To study the prevalence of mood disorders in patients with epilepsy and their relationship with seizure control.
MethodsWe conducted an observational and cross-sectional study of epileptic patients treated in our centre from January-2017 to June-2019. We classify patients into two groups according to crisis control: good control (≤1 crisis/month) and poor control (> 1 crisis/month) and we compare the demographic variables (age, sex, employment status and civil status), clinics (epilepsy type, crisis type, years of evolution of epilepsy), therapeutic (antiepileptic, antidepressant and anxiolytic drugs), presence of depression (NDDIE scale), anxiety (GAD7 scale) and quality of life (QOLIE10). The data was collected by doctors and nurses after informed consent was signed.
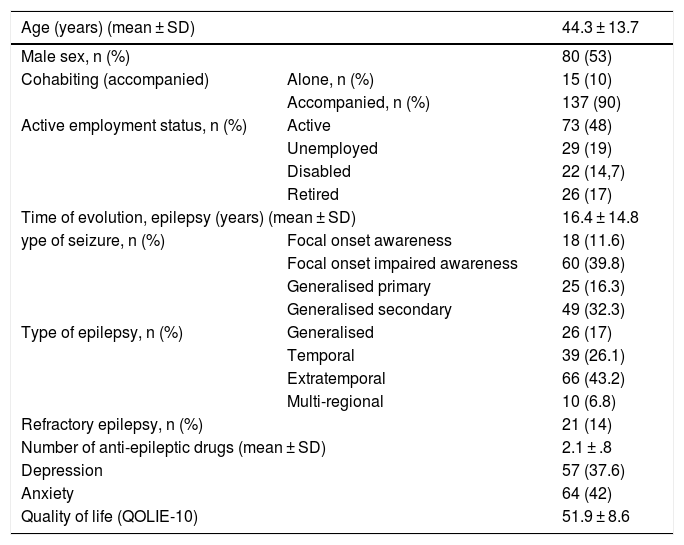
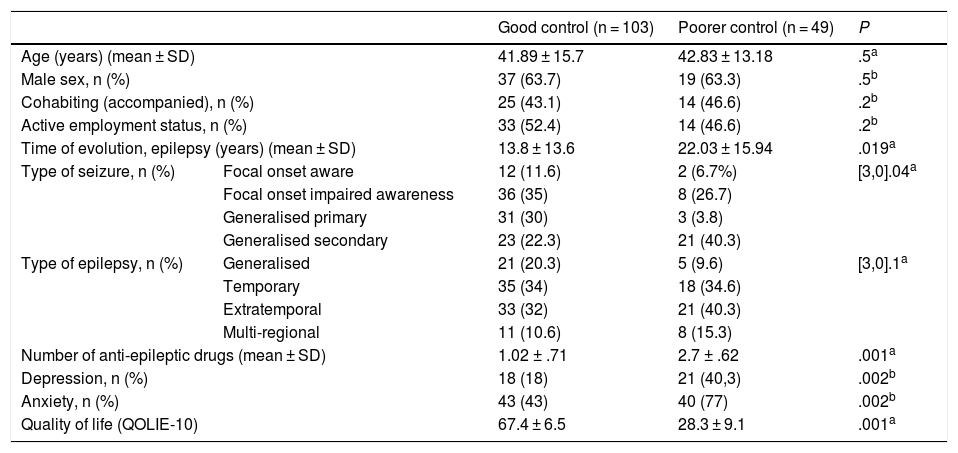
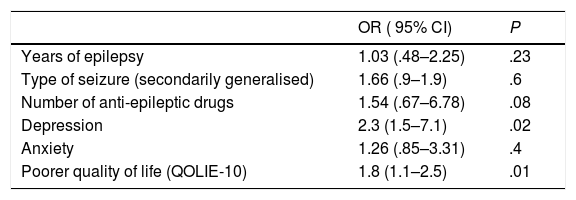
Results152 patients participated, 53% were men with a mean age of 44 ± 11 years. Sixty-eight percent (n = 103) had good crisis control and 32% (n = 49) poor control. Thirty-six point six percent had depression (NDDIE > 15) and 42% anxiety (GAD > 10). Sixty percent of patients with depression and 54% with anxiety did not receive antidepressant and/or anxiolytic treatment. The factors associated with poor crisis control were the presence of depression (OR 2.3, P = .02) and a worse quality of life (OR 1.8, P = .01).
ConclusionsThe presence of altered mood in patients with epilepsy is frequent. In our series, depression and a worse quality of life were related to worse crisis control.
Estudiar la prevalencia de trastornos del ánimo en pacientes con epilepsia y su relación con el control de crisis.
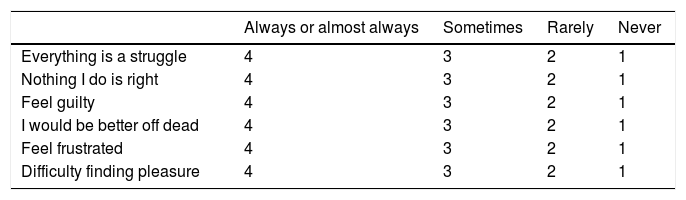
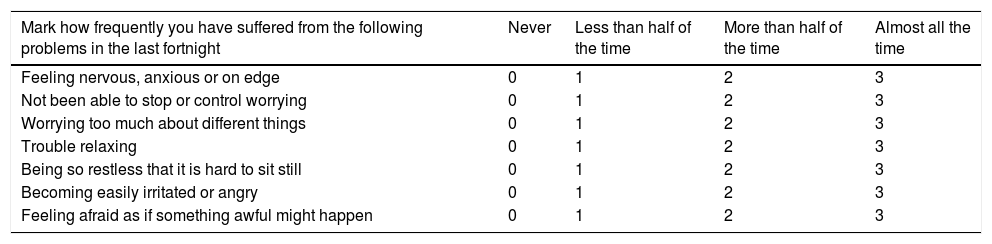
MétodosSe realizó un estudio observacional y transversal de pacientes epilépticos atendidos en nuestro centro desde enero-2017 a junio-2019. Se clasificó a los pacientes en dos grupos según el control de crisis: buen control (≤1 crisis/mes) y mal control (>1 crisis/mes) y se comparó entre ambos grupos las variables demográficas (edad, sexo, estado laboral y civil), clínicas (tipo epilepsia, tipo crisis, años evolución de epilepsia), terapéuticas (fármacos antiepilépticos, antidepresivos y ansiolíticos), presencia de depresión (escala NDDIE), ansiedad (escala GAD7) y calidad de vida (QOLIE10). Los datos fueron recogidos por médicos y enfermeras tras la firma del consentimiento informado.
ResultadosParticiparon 152 pacientes, el 53% fueron varones con edad media de 44 ± 11 años. El 68% (n = 103) tenía buen control de crisis y el 32% (n = 49) mal control. El 37,6% tuvo depresión (NDDIE > 15) y el 42% ansiedad (GAD > 10). El 60% de los pacientes con depresión y el 54% con ansiedad no recibían tratamiento antidepresivo y/o ansiolítico. Los factores asociados a un mal control de crisis fueron la presencia de depresión (OR 2,3, P = ,02) y una peor calidad de vida (OR 1,8, P = ,01).
ConclusionesLa presencia de alteración del estado del ánimo en pacientes con epilepsia es frecuente. En nuestra serie, la depresión y una peor calidad de vida se relacionaron con peor control de crisis.
Article
Diríjase al área privada de socios de la web de la SEDENE, (https://sedene.com/revista-de-sedene/ ) y autentifíquese.