Rotational atherectomy with new ablative strategies have been proposed for the treatment of extremely calcified lesions prior to stent implantation. Nevertheless, few data are available about the adoption of these new strategies in contemporary practice and about late outcomes of patients undergoing this therapy.
MethodsFrom July 2012 to November 2014, a retrospective single center registry was conducted, including all patients undergoing rotational atherectomy as part of the treatment of coronary arteries with heavy calcification or previous failed dilation. We evaluated technical aspects of atherectomy and late outcomes of patients for the occurrence of major adverse cardiovascular events (MACE), defined as death, Q-wave myocardial infarction or repeat target vessel revascularization.
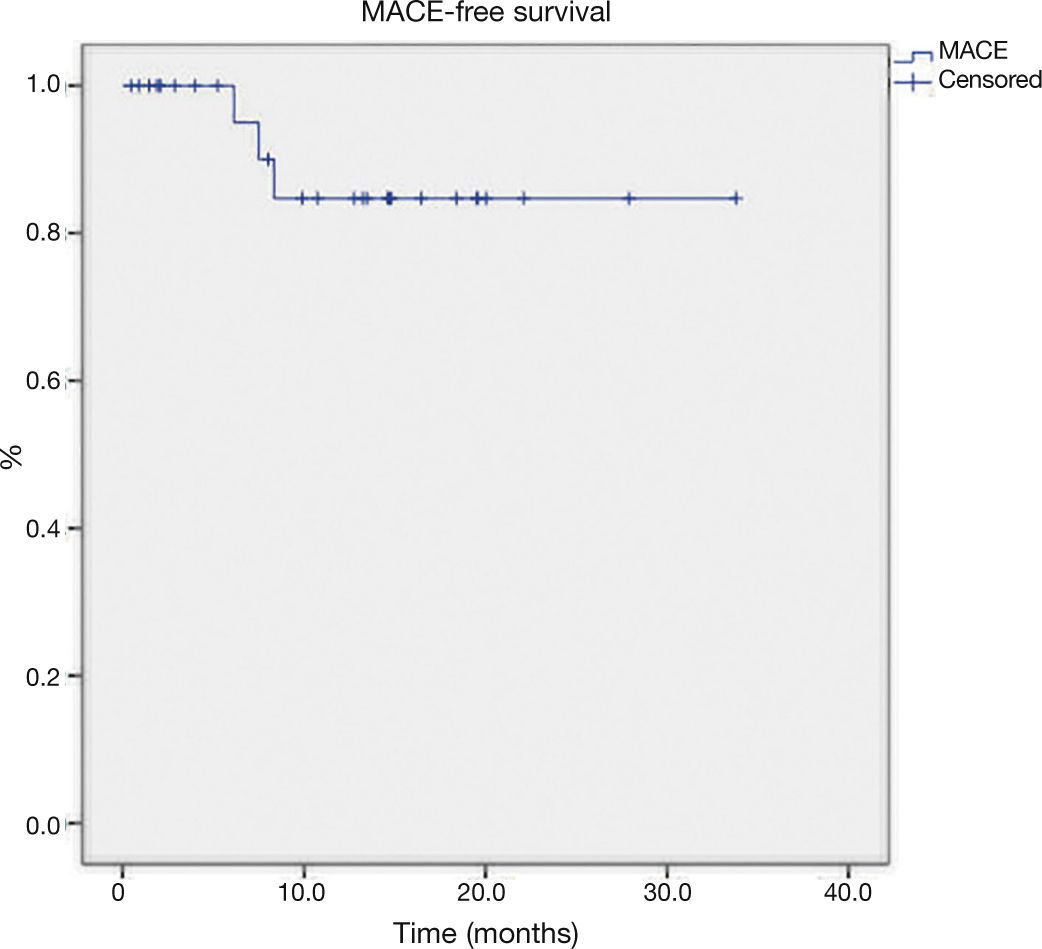
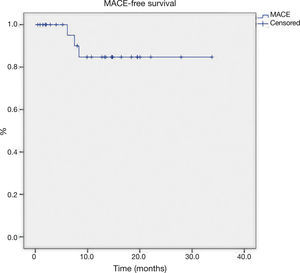
ResultsTwenty-nine patients with a mean age of 69.5 ± 7.6 years, underwent atherectomy. The average burr-to-artery ratio was 0.54 ± 0.07, the initial rotational speed was 161.000 ± 13.928 and the rate of cutting balloon utilization after atherectomy was 45.1%. Angiographic success was achieved in all procedures. The median follow-up time was 13.2 months (IQ: 4.0-17.4) and there were three events: 1 death of non- cardiac cause and 2 new target vessel revascularizations. The mean MACE-free survival time was 29.7 ± 2.1 months.
ConclusionsContemporary rotational atherectomy incorporates less aggressive strategies of ablation with high rates of acute success and low occurrence of major adverse cardiovascular events during late follow-up.
A aterectomia rotacional com incorporação de novas estratégias ablativas tem sido proposta para o preparo de lesões extremamente calcificadas. Entretanto, pouco se conhece a respeito da adoção dessas novas estratégias na prática contemporânea e sobre a evolução tardia dos pacientes submetidos a esse tratamento. Objetivamos avaliar os aspectos técnicos da aterectomia e a evolução tardia dos pacientes quanto à ocorrência de eventos cardiovasculares adversos maiores (ECAM).
MétodosEstudo retrospectivo e unicêntrico, incluindo todos os pacientes submetidos à aterectomia rotacional como parte do tratamento de lesões coronárias com calcificação extrema ou falha de dilatação em procedimento prévio, no período de julho de 2012 a novembro de 2014. Foram definidos como ECAM: óbito, infarto agudo do miocárdio com onda Q e nova revascularização do vaso-alvo.
ResultadosForam submetidos à aterectomia 29 pacientes com idade média de 69,5 ± 7,6 anos. A média da relação oliva/vaso foi de 0,54 ± 0,07; a velocidade de rotação inicial adotada foi de 161.000 ± 13.928 e a taxa de utilização de cutting balloon pós-aterectomia foi de 45,1%. Sucesso angiográfico foi obtido em todos os procedimentos. Na evolução tardia, a mediana de tempo de seguimento foi de 13,2 meses (intervalo interquartil: 4,0 a 17,4 meses). Foram registrados um óbito por causa não cardíaca e duas novas revascularizações do vaso-alvo. A média do tempo de sobrevivência livre de ECAM foi de 29,7 ± 2,1 meses.
ConclusõesA aterectomia rotacional contemporânea incorporou estratégias menos agressivas de ablação, com elevada taxa de sucesso imediato e baixa ocorrência de ECAM na evolução tardia.
Coronary rotational atherectomy (RA), at the time of its introduction, in 1988,1 became popular as an alternative mechanical ablative approach to conventional balloon angioplasty, differentiating itself by obtaining luminal gain, through reduction of the atherosclerotic plaque content.2,3 However, the greater technical complexity and the relatively high incidence of early and late complications in patients treated exclusively with this method determined the decline of its use in the following decade, especially after coronary stents became available.4
Over the past years, there has been a progressive increase in the number of patients and of highly complex lesions considered for percutaneous treatment. Due to this fact, anatomical challenging scenarios became frequent, such as marked coronary calcification, which is associated with difficulty in crossing and dilating the lesions, and has a negative impact on the intervention outcome.5,6
In this context, RA started to be used again as an adjunct tool for percutaneous coronary intervention. Instead of the more aggressive strategy employed in the past, the present proposal includes smaller-caliber burrs and lower rotational velocity, for a geometric modification of the atherosclerotic plaque, followed by lesion dilation with balloons and other devices that, together, allow for stent implantation with adequate expansion of its struts.7,8
There are few data on the use of these new strategies in contemporary practice and on the late evolution of patients undergoing this treatment. Therefore, this study aimed to evaluate the technical aspects of atherectomy and the late evolution of patients regarding the occurrence of major adverse cardiovascular events (MACE).
MethodsStudy design and populationA retrospective study was performed in a single center dedicated to the treatment of high-complexity cardiac diseases. From July 2012 to November 2014, this study included all patients undergoing RA as part of the treatment of coronary lesions with extreme calcification (visible calcification in the fluoroscopic image before contrast injection) or previous failed dilation. Patients with restenotic lesions or with coronary artery bypass grafts were excluded.
Procedure and dual antiplatelet therapyPatients received, prior to the intervention, dual antiplatelet therapy with a loading dose of 200mg acetylsalicylic acid and 600mg clopidogrel. During the procedure, unfractionated heparin was used at the dose of 70 to 100 U/kg, and the use of IIb/IIIa glycoprotein inhibitors was left at the operators’ discretion.
All procedures were performed with the Rotablator RA system (Boston Scientific, Natick, USA), using only the transfemoral access route. The choice of burr size was carried out, considering the distal reference diameter of the target vessel, as assessed by angiography.
Burr size was selected to attain a burr-to-artery ratio of 0.5 (maximum 0.7). The device velocity ranged between 140,000 and 180,000rpm. The atherectomy was interrupted, after a variable number of passages through the lesion or after the occurrence of complications.
The decision about device type and sizes used after ablation was left to the operators’ discretion. After discharge, the recommended daily maintenance dose was 100mg acetylsalicylic acid and 75mg clopidogrel, with thienopyridine withdrawal, according to the attending physician's discretion.
Outcomes and definitionsThe primary outcome was the occurrence of MACE, defined as death, Q-wave acute myocardial infarction (AMI), and target-vessel revascularization in the late follow-up, verified by the institutional electronic medical record and by telephone contact. Secondarily, patients’ hospital outcomes were evaluated regarding the occurrence of death, stroke, periprocedural infarction (type 4a infarction), target vessel revascularization, and contrast-induced nephropathy.
Angiographic success was defined as residual stenosis < 50% and Thrombolysis in Myocardial Infarction 3 (TIMI) coronary flow. Periprocedural infarction was defined as clinical, electrocardiographic, or echocardiographic evidence of ischemia accompanied by an increase of more than five times the 99th percentile of creatine kinase- MB (CK-MB) mass or troponin I values in patients with normal baseline values, or new increase > 20% after stability or early drop, in patients with baseline values higher than the 99th percentile of the method. Q-wave AMI was defined as the appearance of new pathological Q waves in the electrocardiogram, with or without symptoms of ischemia or abnor mal myoc ardial cont rac t i l it y. Contrast-induced nephropathy was defined as serum creatinine increase higher than 25% compared to baseline or greater than 0.5mg/ dL in absolute terms, without other apparent cause after contrast medium administration.
Statistical analysisThe analysis of clinical and angiographic data was performed using SPSS (IBM Corp., New York, USA). Continuous variables were described as means and standard deviations, and categorical variables as absolute numbers and percentages. For time of follow-up, median and interquartile ranges (25th-75th percentiles) were used. Survival analysis was performed using the Kaplan-Meier method.
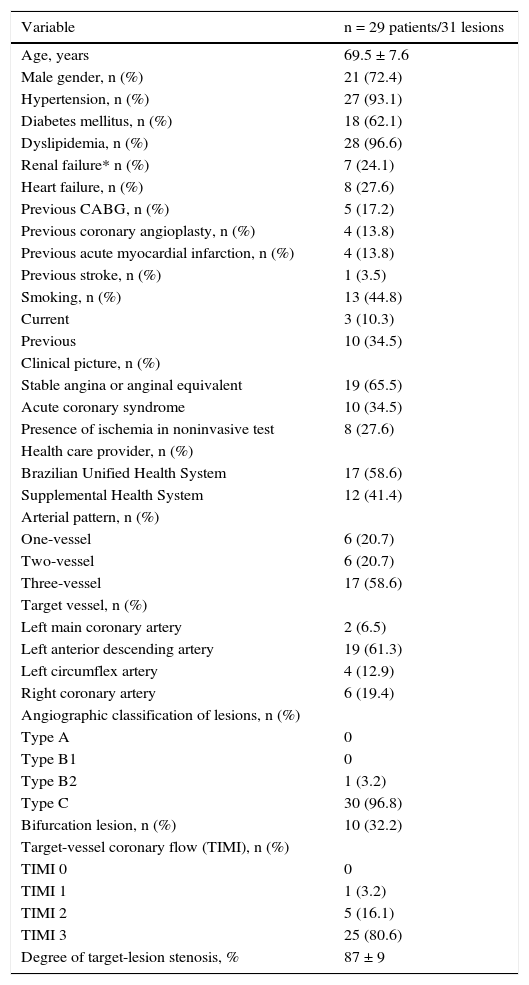
ResultsDuring the analyzed period, 29 patients met the inclusion criteria and were evaluated in this study. The mean age of the population was 69.5 ± 7.6 years, and there was a high percentage of diabetes mellitus (62.1%) and multivessel pattern (79.3%) (Table 1).
Clinical and angiographic characteristics.
| Variable | n = 29 patients/31 lesions |
|---|---|
| Age, years | 69.5 ± 7.6 |
| Male gender, n (%) | 21 (72.4) |
| Hypertension, n (%) | 27 (93.1) |
| Diabetes mellitus, n (%) | 18 (62.1) |
| Dyslipidemia, n (%) | 28 (96.6) |
| Renal failure* n (%) | 7 (24.1) |
| Heart failure, n (%) | 8 (27.6) |
| Previous CABG, n (%) | 5 (17.2) |
| Previous coronary angioplasty, n (%) | 4 (13.8) |
| Previous acute myocardial infarction, n (%) | 4 (13.8) |
| Previous stroke, n (%) | 1 (3.5) |
| Smoking, n (%) | 13 (44.8) |
| Current | 3 (10.3) |
| Previous | 10 (34.5) |
| Clinical picture, n (%) | |
| Stable angina or anginal equivalent | 19 (65.5) |
| Acute coronary syndrome | 10 (34.5) |
| Presence of ischemia in noninvasive test | 8 (27.6) |
| Health care provider, n (%) | |
| Brazilian Unified Health System | 17 (58.6) |
| Supplemental Health System | 12 (41.4) |
| Arterial pattern, n (%) | |
| One-vessel | 6 (20.7) |
| Two-vessel | 6 (20.7) |
| Three-vessel | 17 (58.6) |
| Target vessel, n (%) | |
| Left main coronary artery | 2 (6.5) |
| Left anterior descending artery | 19 (61.3) |
| Left circumflex artery | 4 (12.9) |
| Right coronary artery | 6 (19.4) |
| Angiographic classification of lesions, n (%) | |
| Type A | 0 |
| Type B1 | 0 |
| Type B2 | 1 (3.2) |
| Type C | 30 (96.8) |
| Bifurcation lesion, n (%) | 10 (32.2) |
| Target-vessel coronary flow (TIMI), n (%) | |
| TIMI 0 | 0 |
| TIMI 1 | 1 (3.2) |
| TIMI 2 | 5 (16.1) |
| TIMI 3 | 25 (80.6) |
| Degree of target-lesion stenosis, % | 87 ± 9 |
CABG: coronary artery bypass grafting; TIMI: Thrombolysis in Myocardial Infarction.
* Calculated creatinine clearance < 60 mL/min.
A total of 29 procedures were performed, with 31 lesions treated with the Rotablator. The initial burr diameter was 1.54 ± 0.22mm, with burr-to-artery ratio of 0.54 ± 0.07, and initial rotational speed of 161,000 ± 13,928rpm. In 93.6% of the interventions, balloon dilation of the lesion was performed after RA, using a cutting balloon in 45.1% of cases. Drug-eluting stents were used in 77.4% of patients, with a mean of 1.54 ± 0.66 stents per lesion and mean length of implanted stents of 40.3 ± 20.0mm (Table 2).
Technical aspects of procedure.
| Variable | n = 29 patients/31 lesions |
|---|---|
| Indication of atherectomy, n (%) | |
| Failure to dilate the lesion in a previous procedure | 6 (19.4) |
| Extensive angiographic calcification | 25 (80.6) |
| Burr initial diameter, mm | 1.54 ± 0.22 |
| Use of second burr of larger diameter, n (%) | 4 (12.9) |
| Burr/vessel distal reference ratio | 0.54 ± 0.07 |
| Number of passages through lesion with burr | 3.15 ± 2.01 |
| Total rotation time, seconds | 65 ± 45 |
| Initial rotational velocity, rpm | 161,000 ± 13,928 |
| Rotation increase during atherectomy, n (%) | 5 (16,1) |
| Radiation dose, DAP | 234,400 ± 141,458 |
| Radiation dose, Kerma | 3,839.12 ± 2,404.83 |
| Contrast volume, mL | 260 ± 149 |
| Vascular access, n (%) | |
| Radial | 0 (0) |
| Femoral | 29 (100) |
| Arterial sheath, n (%) | |
| 6 F | 5 (17.2) |
| 7 F | 22 (75.9) |
| 8 F | 2 (6.9) |
| Ad hoc procedure, n (%) | 6 (20.7) |
| Procedures guided by IVUS, n (%) | 6 (20.7) |
| Lesion pre-dilation after atherectomy, n (%) | 29 (93.6) |
| Use of cutting balloon, n (%) | 14 (45.1) |
| Number of treated vessels by procedure | 1.35 ± 0.48 |
| Use of drug-eluting stents, n (%) | 24 (77.4) |
| Number of stents in the target-vessel | 1.54 ± 0.66 |
| Length of stents in the target-vessel, mm | 40.3 ± 20.0 |
| Prophylactic pacemaker, n (%) | 2 (6.9) |
| Intra-aortic balloon, n (%) | 0 |
DAP: dose-area product; IVUS: intravascular ultrasound; RPM: revolutions per minute.
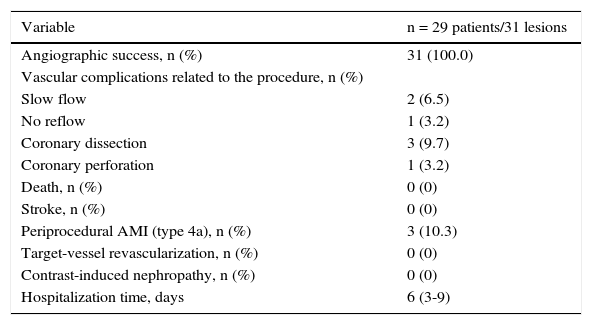
Angiographic success was achieved in 100% of the procedures. Complications associated with atherectomy are shown in Table 3. The periprocedural infarction rate (type 4a) was 10.3%, with no deaths, stroke, or new target-vessel revascularization during inhospital follow-up (Table 4).
Results of the procedure and hospital outcomes.
| Variable | n = 29 patients/31 lesions |
|---|---|
| Angiographic success, n (%) | 31 (100.0) |
| Vascular complications related to the procedure, n (%) | |
| Slow flow | 2 (6.5) |
| No reflow | 1 (3.2) |
| Coronary dissection | 3 (9.7) |
| Coronary perforation | 1 (3.2) |
| Death, n (%) | 0 (0) |
| Stroke, n (%) | 0 (0) |
| Periprocedural AMI (type 4a), n (%) | 3 (10.3) |
| Target-vessel revascularization, n (%) | 0 (0) |
| Contrast-induced nephropathy, n (%) | 0 (0) |
| Hospitalization time, days | 6 (3-9) |
AMI: acute myocardial infarction.
The late follow-up (Table 4) showed two new target-vessel revascularizations and one death from non-cardiac cause (septic shock 6 months after the intervention). The mean MACE-free survival time was 29.7 ± 2.1 months (Fig. 1).
DiscussionThe results of the present study suggest that RA, as an adjunctive technique for percutaneous treatment of lesions with extreme calcification, is safe and effective, with high procedural success rate and favorable evolution in the late follow-up. This registry highlighted the clinical complexity of the population – especially the high percentage of diabetes mellitus (62.1%), chronic renal failure (24.1%), multivessel pattern (79.3%), and previous CABG (31.0%). The atherectomy technique used was characterized by conservative burr-to-artery ratio, initial burr rotation velocity < 180,000rpm, low total ablation time, and high percentage of additional dilation of lesions with significant use of cutting balloon.
Conservative burr-to-artery ratio (< 0.7) has been suggested in the context of RA as a tool to modify plaque compliance, associated to high angiographic success rate and lower incidence of complications, such as coronary perforation and dissection.9 The burr rota-tion velocity, in turn, also appears to influence procedure outcome, as velocities > 180,000rpm have been related to the risk of atherosclerotic debris embolization and elevation of myocardial necrosis markers.10 In spite of these strategies to increase atherectomy safety, recent registries have shown complications rates that are still higher than those observed in other percutaneous coronary interventions.11,12
This series assessed the combined use of the strategies described above, resulting in a single case of perforation, which occurred in an angled left circumflex artery, with a rate of coronary dissection of 9.7%, all percutaneously resolved with stent implantation.
Another characteristic of contemporary ablative strategy is the need for additional plaque preparation prior to stent implantation, due to the smaller luminal gain obtained by using burrs of smaller diameter. The use of a cutting balloon in this context is associated with higher minimum in-stent sectional area, despite the late higher luminal loss.13 In the present study, a 93.6% rate of additional dilation after atherectomy was found, using a cutting balloon in 45.1% of procedures, resulting in immediate angiographic success in all cases, with low incidence of new target-vessel revascularization in the late follow-up.
The retrospective design and the relatively low number of patients were limitations of this study; however, this was a selected population, of high complexity, in which it was possible to describe the technical aspects of the procedure and perform late clinical follow-up.
ConclusionsContemporary rotational atherectomy with the incorporation of conservative ablation strategies demonstrated safety and efficacy in the percutaneous treatment of calcified lesions in high-complexity patients.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.