The current health crisis caused by SARS-CoV-2 and the consequences, which are occasionally fatal, of the cytokine storm associated with it have led to therapies aimed at halting hyperactivation of the immune system being used. Among these therapies, corticosteroids have become one of the treatments of choice when clinical and analytical progress is not favorable.
Like all immunosuppressive regimens, corticosteroids are not exempt from risk and ideally, their use would be able to be prevented. Reactivation of previously-acquired infectious would be one of these risks. Of special interest is hepatitis B, both due to its frequency and the existence of tools to stop it. What’s more, in a recent study, chronic HBV infection has been related to slowed clearance of SARS-CoV-2.1
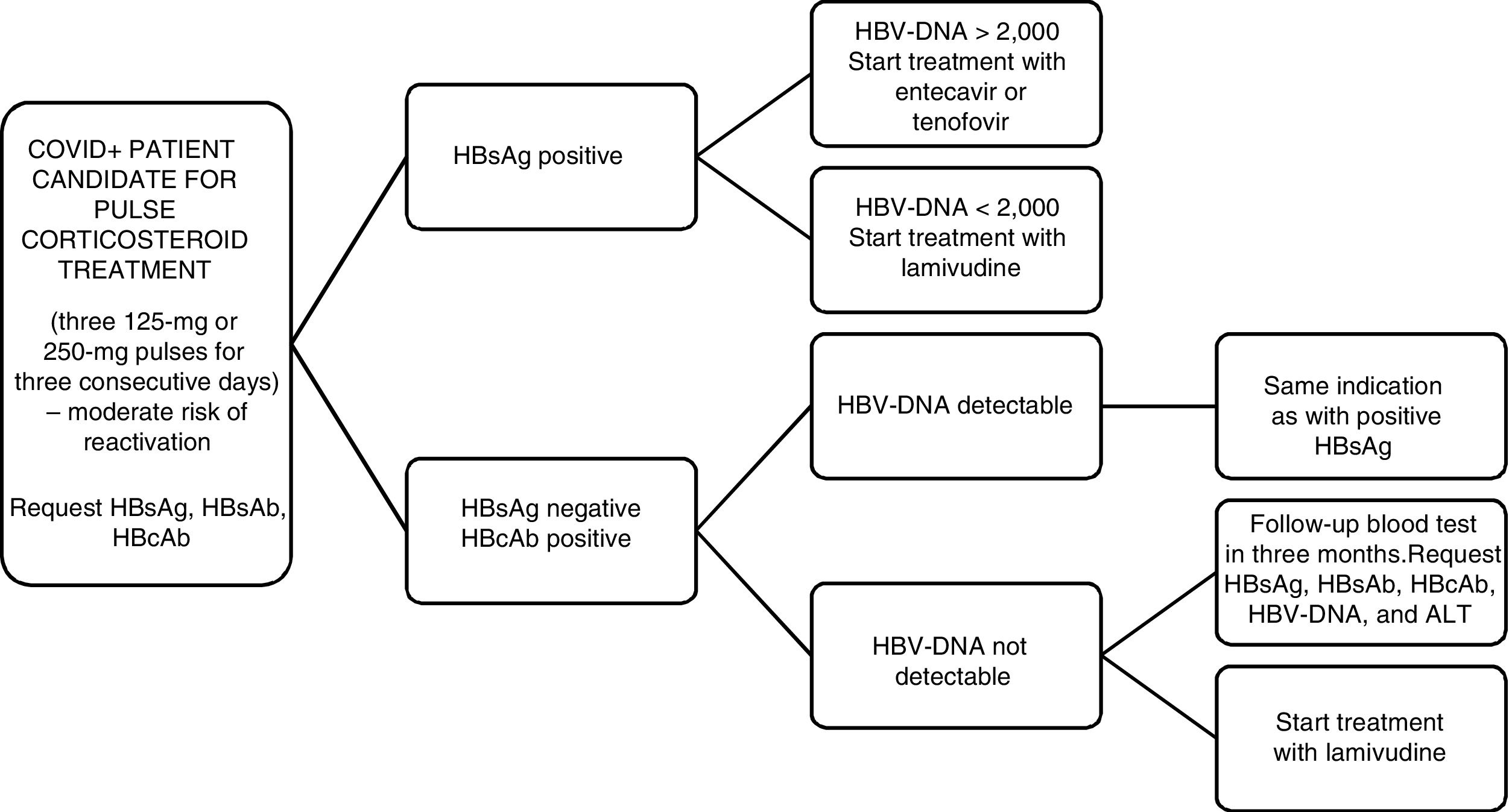
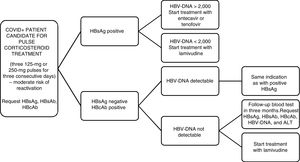
Along these lines, we reviewed the latest recommendations on management of patients who are candidates for receiving corticosteroids at high doses or in pulses (the indication used in our hospital), in this case for the treatment of SARS-CoV-2.
Firstly, the EASL2 and the AGA3 recommend HBV screening by means of determination of HBsAg and anti-HBc for all patients who are going to receive immunosuppressive treatment. According to the recommendations of the AEMPS from July 2014,4 in patients with HBsAg+ and a viral load greater than or equal to 2000 U/mL, we must start treatment with entecavir or tenofovir whereas if the viral load is less than 2000 U/mL and immunosuppressive treatment is going to be used for a short period of time, we must give universal prophylaxis with lamivudine. On the other hand, a patient with HBsAg−, anti-HBc+, and a detectable viral load, the same indication as for HBsAg+ patients should be adopted (Fig. 1).
Doubts arise when we find ourselves before a patient with HBsAg−/anti-HBc+ and an undetectable viral load. Three risk groups are established based on the probability of virus reactivation: high (>10%), moderate (1-10%), and low (<1%). There are tables that summarize the risk of reactivation based on the treatment administered. The wide variety of treatment regimens, in terms of the doses and times indicated, make it more difficult to determine the evidence.
The AGA includes patients who receive low doses (<10 mg/day) or moderate doses (10–20 mg/day) of prednisone or an equivalent in the low-risk group. Patients who have been treated with high doses of corticosteroids (>20 mg/day) belong to the moderate-risk group. This review does not make reference to the duration of the corticosteroid therapy indicated in previous documents, which arbitrarily indicated 4 weeks in order to define prolonged regimens. No specific mention to pulse therapy was made at any time.
Recently, a retrospective study was published in the Journal of Hepatology that analyzed the risk of seroreversion of HBsAg in 12,997 patients exposed to at least one dose of systemic corticosteroids in the period between 2001 and 2010.5 Of them, 1800 presented with anti-HBc+. Among those, 830 also had anti-HBs+, which constitutes a protective factor. It was observed that in the remaining group of 970 anti-HBc+/anti-HBs−patients, the annual risk of presenting with a hepatitis flare is 16.2%, independently of the time of corticosteroid treatment. Greater risk is indeed observed in the group that received doses of prednisone that were greater than 40 mg/day. However, it has not been demonstrated that the differences in the dose or duration of corticosteroid therapy have an influence on risk of seroreversion (defined as a switch in HBsAg from negative to positive), which is 1.8% annually in the anti-HBc+/anti-HBs− group. Thus, the AGA’s proposal is strengthened and patients who receive pulse corticosteroid therapy may be included in the group at moderate risk of reactivation.
In conclusion, in the context of the COVID-19 pandemic, systemic corticosteroids are one of the treatment of choice. In all patients for whom they are indicated, a HBV screening should be performed via determination of HBsAg and anti-HBc.
Evidence exists that supports prophylactic treatment with lamivudine in HBsAg−/anti-HBc+/undetectable HBV DNA patients who are at high risk of HBV reactivation. Likewise, its use is not advised in those who are at low risk. And in the moderate-risk group? Universal prophylaxis has a weaker degree of recommendation. Quarterly monitoring is also accepted for starting early treatment if it were necessary.
Furthermore, it would be interesting to know the risk of HBV reactivation in the context of treatment with other immunosuppressive drugs used, such as tocilizumab or baricitinib. There are previous studies on both drugs in this regard in rheumatoid arthritis, but they are limited in the number of patients and the previous or concomitant use of DMARDs.6,7
Please cite this article as: Varona Pérez J, Rodriguez Chinesta JM. Riesgo de reactivación de la hepatitis B asociado al tratamiento con corticoides frente a SARS-CoV-2 (COVID-19). Rev Clin Esp. 2020;220:535–536.