Despite advances in perioperative management, acute pain and postoperative nausea and vomiting continue to be significant complications worldwide. The frequency and the implications of these complications for the process of recovery impact clinical findings, patient quality of care, and hospital costs.
Materials and methodsA search and systematic review of the literature after 2011 was conducted. Three international guidelines were selected and they were paired-rated for quality using the AGREE II tool. Management recommendations, adjusted to the Colombian setting, were adopted on the basis of expert consensus, using the Delphi methodology.
ResultsRecommendations were generated for adult patients based on the international pain management guidelines for acute pain, postoperative nausea and vomiting, and transfer of critically ill patients. Some of the recommendations are of general nature while others are specific for particular situations. They were all adapted to the Colombian context, bearing in mind the use of drugs which have not received approval from the healthcare authorities or which are not included in the Mandatory Healthcare Plan.
ConclusionsUpdating and standardizing clinical management recommendations based on the literature on international guidelines is a useful process, provided it is adapted to the national context. This process and its outcome may be useful for healthcare providers and has a positive effect on patient safety, practitioner performance and efficient use of resources.
A pesar de los avances en el manejo perioperatorio, el dolor agudo y las náuseas y vómito posoperatorio aún son importantes complicaciones a nivel mundial. Su frecuencia de presentación y el grado de afectación en el proceso de recuperación impactan aspectos clínicos, la calidad de la atención de los pacientes y los costos hospitalarios.
Materiales y métodosSe realizó búsqueda y revisión sistemática de la literatura a partir de 2011. Se seleccionaron tres guías internacionales y se calificó la calidad de manera pareada con el instrumento AGREE II. Mediante consenso de expertos y utilizando metodología Delphi, se adaptaron las recomendaciones de manejo adaptadas al medio colombiano.
ResultadosSe generaron recomendaciones para pacientes adultos extraídas de las guías de manejo internacional de dolor agudo, náuseas y vómito posoperatorio y transporte de paciente complicado. Algunas de las recomendaciones son generales y otras especificas para situaciones particulares. Todas fueron adaptadas al contexto colombiano teniendo en cuenta medicamentos que no cuentan con registro sanitario o no están incluidos en el Plan Obligatorio de Salud.
ConclusionesLa actualización y estandarización de recomendaciones de manejo clínico basadas en la literatura de guías internacionales es un proceso útil siempre y cuando se adapte al contexto nacional. Este proceso y su resultado puede ser utilizado por prestadores de salud e impactar positivamente la seguridad del paciente, el desempeño de los profesionales sanitarios y la eficiencia de los recursos.
Despite advances in perioperative management, post-operative complications continue to affect patient care and recovery, reduce healthcare quality, alter quality of life, and have a significant impact on costs.1
A key factor in ensuring the effectiveness of the Post-anaesthetic Care Unit (PACU) is to maintain a balance in the use of resources between patients requiring more care and others who do not.2 Different tools have been developed, including checklists3 and various clinical protocols that include detailed steps for patient care on the basis of their clinical status. The use of these tools may shorten length of stay significantly and improve postoperative results.4
Post-operative complications may be general or specific for the type of surgery performed and, when they occur, they must be managed taking into consideration the specific clinical characteristics of the individual patient. The group of postoperative complications being broad, we decided to describe only two in this paper, because of their frequency and the degree to which they affect the recovery process: acute postoperative pain and postoperative nausea and vomiting (PONV).
The incidence of severe post-operative pain reported in the literature varies widely, with reports of 75% (Cohen 1980, USA), 33% (Oates 1994, United Kingdom), 46% (Poisson–Saloman 1999, France), 68% (Spanish Society of Pain, 2003), 59% (Apfelbaum 2003, USA).5 In Colombia, with data from the San Vicente Paul Hospital, the prevalence of moderate pain 24h after the procedure has been estimated at 31%, and the prevalence of severe pain at rest has been estimated at 22.3%.6 The San José Hospital in Popayán reports an incidence of severe postoperative pain within the first hour after surgery of 12.3%, 95%CI (7.1–18.2) and of 4.5%, 95%CI (1.3–8.4) at the 30min assessment. In the PACU, 48.7% of the patients required rescue analgesia.7 Moreover, the University Hospital in Pereira reports that 51.4% patients did not have pain control 4h after the procedure.8 Adverse outcomes resulting from inadequate management of perioperative pain include thromboembolic and pulmonary complications, longer Intensive Care Unit (ICU) stay, readmission for pain management, unnecessary suffering, deterioration of the quality of life, and development of chronic pain.9
The global incidence of postoperative nausea and vomiting is approximately 50% and 30%, respectively. In patients with a high risk of PONV, the incidence may be as high as 80%.10 The presence of PONV increases the risk of aspiration of gastric contents and is associated with other types of complications, including evisceration and suture dehiscence. PONV delays discharge from the PACU and increases the rate of readmissions after surgery.11
The management of pain and PONV must be a priority for the different healthcare institutions as an integral part of healthcare quality. The purpose of this manual was to update and standardize PONV and pain management as part of postoperative care, on the basis of the recent literature and recommendations from Colombian experts.
MethodologyThe process was divided into four phases. During each of the phases, standardized techniques and procedures used as part of the development of evidence-based guidelines and protocols were considered.
Team membershipA group of experts in anaesthesiology and epidemiology was put together to provide the methodological and technical guidelines for the development of the protocol. The members included two methodology coordinators with experience in the development of clinical practice guidelines and evidence-based management protocols who were responsible for coordinating the methodology of the process. Another group consisting of a physician specialized in anaesthesiology and an epidemiologist with experience in critical analysis of scientific evidence was also created. All of the members of the team that prepared the guidelines agreed to participate in the process and signed the disclosure form, which is consistent with the regulations pertaining to the development of evidence-based guidelines and protocols.
Systematic review of the secondary literatureThe systematic review was conducted in order to identify the clinical protocols and practice guidelines. The unit of analysis for the review was based on articles published in scientific journals or on technical documents found in the grey literature:
- a)
Evidence-based management protocols (or clinical practice Guidelines) with indications or recommendations regarding clinical management by the anaesthesia group
- b)
Published since 2011
- c)
Published in English and Spanish
A sensitive digital search strategy was designed and was conducted on the 19th of August 2014. The sources of information included the Medline and Embase databases of the biomedical scientific literature, as well as the grey literature in Google. Additional sources included international agencies specialized in anaesthesia (Australian and New Zealand College of Anaesthetists (ANZCA), The Association of Anaesthetists of Great Britain and Ireland, Royal College of Anaesthetists, Agency for Health care Research and Quality, American Society of Anaesthesiologists, NICE, Scottish Intercollegiate Guidelines Network).
Selection of the evidenceBased on the results of the search strategies, a database of the potential documents was built and given to two reviewers who worked independently to read the titles and abstracts, and identified the documents that met the requirements. The full texts of the selected documents were downloaded and subjected to final screening.
Quality assessmentThe AGREE II tool was used to assess the quality of the evidence found in the previous step. This was a paired quality analysis.
Finally, three documents that met the requirements for use as source documents were selected for adaptation to clinical management. The final identification of the source document was done on the basis of the clinical judgement of the expert, the currency, the detail of the indications (recommendations), and the quality.
Participatory methodA modified Delphi methodology was used (face to face or in real time). The group in charge of preparing the guidelines selected the experts with whom a meeting was then held on Thursday September 18, 2014, at the S.C.A.R.E. facilities. Overall, 28 experts in anaesthesiology and epidemiology participated.
The agenda of the meeting was the following:
- i.
Presentation of the project.
- ii.
Delphi methodology.
- iii.
Results of the evidence.
- iv.
Protocol proposal.
- v.
Vote.
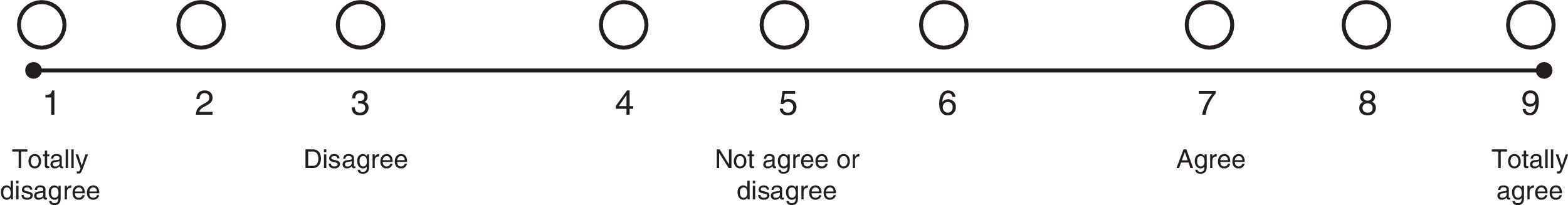
A nine-category ordinal scale was developed to rate each of the questions (Fig. 1). Bearing this in mind, each of the indications proposed was rated as recommended (appropriate), contraindicated (inappropriate) or within a level of uncertainty, in accordance with the median value of the answers given by the experts. Information on the degree of agreement or consensus was presented, together with the results of the response ranges for each of the questions. This rating was based on the descriptive method proposed by Sánchez et al.12
According to this methodology, the anonymous responses of the experts were collected first, and then the median and the extremes in the range of responses were calculated. When the extreme points in the range fell within one of the three regions of the scale (1–3; 4–6; 7–9), strong agreement was considered to exist and consensus was declared. When the extreme points in the range fell within two consecutive regions (1–3 and 4–6), a relative agreement was considered to exist. When the extreme points in the region were scattered between two non-consecutive regions (1–3 and 7–9) no consensus was considered to exist (Fig. 2).
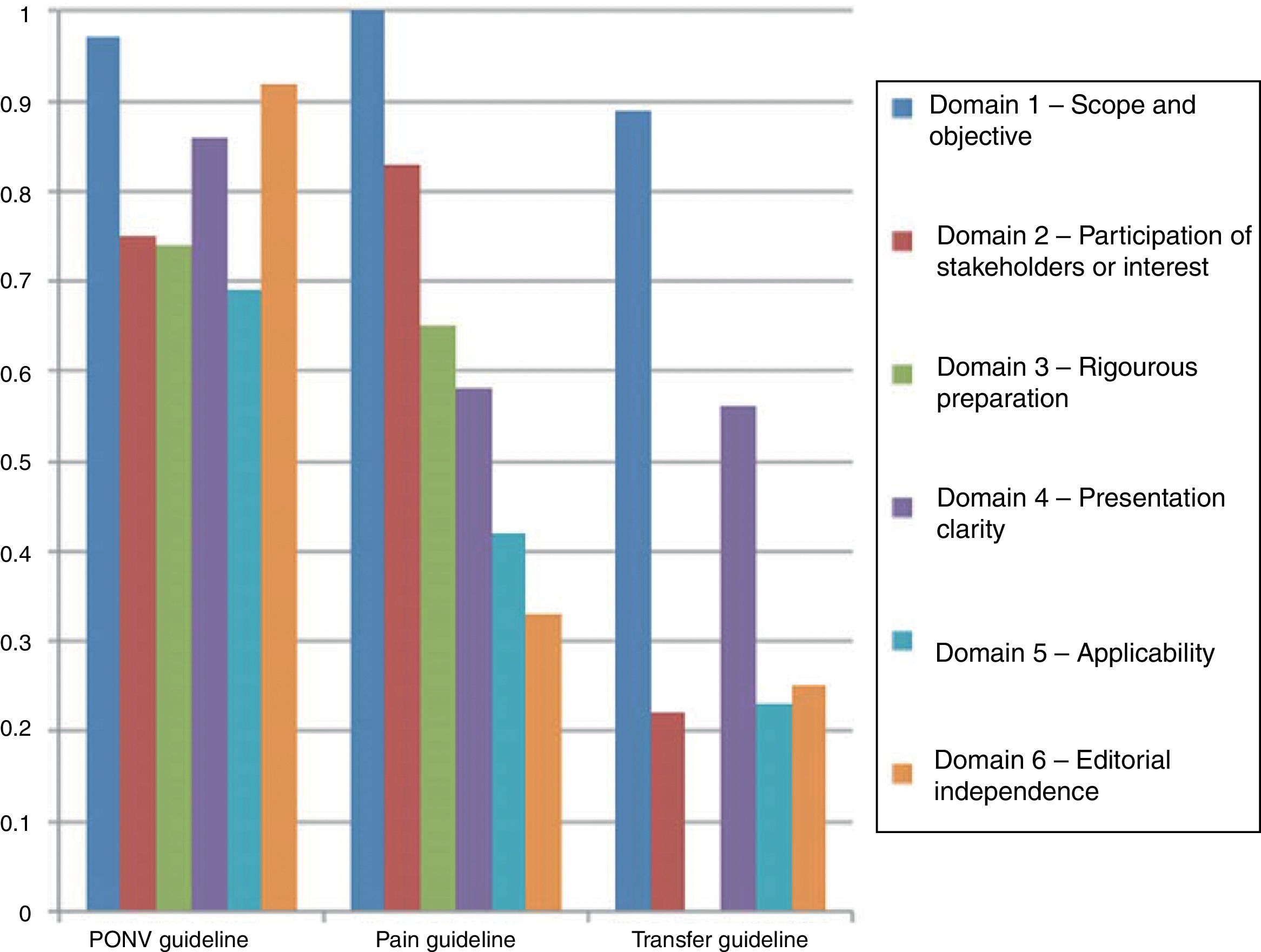
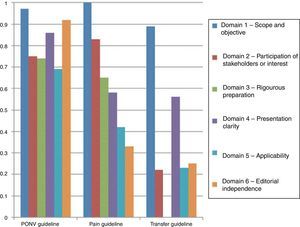
Fig. 3 shows the results of agreement among the participants in the consensus meeting.
Preparation and drafting of the final documentA final protocol model document was prepared which included the justification for developing the document, the methodology used, and the adaptation of the proposed protocol, following the recommendations from the experts in the participatory method.
Conflicts of interestAll of the participants in the writing group and in the expert consensus meeting filled and signed the disclosure form in accordance with the format and recommendation from the Cochrane group.
CopyrightPermission was obtained for the use and translation of the guidelines contained in the series of manuals. Translation and partial reproduction were authorized by Lippincott Williams and Wilkins/Wolters Kluwer Health, Association of Anaesthetists of Great Britain & Ireland & the AAGBI Foundation.
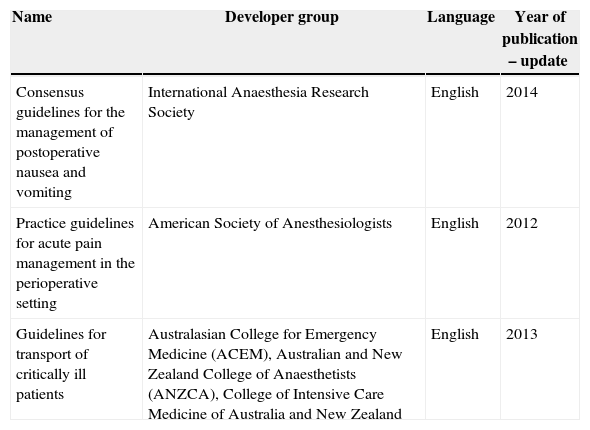
ResultsRating of the evidenceThree guidelines were selected, as shown in Table 1.
Guidelines for the management of post-operative complications.
| Name | Developer group | Language | Year of publication – update |
|---|---|---|---|
| Consensus guidelines for the management of postoperative nausea and vomiting | International Anaesthesia Research Society | English | 2014 |
| Practice guidelines for acute pain management in the perioperative setting | American Society of Anesthesiologists | English | 2012 |
| Guidelines for transport of critically ill patients | Australasian College for Emergency Medicine (ACEM), Australian and New Zealand College of Anaesthetists (ANZCA), College of Intensive Care Medicine of Australia and New Zealand | English | 2013 |
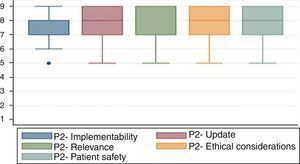
As was described in the section on the methodology, the guidelines were assessed using the AGREE II tool. Fig. 3 shows the results of the paired review by the authors. The PONV had a higher rating in most of the domains. However, the transfer guidelines reflect a significant gap in the rating of the third domain.
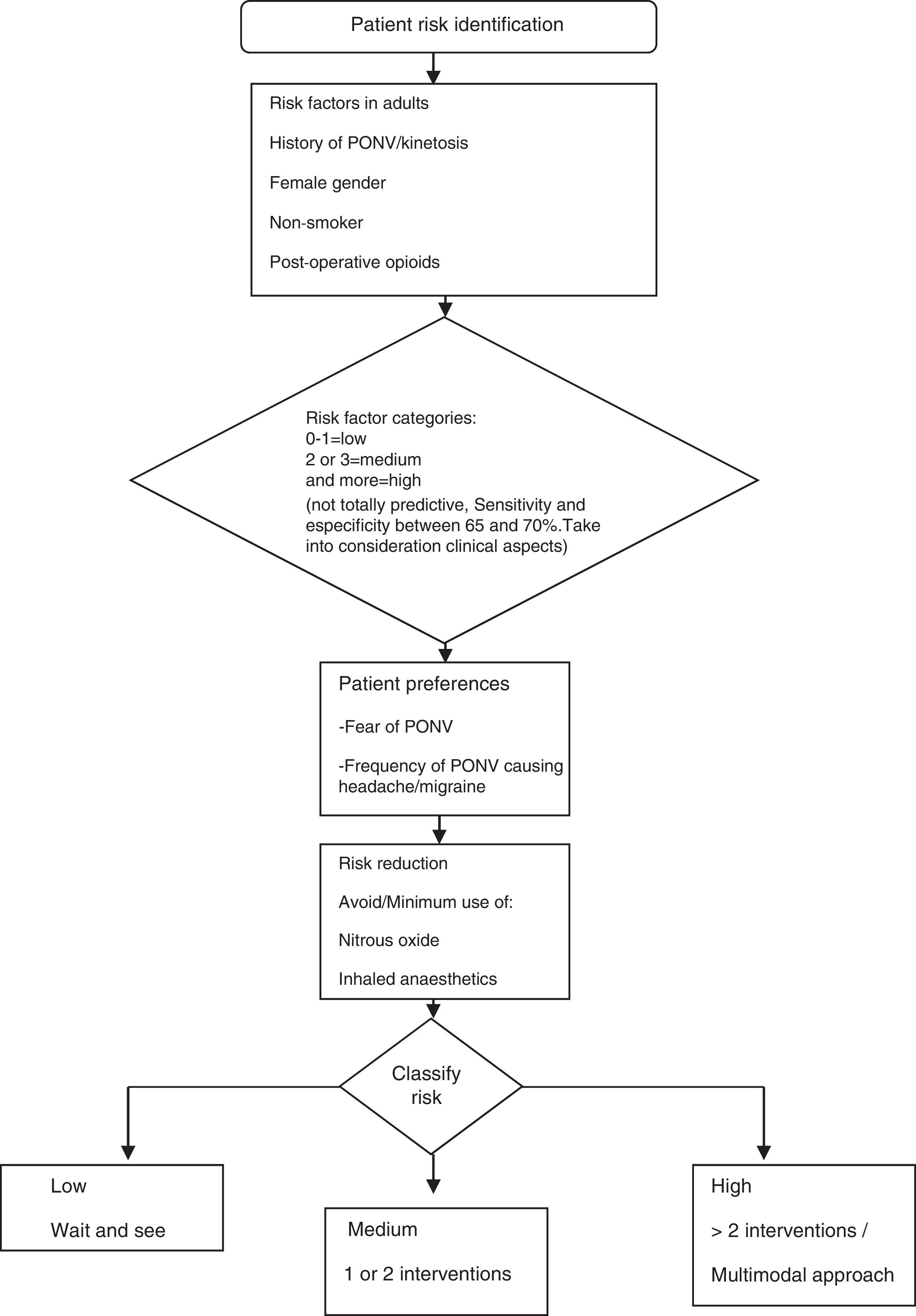
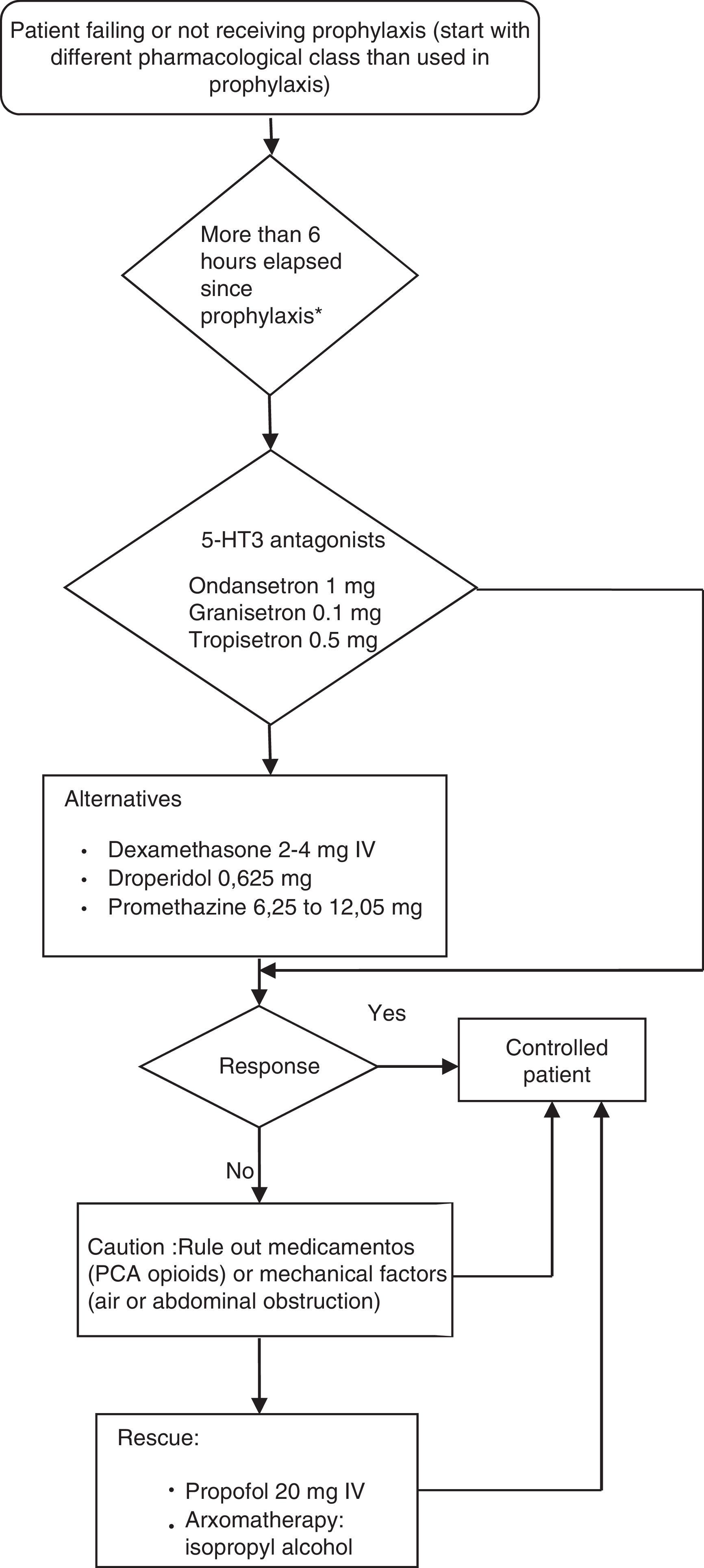
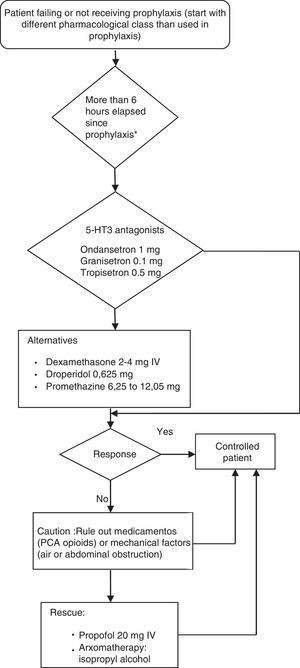
Postoperative nausea and vomiting (PONV) guidelinesProvision of antiemetic treatment for patients with PONV not receiving prophylaxis or for patients failing prevention regimensWhen PONV occurs after a surgical procedure, the treatment must use an anti-emetic of a different pharmacological class from the prophylactic agent given intra-operatively. If no anti-emetic prophylaxis is given, the recommendation for treatment is low-dose 5-HT antagonists.
Established alternative treatments for PONV include dexamethasone 2–4mg IV, droperidol, 0.625mg IV, or promethazine 6.25–12.05mg IV. When necessary, propofol 20mg IV may be considered for rescue therapy in patients in the PACU, and is as effective as ondansetron. However, the anti-emetic effect with low-dose propofol is probably short acting.
Aromatherapy with isopropyl alcohol was effective in achieving faster reduction of nausea severity compared to promethazine or ondansetron. However, the relevant studies had limitations and it is not clear whether this is an effective modality for achieving total control of PONV.
A repeat dose for PONV prophylaxis given within the first six hours after the initial dose does not provide additional benefit. After more than six hours, it may be possible to achieve an effect with a second dose of an 5-HT3 antagonist or with the use of butyrophenones (droperidol and haloperidol), although this has not been demonstrated in clinical trials and must be tried only if triple therapy has been used for prophylaxis and if there are no alternatives available for rescue in those cases in which prophylaxis has not been used. The repeated administration of longer-acting drugs like dexamethasone, aprepitant and palonosetron is not recommended.
The rescue attempt must be initiated when the patient presents with PONV and, at the same time, an assessment must be performed in order to rule out a medication or mechanical factor which may be causing nausea and vomiting. Contributing factors may include PCA (patient controlled analgesia) with opioids, blood drainage through the pharyngeal airway, or abdominal factors.
Nausea and vomiting after PACU or hospital dischargeThe combination of oral and IV anti-emetics at various time points in the perioperative period reduces this complication. A study found that dexamethasone 8mg IV on induction plus ondansetron 4mg IV at the end of surgery, plus oral ondansetron 8mg after the operation, was more effective at reducing nausea and vomiting after discharge from the PACU or from the hospital than ondansetron 4mg IV alone at the end of surgery.
The combination of haloperidol 2.5mg plus dexamethasone 5mg IV after induction was more effective than droperidol 1.25mg, haloperidol 2mg, or dexamethasone 5mg, alone. All of them were more effective than placebo.
Aprepitant 40mg and 120mg, and ondansetron 4mg reduced PONV to a similar degree during the postoperative period between 0 and 24h; however, 24–48h after surgery, aprepitant 40mg and 120mg had a similar effect, and they were more effective than ondansetron 4mg.
The prophylactic administration of anti-emetics may be justified in patients at high risk of developing nausea and vomiting after PACU or hospital discharge. A systematic review showed that the use of propofol, compared to inhaled anaesthetics, also reduced the incidence of nausea and vomiting (P<0.05). Some small RCTs have shown effectiveness for prevention with oral disintegration of ondansetron tablets and stimulation of the P6 acupuncture point.
Adaptation of the PONV guidelines to the Colombian contextConsensus recommendations of Colombian anaesthetists- •
Another pharmacological management strategy for PONV is metoclopramide 10mg IV. A recent meta-analysis reported that metoclopramide reduces the incidence of PONV within the first 24h after surgery compared to the control (OR=0.58; 95% CI (0.43–0.78).13 Metoclopramide is an alternative to ondansetron and dexamethasone in countries that have experienced shortages of these latter agents or in situations where cost is a barrier to access.13
- •
Although ondansetron (IV and tablets) has been approved by INVIMA,14 it is listed in the Mandatory Health Plan (POS) for the indication of use in anti-neoplastic chemotherapy.15
- •
Granisetron, tropisetron, propofol, promethazine and droperidol are approved by INVIMA,14 but they are not on the POS schedule. Their availability in the clinical setting depends on local hospital factors.
- •
Anaesthetists in charge of managing perioperative pain must use therapeutic options such as epidural or intra-thecal opioids, patient controlled analgesia (PCA) with systemic opioids, and regional techniques, after conducting an analysis and bearing in mind the risks and benefits for the individual patient.
- –
These modalities should be used preferably over IM opioids prescribed PRN.
- –
- •
The selected therapy must be based on the anaesthetist's own individual experience as well as on the ability to apply each modality safely in every scenario.
- –
This includes the ability to recognize and manage adverse events occurring after the initiation of therapy.
- –
- •
Care is required when using continuous infusion modalities because the cumulative effect of the drug may give rise to adverse events.
- •
Whenever possible, anaesthetists must use multimodal pain management approaches.
- –
Unless there is a contraindication, patients must receive a regimen based on a time scheme consisting of NSAIDs, COXIBs or acetaminophen and calcium-channel antagonists (gabapentin and pregabalina).
- –
A regional block with local anaesthetics must be considered.
- –
- •
Dose regimens must be given with the aim of optimizing efficacy and reducing the risk of adverse events down to a minimum.
- •
The choice of medication, route of administration, and duration of the therapy must be individualized.
Transfer of critically ill patients may be required in three instances: pre-hospital transfer, inter-hospital transfer, and intra-hospital transfer.
Aspects to consider:
Staff
The team must be trained in all aspects of patient transfer and participate in quality and organization training activities, as well as continuous professional development. There must be at least one qualified nurse, an aide and a physician with the specific skills and training for this type of procedure.
Every team must be familiar with the means used for transfer and have the necessary experience in airway management procedures, pulmonary ventilation, cardio-cerebro-pulmonary resuscitation and other foreseen emergency procedures.
Transfer
With all forms of transfer, securing the airway, intravenous access and all the catheters, and providing adequate follow-up before exiting are all critical for effective transfers. Vital sign stabilization must occur before transfer.
Equipment
The required equipment must include:
Ventilation support equipment
- •
Airway (oral, nasopharyngeal and supraglottic [laryngeal masks] airway management devices)
- •
Oxygen source, masks, nebulizer
- •
Self-inflatable bag or manual ventilation devices
- •
Positive end-expiratory pressure valve
- •
Suction devices
- •
Portable ventilator with disconnection and high pressure alarms
- •
Intubation devices and endotracheal tubes
- •
Emergency surgical airway equipment
- •
Difficult airway management equipment
- •
Pleural drainage equipment
- •
Oxygen supply for the longest estimated transfer time
Circulatory support equipment
- •
Monitor/defibrillator
- •
Pulse oxymeter
- •
Aneroid sphygmomanometer with a range of cuff sizes
- •
Peripheral and central vascular cannulas
- •
Intravenous fluids and pressure infusion set
- •
Infusion pumps
- •
Arterial cannulas and blood pressure transducer kit
- •
Syringes and needles
- •
Thoracotomy and pericardiocentesis equipment
- •
Sharps container and a bag for biologic waste
Other equipment
- •
Nasogastric tube and bag
- •
Urinary drainage catheter and bag
- •
Decongestant nasal spray
- •
Instruments, sutures, gauze, antiseptic lotions, gloves
- •
Thermal isolation and temperature control
- •
Splints, spinal immobilization and physical integrity maintenance devices
- •
Neonatal/paediatric/obstetric transfer equipment when applicable
- •
Dressings, bandages, slings, splints and tape
- •
Cutting scissors and portable flashlight
- •
Use of personal protection gloves and goggles
- •
Consider:
- –
Alternative vascular access such as intra-osseous devices for adults and children
- –
Blood for transfusion in case it is indicated
All medications must be checked and labelled clearly before administration. The range of medications available must include all those required for the treatment of life-threatening medical emergencies and for the treatment of the patient's individual clinical condition.
DiscussionThe focus of this document is the treatment in adult patients of the two most common perioperative complications: PONV and acute post-operative pain. They have both been studied in different populations and management strategies have been published for the management of specific surgical procedures.16–19 There are reports in the literature on the use of protocols for Enhanced Recovery after Surgery (ERAS) which still are in need of additional information for the assessment of assess their clinical usefulness.20 Although they are important tools, they must evolve continuously.21
The European Society of Regional Anaesthesia and Pain Therapy developed the Procedure Specific Postoperative Pain Management (PROSPECT) initiative to establish specific treatment strategies for post-operative pain management on the basis of the individual surgical procedure.22 The adaptation of these initiatives to the Colombian setting is an important strategy for tackling a problem that still prevails in our operating rooms, considering that acute pain is frequently underestimated and inadequately treated.8 The published international guidelines on post-operative pain management, used as the basis for this Manual, contain several general recommendations for approaching the management of post-operative pain. Depending on the context and the specific logistic conditions of each institution, these recommendations ought to be considered an integral part of institutional and patient care policies. These guidelines do not make concrete recommendations (e.g., the use of a specific drug), but rather emphasize the importance of the process more so than of the specific interventions.
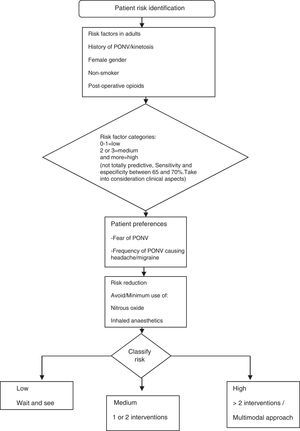
The guidelines for the management of PONV focus in particular on the perioperative situation and consider the individual risk factors reported in the literature (Fig. 4). The full adaptation of the pharmacological management to the Colombian context poses some difficulty, given the limited availability of certain drugs (e.g., transdermal scopolamine). Moreover, other pharmacological compounds recommended by international guidelines are not included in the Colombian Mandatory Healthcare Plan (POS).15 These are described in this document because they are approved by INVIMA and may, therefore, be marketed in this country and used in clinical practice depending on individual hospital settings (Fig. 5).
The expert consensus meeting ratified the need to include in the recommendations the use of metoclopramide for the management of PONV. Although this medication was not included in the guidelines described, a recent meta-analysis has reported its effectiveness in the reduction of this complication. This analysis did not include the studies by Fujii, which were challenged on the grounds of validity.13 Consequently, the use of metoclopramide is reaffirmed for the Colombian context, particularly because of the high cost of other anti-emetic drugs or because of their non-inclusion in the POS listing.
The description regarding transfer of critically ill patients compiles the information required at an international level. The guidelines include items pertaining to pre-, intra, and inter-hospital transfers. This document focused on recommendations pertaining to staff and equipment required for intra-hospital transfers, based on information endorsed by experts from Australia and New Zealand.23 It is important to develop guidelines specifically adapted for local settings, bearing in mind the peculiarities of the level of care and the limitations inherent to the healthcare system.
There is a need to set up organized teams to work on a continuous basis updating the information related to the management of post-operative complications. It is important to conduct studies describing the use of protocols and checklists at a national level in order to obtain information about weaknesses and opportunities for improving patient safety in anaesthesia. Consequently, direct healthcare providers must do the job of updating the appropriate pharmacological and non-pharmacological strategies for the management of complications using a multidisciplinary approach.24
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Calvache JA, Leon E, Gomez LM, Garcia C, Torres M, Buitrago G, et al. Recomendaciones basadas en la evidencia del manejo de complicaciones posquirúrgicas en el contexto colombiano. Rev Colomb Anestesiol. 2015;43:51–60.
Although gabapentin and pregabaline are approved by INVIma, they are not listed in the Mandatory Healthcare Plan (POS).15