-E. Experts do not feel that this improves patient comfort or satisfaction.17
Requirement for the patient to have a responsible companion prior to home dischargeIt has been considered that the need to have a responsible companion at discharge of the outpatient reduces the risk of complications and improves patient satisfaction and wellbeing. In the opinion of experts his should be a compulsory requirement. However, scientific evidence is insufficient.
Need for minimum length of stay at the post-anesthetic care unitEvidence is insufficient and experts do not feel that a minimum length of stay is required. The LOS shall be determined on a case-by-case basis. There is no consensus regarding whether a minimum length of stay reduces the number of adverse events or complications.
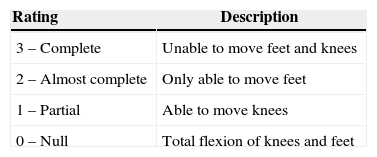
Motor activity assessment following regional anesthesiaAccording to S.C.A.R.E's minimum anesthesia safety rules 2013,85 the use of a scale to measure the recovery of motor activity is suggested. Bromage scale86 is the most widely used (Table 5).
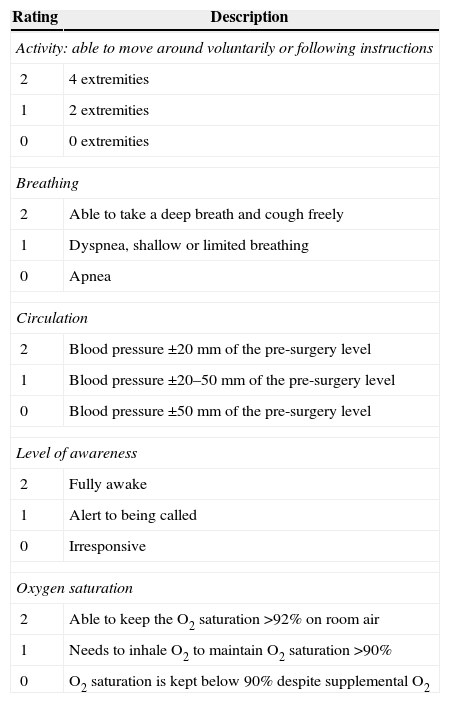
Evaluation of discharge criteriaA systematic review concluded that every discharge evaluation should include the awareness status, blood pressure, pain, and nausea/vomiting assessment.87 Aldrete's scale covers all these aspects and hence could be considered the scale of choice for this purpose88 (Table 6).
Modified Aldrete score for authorizing postoperative care unit discharge.
| Rating | Description |
|---|---|
| Activity: able to move around voluntarily or following instructions | |
| 2 | 4 extremities |
| 1 | 2 extremities |
| 0 | 0 extremities |
| Breathing | |
| 2 | Able to take a deep breath and cough freely |
| 1 | Dyspnea, shallow or limited breathing |
| 0 | Apnea |
| Circulation | |
| 2 | Blood pressure ±20mm of the pre-surgery level |
| 1 | Blood pressure ±20–50mm of the pre-surgery level |
| 0 | Blood pressure ±50mm of the pre-surgery level |
| Level of awareness | |
| 2 | Fully awake |
| 1 | Alert to being called |
| 0 | Irresponsive |
| Oxygen saturation | |
| 2 | Able to keep the O2 saturation >92% on room air |
| 1 | Needs to inhale O2 to maintain O2 saturation >90% |
| 0 | O2 saturation is kept below 90% despite supplemental O2 |
Source: Authors.
The consensus group advices to have an institution staff member accompany the patient to the exit.
Indications- •
The requirement to void or drink fluids prior to discharge may be obligatory for particular patients.
- •
As part of the discharge protocols at every institution, all patients discharged must have a responsible companion at the time of discharge.
- •
A minimum length of stay at the postoperative care unit is not recommended as a routine. The length of stay shall be determined on a patient-by-patient basis.
- •
Consider checking the patency of the airway, drains and catheters and needed.
- •
Check the complete records.
- •
Use an Aldrete type scale to assess every patient prior to discharge from the postoperative care unit.
If the patient is discharged home from the postoperative care unit, all the surgery-related recommendations, the alarm signs and unexpected adverse events shall be submitted to the patient in writing.19,20
Funding sourcesThis project was Colombian society of Anesthesiology and Resuscitation – S.C.A.R.E. in collaboration agreement with the School of Medicine, Universidad Nacional de Colombia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caro CAB, Alvarado FEP, Torres M, Buitrago G, Duarte HG, García C, et al. Manual de práctica clínica basado en la evidencia: controles posquirúrgicos. Rev Colomb Anestesiol. 2015;43:20–31.