Stroke represents the main cause of functional dependence and in the Portuguese adult population.
Objectiveto analyse the impact of rehabilitation on functional state and basic activities of daily life (ABVD), 8 weeks following a stroke, in a population of elderly people in north-western Portugal.
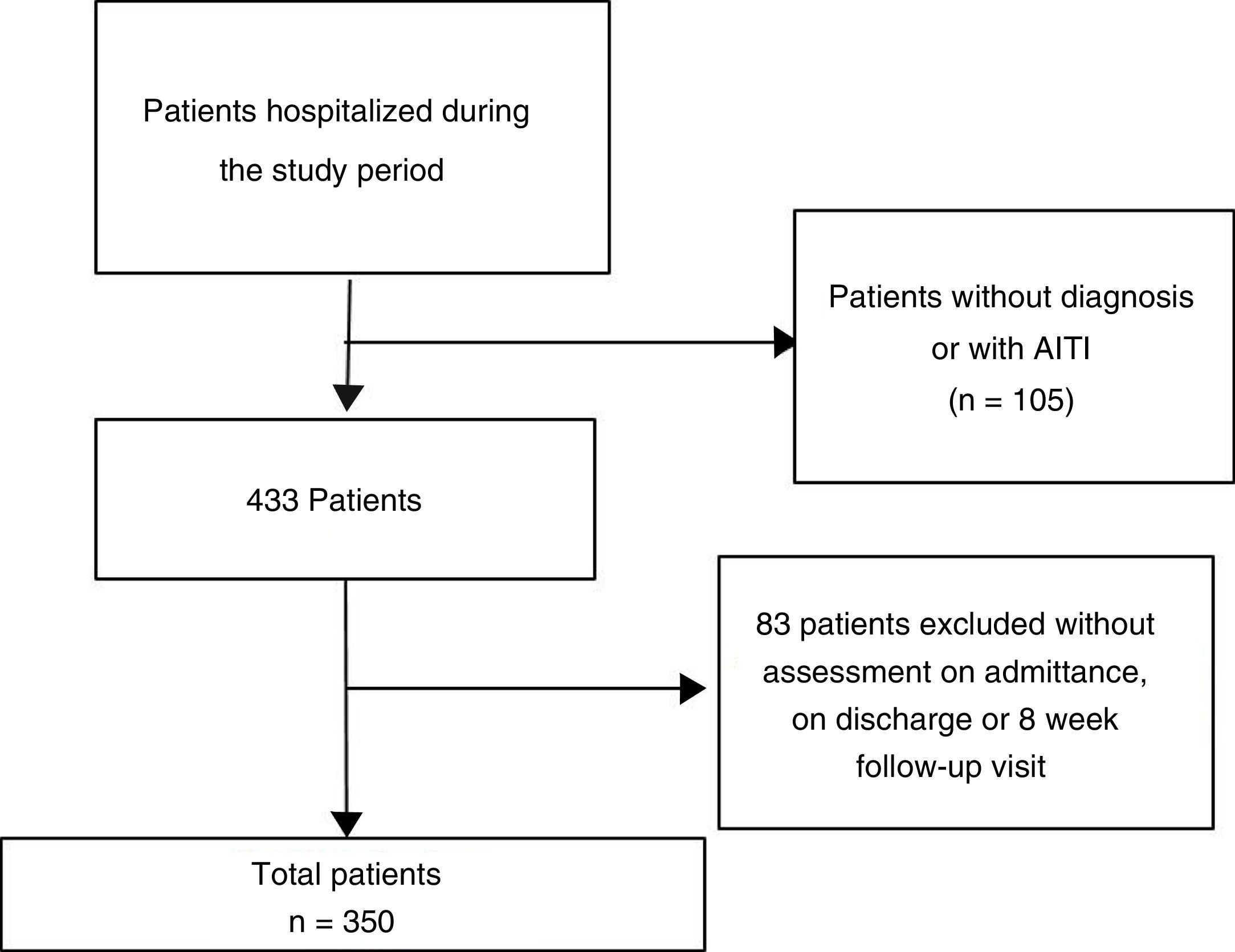
MethodologyObservational, longitudinal and retrospective study. The patients were grouped into 3 groups according to the rehabilitation treatment received: Non-rehabilitation (NR), light rehabilitation (RL) and intense rehabilitation (RI).
Sociodemographic data, clinical variables (on stroke), hospital stay, rehabilitative treatment, and functional status (Barthel Index) were collected.
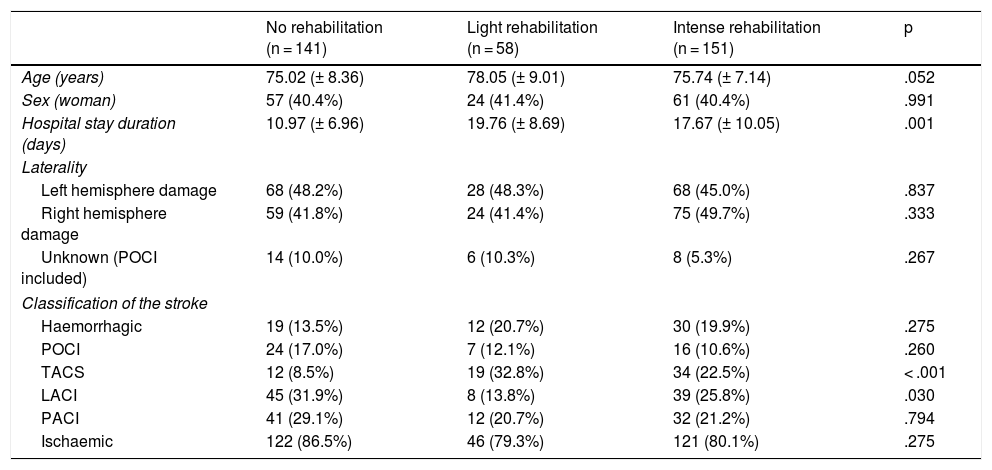
Results350 patients, with a mean age of 75.83 (±8.02) years. The hospital stay was longer in the group of RL (19.7 (±8.69)), RI (17.67 (±10.05)) and of those who did not undergo rehabilitation (10.97 (±6.96)), (p = .001).
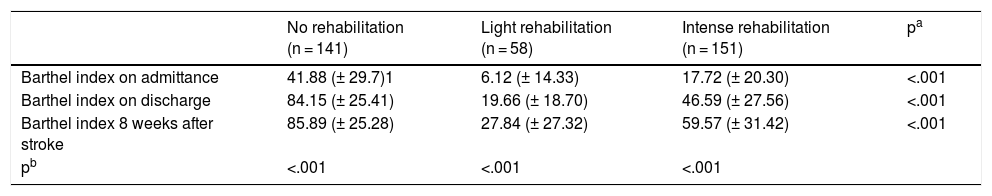
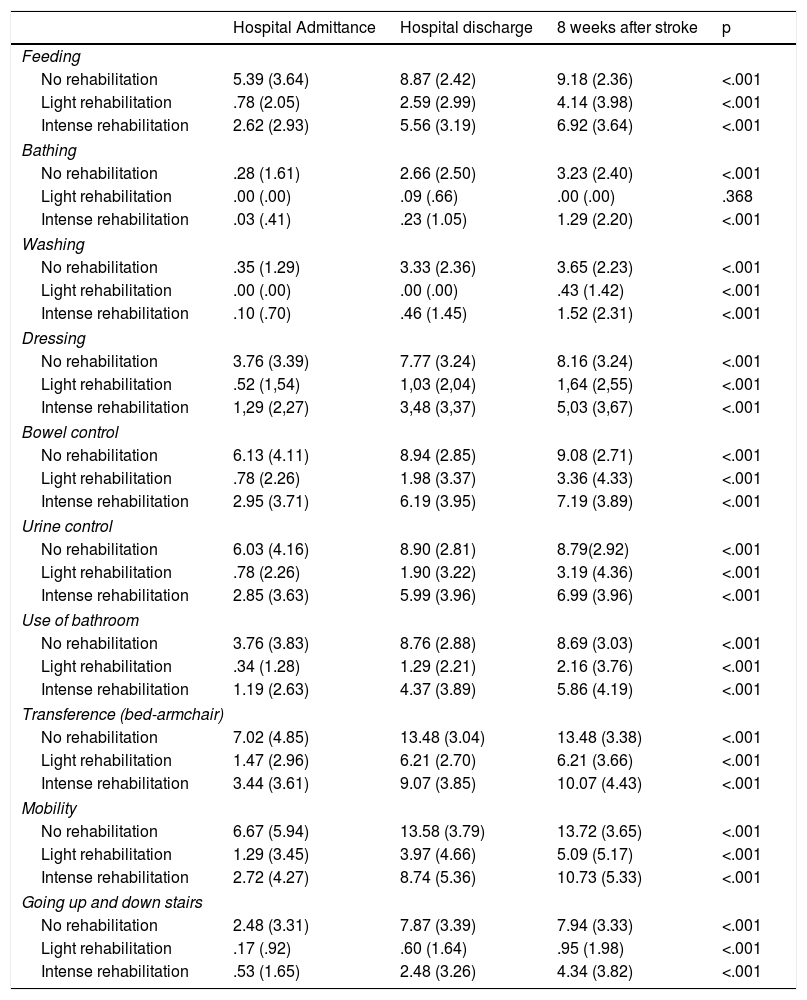
A significant increase (p < .001) was observed in the Barthel index scores from admission to 8 weeks after the stroke.
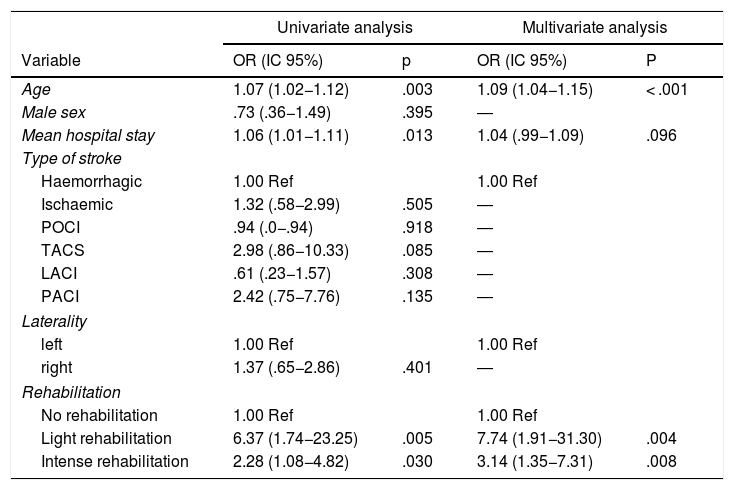
Age (p = .003) and hospital stay (p = .013) were shown as risk factors for functional dependence. Similarly, taking as a reference the patients who did not undergo rehabilitation, the subjects who underwent light rehabilitation (OR (95% CI): 6.37 (1.74−23.25), p = .005) and intensive rehabilitation (OR (95% CI): 2.28 (1.08−4.82, p = .030), had a significantly higher risk of presenting functional dependence
Conclusionundergoing intensive rehabilitation improves functional state and ABVD compared to light rehabilitation, 8 weeks following a stroke in elderly patients.
El ictus representa la principal causa de dependencia funcional y en la población adulta Portuguesa.
ObjetivoAanalizar el impacto de la rehabilitación en el estado funcional y en las actividades básicas de la vida diaria (ABVD), tras 8 semanas de sufrir un ictus, en una población de ancianos del noroeste de Portugal.
MetodologíaEstudio observacional, longitudinal y retrospectivo. Los pacientes fueron agrupados en 3 grupos de acuerdo con el tratamiento rehabilitador recibido: No rehabilitación (NR), rehabilitación ligera (RL) y rehabilitación intensa (RI).
Se recogieron datos sociodemográficos, variables clínicas (sobre el ictus), estancia hospitalaria, tratamiento rehabilitador, y estado funcional (Índice Barthel).
Resultados350 pacientes con edad media de 75,83 (±8,02) años. La estancia hospitalaria fue mayor en el grupo de RL (19,7(±8,69)), RI (17,67(±10,05)) y del que no realizó rehabilitación (10,97(±6,96)), (p = 0,001).
Se observó un aumento significativo (p < 0.001) en las puntuaciones del índice de Barthel desde el ingreso hasta las 8 semanas tras el ictus
La edad (p = 0,003) y la estancia hospitalaria (p = 0,013) se presentaron como factores de riesgo de presentar dependencia funcional. De igual manera, teniendo como referencia a los pacientes que no se sometieron a rehabilitación, aquellos sujetos que realizaron rehabilitación ligera (OR (IC 95%): 6,37 (1,74−23,25), p = 0,005) y rehabilitación intensa (OR (IC 95%): 2,28 (1,08−4,82, p = 0,030), tuvieron un riesgo significativamente mayor de presentar dependencia funcional
Conclusiónla realización de una rehabilitación intensa mejora el estado funcional y las ABVD con respecto a la rehabilitación ligera, a las 8 semanas de sufrir un ictus en pacientes ancianos.
Article
Diríjase al área privada de socios de la web de la SEDENE, (https://sedene.com/revista-de-sedene/ ) y autentifíquese.