Non-compressible torso haemorrhage is the leading cause of death in trauma cases. This has led to the development of new devices to control bleeding, including Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA).
ObjectiveTo perform a non-systematic review of the literature on the use of Resuscitative Endovascular Balloon Occlusion of the Aorta in trauma.
Materials and methodsA systematic literature search through Medline was conducted. Articles relevant to our objective were selected. A qualitative and narrative synthesis of results is presented.
ResultsOur qualitative and narrative results show that Resuscitative Endovascular Balloon Occlusion of the Aorta could be a safe and effective intervention for the control of haemorrhage in abdomino-pelvic trauma. Its use is controversial in thoracic trauma. Finally, the performance of this intervention may cause complications.
ConclusionResuscitative Endovascular Balloon Occlusion of the Aorta is an alternative that can be used in damage control surgery. It could be effective for early control of bleeding in patients with non-compressible torso haemorrhage. As a complex intervention, REBOA is in its development phase, and the evidence available preclude us from providing strong recommendations.
La hemorragia no compresible del torso es la principal causa de muerte asociada al trauma. Esto ha llevado al desarrollo de nuevos dispositivos para el control hemorrágico, uno de estos es el balón de resucitación aórtico endovascular.
ObjetivoEl objetivo de este trabajo fue realizar una revisión no sistemática de la literatura con respecto al uso del REBOA en trauma.
Materiales y métodosSe realizó una búsqueda sistemática de la literatura en MEDLINE, se seleccionaron los artículos relevantes para el logro de nuestro objetivo y con estos se realizó una síntesis cualitativa y narrativa de la literatura disponible.
ResultadosNuestra síntesis cualitativa y narrativa muestra que el balón de resucitación aórtico endovascular podría ser una intervención segura y efectiva para el control de la hemorragia en trauma abdomino-pélvico. Su uso es controvertido en trauma torácico. Finalmente, el uso del balón de resucitación aórtico endovascular puede causar complicaciones relacionadas con su aplicación.
ConclusiónEl balón de resucitación aórtico endovascular (REBOA) es una alternativa en la cirugía de control de daños que podría ser efectiva en el control de la hemorragia no compresible del torso de origen abdomino-pélvico. Al ser una intervención compleja, REBOA se encuentra todavía en fase de desarrollo y la evidencia disponible no es suficiente para proveer recomendaciones fuertes.
Trauma is a public health problem globally and has been considered an unattended epidemic in the developing countries.1 According to data from the “Global Burden of Disease Study”,2 close to 973 million people suffered injuries requiring some form of medical intervention in 2013: of these, 4.8 million died and 56.2 million required hospital care. The causes of death associated with trauma were motor vehicle accidents, suicide, falls, and inter-personal violence.2
Vascular injury is one of the main causes of death in civil and military trauma.3,4 Bleeding secondary to vascular injury may come from sites amenable to direct compression or sites where direct compression is not possible; the latter is known as non-compressible torso haemorrhage (NCTH).5
NCTH is defined as massive thoracic, abdominal and/or pelvic bleeding not amenable to direct compression. Mortality resulting from this condition can be as high as 45%,6 and it has been estimated that the majority of these deaths are potentially preventable.
Advances in the study of trauma have shed light on the pathophysiology of haemorrhagic shock, leading to improvements in the care of critically ill patients. This has resulted in a greater proportion of patients with non-compressible torso haemorrhage surviving after admission to hospital and taken to the operating room for bleeding control.7
Clinical and contemporary translational research in trauma has focused on the development of methods and devices to improve prognosis in patients with severe trauma.8 One of the new devices is REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta), which could be a safe and effective alternative to emergency thoracotomy in patients with haemorrhagic shock secondary to non-compressible torso haemorrhage.
The objective of this study was to conduct a non-systematic review of the literature on the use of REBOA in trauma.
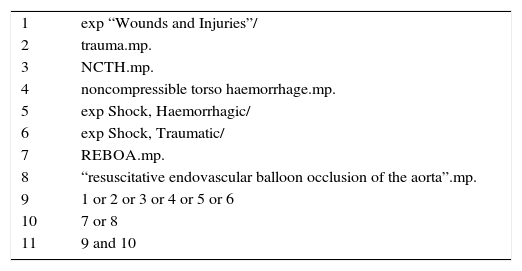
MethodsA systematic review of the literature was conducted in the Medline database through the OVID interface. A search formula was built based on the condition (Trauma) and the intervention of interest (REBOA). The search formula is shown in Table 1. Articles on the use of REBOA in trauma patients were selected, and a qualitative and narrative synthesis of the literature was made.
Formula for the systematic search of the literature in the Medline database.
| 1 | exp “Wounds and Injuries”/ |
| 2 | trauma.mp. |
| 3 | NCTH.mp. |
| 4 | noncompressible torso haemorrhage.mp. |
| 5 | exp Shock, Haemorrhagic/ |
| 6 | exp Shock, Traumatic/ |
| 7 | REBOA.mp. |
| 8 | “resuscitative endovascular balloon occlusion of the aorta”.mp. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 |
| 10 | 7 or 8 |
| 11 | 9 and 10 |
Considering that Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is still under development, a systematic review using a typical PICO question is not possible.
Consequently, the review of the literature is designed to answer the question on “What has been described in the literature regarding the use of REBOA in trauma patients?”
In order to answer this question, the search included controlled clinical trials, case series, cohort studies and case control studies that reported or studied the use of REBOA in trauma patients. Studies in which REBOA was used in conditions other than trauma were excluded.
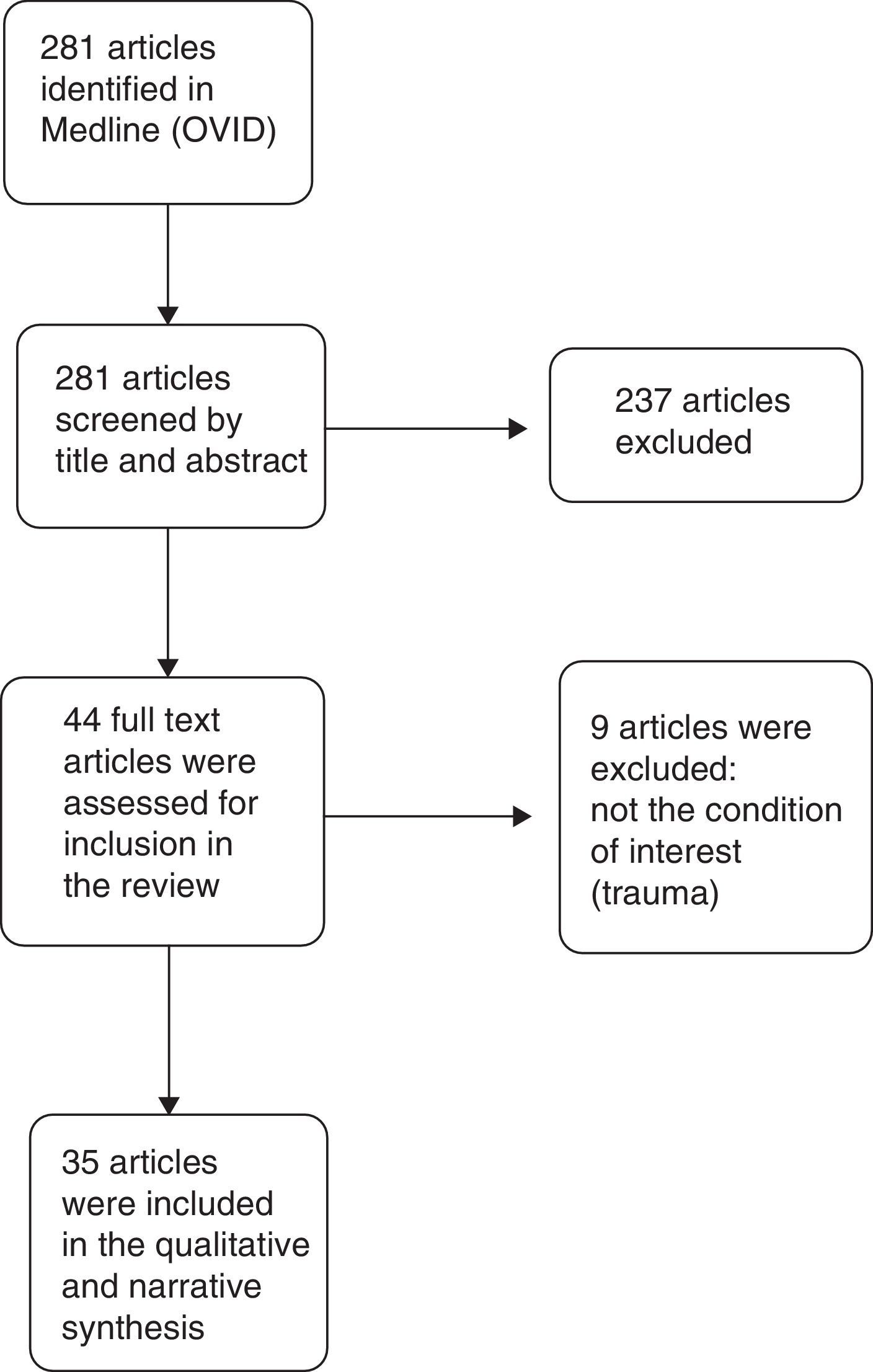
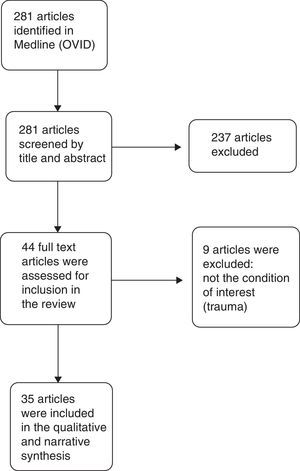
Results of the qualitative synthesisThe search in Medline provided 281 results. Of these, 246 were excluded, and 35 articles were finally included in the narrative and qualitative synthesis. The PRISMA flow diagram used for article selection is shown in Fig. 1.
Evolution and historyVascular occlusion for temporary control of bleeding has been one of the mainstays of damage control surgery, particularly in patients in shock secondary to non-compressible torso haemorrhage.5
Emergency thoracotomy is the technique traditionally performed for direct aortic occlusion.9 It was first described in humans by Paul Nihans in 1880. In 1967, Beall reported its use in dying patients and, in 1976, the performance of this procedure in trauma patients became widespread when Ledgerwood10 described the use of pre-laparotomy thoracotomy for the management of massive bleeding of abdominal origin.
The use of thoracotomy in emergency situations is not limited to resuscitation of the dying patient by means of direct cardiac massage. This procedure is also useful for the control of bleeding from chest injuries, facilitating drainage of the hemopericardium in cases of associated heart injuries. Aortic clamping through a left thoracotomy provides some degree of control in abdominopelvic injuries and increases coronary and cerebra blood flow.11 Emergency thoracotomy with aortic clamping is a simple intervention that can be readily performed in expert hands; however, it is an invasive and bloody technique that may increase morbidity and mortality in critically ill patients.
Endovascular aortic occlusion using the REBOA technique in patients with abdominal and pelvic bleeding at risk of cardiocirculatory shutdown is a less invasive method than emergency thoracotomy, with a history that dates back to the mid-twentieth century.11,12 The first description of the use of REBOA in patients with fatal bleeding was made by Colonel Hughes13 in 1954 during the Korean War. In that report, aortic occlusion was accomplished by means of the Dotter-Lukas balloon14; although the 3 patients who were intervened died, the use of the balloon was effective at restoring arterial pressure in one patient.
Although the initial reports showed effective recovery of arterial blood pressure and temporary haemorrhage control, this technique did not gain much attention and was not adopted in routine clinical practice due to the lack of endovascular technology at the time8 and the limitations for demonstrating its effectiveness.15
With the new and mature endovascular techniques, there has been a renewed interest in REBOA.8 Brenner et al.16 described 5 cases of blunt trauma and 2 cases of penetrating trauma in which they applied endovascular aortic occlusion for bleeding control. The authors concluded that REBOA is a safe manoeuvre for resuscitation and bleeding control in patients with non-compressible thoracic haemorrhage.
Additionally, success with this method has been reported in cases of non-traumatic bleeding such as postpartum haemorrhage,17 oncologic pelvic surgery,18 elective orthopaedic surgery, and ruptured aneurysms of the abdominal aorta, in which it is now in routine use.8,19
Physiological principlesThe goal of resuscitation in patients in shock secondary to trauma is to restore intravascular volume, maintain adequate cell metabolism, and address blood loss.20
Open or endovascular aortic occlusion in abdominal or pelvic bleeding is designed to redistribute circulating volume and prevent circulatory arrest, allowing for a greater opportunity to perform definitive control of bleeding by means of surgery or angioembolization.
Aortic flow obstruction using REBOA in patients in shock and at risk of cardiocirculatory collapse results in a change in volume distribution and increased arterial pressure,18 improving coronary and carotid perfusion.21,22
Despite the benefit of circulating volume redistribution and the subsequent increase in cerebral and coronary perfusion, REBOA may produce ischaemia of the tissues distal to the occlusion. Studies of REBOA in animal models have shown that prolonged aortic occlusion has deleterious physiological effects such as increased acidosis, hypoxia and inflammatory response.23 However, this physiological abnormality appears to be less severe with REBOA than with traditional aortic occlusion through a thoracotomy.23,24
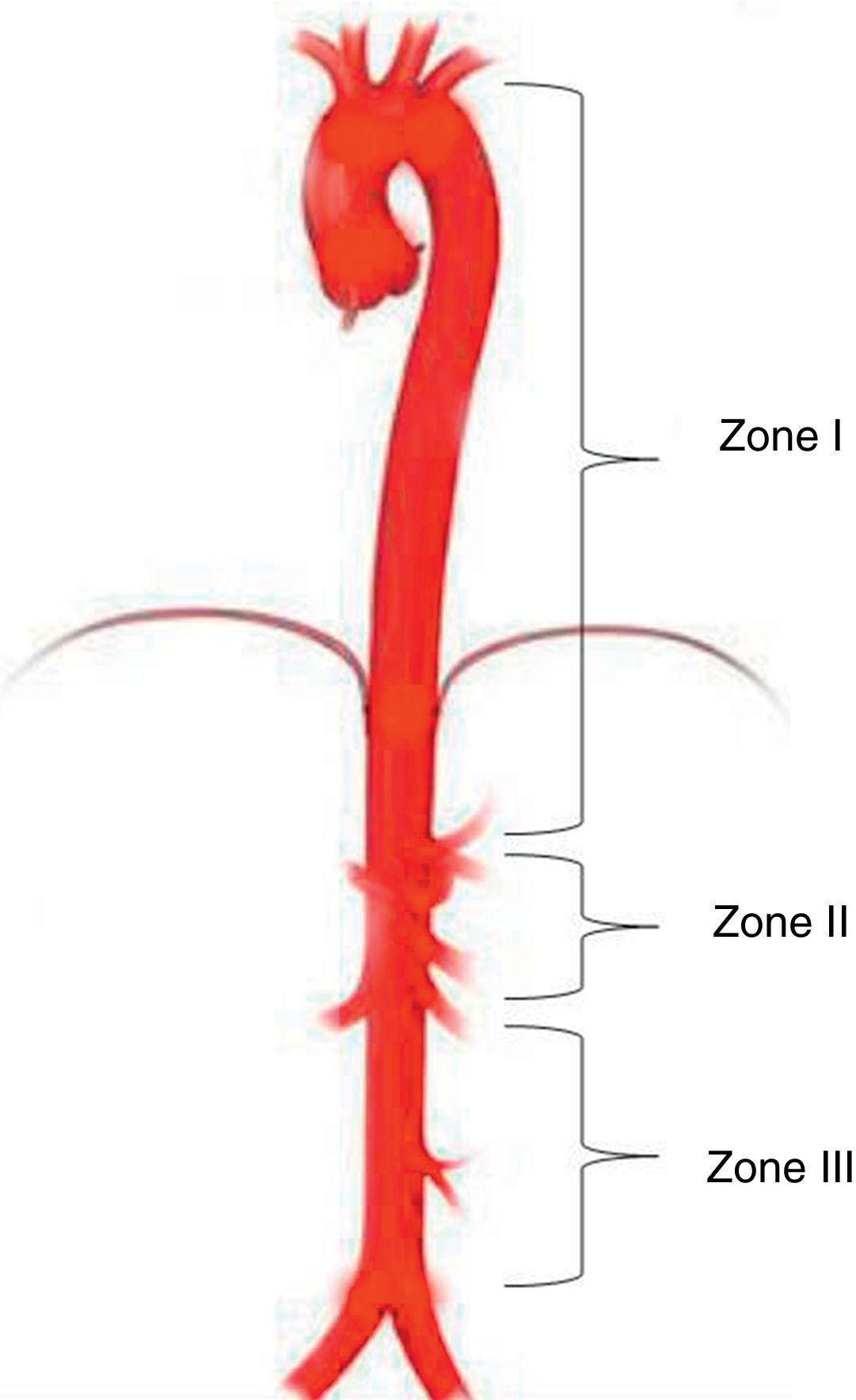
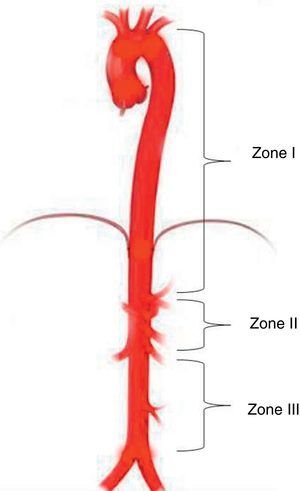
Aortic occlusion sitesREBOA level depends on the indication and the source of bleeding. Three different anatomical sites have been identified for deploying the balloon in the aorta25: Zone I between the left subclavian artery and the celiac trunk; Zone II characterised as a non-occlusion region extending from the celiac trunk to the most distal renal artery; and Zone III from the renal arteries to the aortic bifurcation (Fig. 2).25
Indications for REBOA in traumaBased on military studies, suggested potential indications26 include injuries with a score of 3 or more on the Abbreviated Injury Scale (AIS), equivalent to a severe war injury that places the patient at an impeding risk of dying.26,27
Potential indications include: (1) REBOA deployment in Zone I in abdominal bleeding secondary to severe injury (AIS≥3) to a solid organ, mesenteric injury or vascular injury proximal to the aortic bifurcation; and (2) use of REBOA in Zone III in pelvic or inguinal bleeding and severe injury (AIS≥3) of the pelvic rim associated with fracture, amputation close to the hip joint, or vascular injury proximal to the femoral artery26,27; these two settings accompanied by systolic blood pressure under 90mmHg at the time of arrival at the hospital.
Existing clinical reports in civilians have confirmed that patients with abdominal or pelvic injuries associated with haemorrhagic shock and impending circulatory shutdown are the population that benefits the most from the use of REBOA in trauma.12,28,29
Based on the available evidence in military and civilian patients, Biffl et al.30 propose an algorithm for bleeding control. They suggest considering the use of REBOA in patients with non-compressible abdominal and/or pelvic bleeding and haemorrhagic shock, as long as they present with a palpable pulse.
There is no published consensus to date regarding the indications for use of REBOA in trauma.27 REBOA being a complex intervention is still under development and the evidence available is not sufficient to provide strong and compelling recommendations regarding indications and contraindications.18,31
Abdominal traumaTraumatic abdominal injuries account for 15–20% of overall mortality in trauma.32 The abdomen is a highly vascularised structure and patients sustaining severe abdominal trauma are at a high risk of presenting with massive bleeding requiring immediate surgical repair of the vascular injuries.33
A patient with abdominal trauma, haemodynamic instability and suspected intra-abdominal injury must be taken to exploratory laparotomy for definitive control of bleeding and injury repair, or damage control.33,34 Many of these patients require thoracotomy and aortic clamping, a technique developed initially under the concept of pre-laparotomy thoracotomy with the aim of preventing cardiocirculatory collapse and assisting in the control of intra-abdominal haemorrhage.10 However, studies that came later showed that simultaneous exploration of two cavities (thoracotomy+laparotomy) is an independent predictor of mortality.35 Hence the suggested need of finding less invasive options than traditional thoracotomy for aortic occlusion.
Consequently, in extreme cases of abdominal trauma associated with haemodynamic instability, REBOA would be indicated before traditional aortic occlusion through a thoracotomy.
In cases of haemorrhagic shock in blunt or penetrating abdominal trauma, it has been shown that REBOA deployment in Zone I may contribute to haemodynamic stabilisation and reduction of haemoperitoneum, making it easier to perform damage control laparotomy and identify the source of bleeding.25,36
REBOA has been used in non-operative management of blunt abdominal trauma associated with haemodynamic instability. Ogura et al.,37 in a series of 7 patients with these characteristics treated with REBOA and angioembolisation, reported a 28-day survival of 86%, and concluded that the combined use of REBOA and angioembolisation could be an effective alternative in those cases. Another recent experience with a case of grade V liver trauma associated with massive abdominal bleeding showed that REBOA is an effective intervention for the management of severe abdominal trauma.38
Pelvic traumaApproximately 15% of patients with pelvic fractures present with complex pelvic injuries and, of them, 4% have associated arterial injuries.39 Mortality in these patients is high, and it has been shown that the volume of transfused blood is an independent predictor of mortality.40 For this reason, proximal control of the bleeding is a critical requirement for improving prognosis and survival.
Translational research shows that the use of REBOA provides better control of blood loss and is associated with lower mortality in animal models with pelvic bleeding associated with coagulopathy.41 Likewise, clinical research has confirmed the findings in animal models and shown that REBOA could be an effective technique for controlling haemorrhage in patients with pelvic fractures and haemorrhagic shock.18,42
In patients with pelvic fracture and haemodynamic instability (SBP<90mmHg), pre-hospital mortality may be as high as 78%.29 In these patients, haemorrhagic shock is usually of arterial origin and, consequently, REBOA associated with pre-peritoneal packing and/or angioembolisation, is a therapeutic option to consider.29,43
In trauma, a patient with severe pelvic fracture and haemodynamic instability has an indication for surgical control of bleeding and damage control. In the presence of marked haemodynamic instability, REBOA must be considered as a means to maintain proximal perfusion while control of bleeding is achieved.29,43 REBOA deployment in Zone III in cases of pelvic rim fractures with haemorrhagic shock could be effective for controlling haemorrhage in the absence of abdominal bleeding.18 Moreover, infra-renal aortic occlusion has the advantage of maintaining renal and visceral perfusion, reducing complications following the use of the balloon.
Related to the above, the Western Trauma Association included the use of REBOA in its guidelines for pelvic fractures associated with haemodynamic instability as an adjunct or alternative to pre-peritoneal pelvic packing.44
ContraindicationsBased on the available evidence, it has been suggested that REBOA could be harmful in haemorrhagic shock associated with penetrating or blunt cervicothoracic trauma, specifically in cardiac or aortic injuries, considering that Zone I occlusion could exacerbate bleeding and accelerate cardio-circulatory collapse.8 Consequently, the current consensus recommendation is to take these patients directly to the operating theatre for emergent thoracotomy.30
Comparative studies on REBOAFew studies have been developed with the aim of studying the effectiveness of REBOA compared to traditional aortic occlusion through thoracotomy. Abe et al.45 compared outcomes associated with the use of REBOA and traditional aortic occlusion in trauma patients. In that study, the use of REBOA was associated with lower mortality when compared with traditional aortic occlusion through thoracotomy. Additionally, patients taken to REBOA had a lower incidence of thoracic complications. In other studies in which the propensity score was used to study the effect of REBOA on mortality, an association was found between REBOA and higher mortality.46,47
ComplicationsBalloon inflationIschaemic injury to the tissues distal to the occlusion is the main source of concern with REBOA. In this regard, research in animal models has focused on determining safe occlusion time in order to ensure that the risk of physiological alterations is minimum or reversible. In this regard, Avaro et al.,21 used an animal model of haemorrhagic shock and determined that 40min of aortic occlusion is a safe time period. Along the same lines, Scott et al.48 reported that 60min of aortic occlusion with REBOA is tolerable and does not lead to irreversible organ damage despite the physiological alterations found to occur. Markov et al.49 assessed physiological tolerance to endovascular occlusion in trauma and found that 90min of occlusion time produces irreversible organ damage such as liver necrosis and acute renal injury.
Studies in humans have shown that aortic occlusion time using REBOA is much shorter in survivors, confirming that prolonged occlusion is associated with increased mortality.28,50,51 Additionally, of all cases published regarding the use of REBOA in humans, only two have survived occlusion times of more than 90min.37,51
Besides liver and kidney injury, there may be other ischaemic injuries associated with prolonged aortic occlusion. These include paralysis due to spinal cord ischaemia, lower limb amputation, intestinal ischaemia, and multi-organ failure.8,28,52,53
As already mentioned, aortic occlusion in trauma restores cardiac and cerebral perfusion for short periods of time. However, sustained elevation of arterial blood pressure may be harmful for organs that lie proximal to the site of balloon deployment.54
Regarding this occurrence, Uchino et al.55 reported one case of massive intracranial haemorrhage secondary to the use of REBOA. In that case, REBOA was used to control bleeding associated with a complex pelvic fracture. During angioembolisation following REBOA, systolic blood pressure rose above 180mmHg. This supraphysiologic increase in blood pressure was associated with massive intracranial bleeding not reported upon patient admission.
Consequently, avoiding supraphysiologic hypertension could diminish the risk of complications triggered by increased cerebral, cardiac and pulmonary flows.24,52,55,56
Balloon decompressionDistal ischaemia due to prolonged endovascular occlusion alters cell physiology, reducing energy production and increasing lactic acid production as a result of the transition from aerobic to anaerobic metabolism. Flow restoration to the tissues causes the release of free radicals and inflammatory mediators that give rise to reperfusion injury.54,56 The inflammatory reaction associated with ischaemia-reperfusion tissue injury worsens the systemic inflammatory response syndrome already initiated by the traumatic injury and the surgical interventions for damage control.25,54
Kralovich et al.57 studied the haemodynamic effects of aortic occlusion in haemorrhagic shock in an animal model. They found that after restoring aortic flow during resuscitation there were abnormalities of left ventricular function, oxygen consumption and coronary perfusion pressure. This means that caution must be exercised when deflating the balloon, in particular because of the abrupt fall in blood pressure together with reduced myocardial contractility and the ischaemia-reperfusion injury, which put the patient at risk of a haemodynamic collapse, when the necessary preventive measures are not adopted.25,54 Intermittent balloon inflation and deflation is recommended in order to reduce the intensity of the deleterious effects associated with REBOA decompression.25
Technique-related complicationsThe procedure for inserting the endovascular aortic balloon consists of five steps: arterial access, balloon positioning, inflation, decompression, and sheath removal.25 REBOA is performed using the Seldinger technique.58 Technique-related complications have been found to occur mostly at the time of arterial access and sheath introduction or removal.50
The approach to arterial access is percutaneous through the common femoral artery. This access can also be done under ultrasound guidance or direct identification of the artery in surgery.25 During access, there is a risk of vascular injury from direct puncture or catheter insertion. The potential complications reported include femoral artery injury, aortic dissection and perforation, embolisation, air embolism, and peripheral ischaemia.18,50 However, it has been described that these complications are rare and usually related to multiple puncture attempts.52
After this step, the sheath is introduced over a guide wire.25 Traditionally, REBOA is performed using large 10–14 Fr sheaths. This has been associated with severe complications such as embolism and secondary lower limb amputation, dissection, pseudo-aneurysm, and femoral arteriovenous fistula.50,52,59
Balloon rupture has also been reported as a complication. Matsuda et al.60 reported three balloon ruptures in 125 patients with abdominal aortic aneurysm.
The futureIn the short term, the objective is to validate safety, effectiveness and viability of the use of REBOA in trauma before this procedure can be adopted universally. Efforts are focusing on reducing complications. The development of new devices, shorter ischaemia time, an enhanced technique, and a larger number of trained staff, among other factors, could help reduce complications.
There is a need to develop a specific kit or device for REBOA in trauma. Catheters currently available for the endovascular balloon (CODA balloon catheter [Cook Medical, Bloomington, IN]) are very large (9 and 10 Fr) and are designed for different indications than trauma. A smaller catheter will have less contact with the intima, reducing the risk of thromboembolic events during sheath removal.59 The 7-Fr catheter (ER-REBOA [Prytime Medical Devices Inc., Boerne, TX]) is among the new devices and, so far, it has not been associated with vascular injury, haematoma or embolic complications. Because of its smaller size, removal of the device and direct compression are easier, and there is no need to perform arteriotomy as is recommended for larger sheaths.59,61
As a result of the concern with prolonged ischaemia and its implications, new alternative techniques have been developed. These include partial REBOA (P-REBOA) which maintains some distal perfusion because the balloon is not fully inflated,54 and intermittent REBOA (I-REBOA) in which there is alternating full occlusion and no occlusion.8
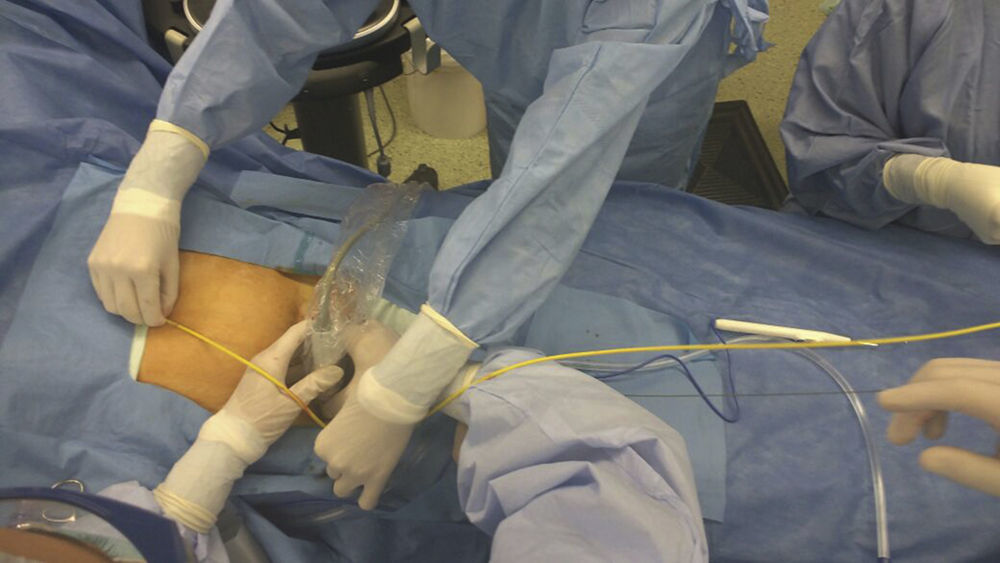
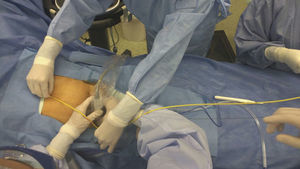
Verification of balloon positioning is very important to prevent complications. Despite its reliability, the use of fluoroscopy in the emergency room may be challenging because of availability and the time needed for verification.62 For this reason, the use of external anatomical landmarks has been proposed for balloon placement, without the need for imaging.52 A study conducted in cadavers by Linnebur et al.63 reported a 100% success rate in REBOA deployment in Zone I using the distance between the puncture site and the middle of the sternum as a landmark. In our experience, the use of anatomical landmarks for measurement is reliable and safe for REBOA insertion in Zones I and III (Fig. 3).
Timely access is required in order to increase the successful use of REBOA in trauma and to ensure that it becomes part of the standard resuscitation protocol. This requires adequate, standardised and accredited training for emergency room staff.15,62 Moreover, exposure to endovascular procedures must be reinforced in residency programmes, ideally including them as part of the academic curriculum.64 Virtual reality simulation has also been shown to be effective in developing skills for endovascular procedures in damage control.65
The future might also include pre-hospital use of REBOA in trauma. In this regard, Sadek et al.66 reported the first successful case of pre-hospital aortic occlusion in a patient with non-compressible bleeding of pelvic origin.
ConclusionREBOA may be a safe and effective manoeuvre for haemorrhage control and prevention of cardiocirculatory collapse in trauma patients. It would appear that its most useful application is in patients with non-compressible torso haemorrhage of pelvic or abdominal origin.
There are doubts regarding its safety in patients with thoracic trauma and, consequently, it is contraindicated in trauma associated with non-compressible haemorrhage of the chest. In the future, REBOA could take the place of emergent thoracotomy in patients with non-compressible haemorrhage of abdominal and pelvic origin when it is associated with a risk of cardiocirculatory collapse. However, further prospective and comparative studies are required in order to demonstrate its effectiveness.
FinancingThe authors were not sponsored to carry out this article.
Conflicts of interestNone. The authors declare having no commercial or associative interest that might be in conflict with the work presented.
We are grateful to Jesús Edigio Solís, M.D. for his recommendations during the preparation of this manuscript.
Please cite this article as: Ordoñez CA, Manzano-Nunez R, del Valle AM, Rodriguez F, Burbano P, Naranjo MP, et al. Uso actual del Balón de Resuscitación Aórtico Endovascular (REBOA) en trauma. Rev Colomb Anestesiol. 2017;45:30–38.