Implantable cardioverter-defibrillators (ICDs) are usually indicated for patients with malignant arrhythmias considered as high risk. Sympathetic hyperactivity plays a critical role in the development, maintenance, and worsening of ventricular arrhythmias. New treatment options in this population represent a clinical necessity. This study's objective was to report the outcomes of patients with ICDs and electrical storm submitted to renal sympathetic denervation for arrhythmia control.
MethodsEight patients with ICDs admitted for electrical storm refractory to optimal medical therapy underwent renal sympathetic denervation. Underlying diseases included Chagas disease (n=6), non-ischemic dilated cardiomyopathy (n=1), and ischemic cardiomyopathy (n=1). Information on the number of episodes of ventricular tachycardia/ventricular fibrillation and antitachycardia therapies in the week before the procedure and 30 days after treatment were obtained through interrogation of the ICDs.
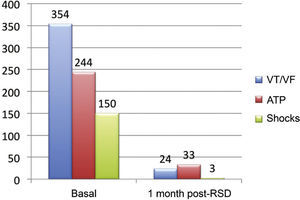
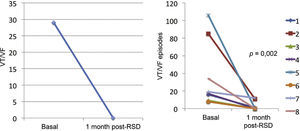
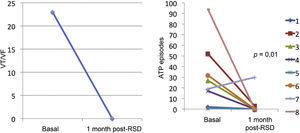
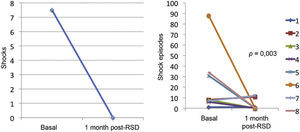
ResultsThe median numbers of episodes of ventricular tachycardia/ventricular fibrillation, antitachycardia pacing, and shocks in the week before renal sympathetic denervation were 29 (9 to 106), 23 (2 to 94), and 7.5 (1 to 88), and significantly reduced to 0 (0 to 12), 0 (0 to 30), and 0 (0 to 1), respectively, 1 month after the procedure (p=0.002; p=0.01; p=0.003, respectively). No patients died during follow-up. There were no major complications related to the procedure.
ConclusionsIn patients with ICDs and electrical storm refractory to optimal medical treatment, renal sympathetic denervation significantly reduced arrhythmia load and, consequently, antitachycardia pacing and shocks. Randomized clinical trials in the context of renal sympathetic denervation to control refractory cardiac arrhythmias are needed to further support these findings.
Cardiodesfibriladores implantáveis (CDIs) são geralmente indicados para pacientes com arritmias malignas considerados de alto risco. A hiperatividade simpática desempenha um papel crítico no desenvolvimento, na manutenção e no agravamento de arritmias ventriculares. Novas opções de tratamento nessa população representam uma necessidade clínica. Nosso objetivo foi relatar os resultados de pacientes com CDIs e tempestade elétrica submetidos à denervação simpática renal para controle da arritmia.
MétodosOito pacientes com CDIs internados por tempestade elétrica refratária ao tratamento médico otimizado foram submetidos à denervação simpática renal. Condições subjacentes foram: doença de Chagas (n=6), cardiomiopatia dilatada não isquêmica (n=1) e cardiomiopatia isquêmica (n=1). As informações sobre o número de taquicardias ventriculares/fibrilações ventriculares e episódios de terapias antitaquicardia na última semana pré-procedimento e nos 30 dias pós-tratamento foram obtidas por meio de interrogação dos CDIs.
ResultadosAs medianas dos episódios de taquicardias ventriculares/fibrilações ventriculares, sobre-estimulação e choques na semana que antecedeu a denervação simpática renal foram de 29 (9 a 106), 23 (2 a 94) e 7,5 (1 a 88), sendo significativamente reduzidas para 0 (0 a 12), 0 (0 a 30) e 0 (0 a 1), respectivamente, 1 mês após o procedimento (p=0,002; p=0,01; p=0,003). Nenhum paciente morreu durante o acompanhamento. Não ocorreram complicações maiores relacionadas ao procedimento.
ConclusõesEm pacientes com CDIs e tempestade elétrica refratária ao tratamento médico otimizado, a denervação simpática renal reduziu significativamente a carga de arritmia e, consequentemente, as sobre-estimulações e os choques. Ensaios clínicos randomizados, no contexto de denervação simpática renal para controle de arritmias cardíacas refratárias, são necessários para trazer maior robustez aos nossos achados.
Implantable cardioverter-defibrillators (ICDs) have shown to be effective in primary and secondary prevention of sudden cardiac death and are usually indicated for patients with malignant arrhythmias considered as high risk.1 Electrical storm is defined as the occurrence of three or more episodes of potentially malignant ventricular arrhythmias (VAs) within a 24-hour period, whose reversion requires intervention with antitachycardia therapy, i.e., antitachycardia pacing (ATP) or shock. This is a severe and dramatic event when repeated shocks are required, a fact that leads to hospital admission at the intensive care unit, causing considerable discomfort to patients in addition to premature ICD battery wear. The therapeutic options for patients with recurrent ICD shocks include drug treatment with antiarrhythmics and beta-blockers,2–4 and catheter ablation.5,6 However, both approaches are associated with low efficacy in the long term, and in case of ablation, it may be associated with potential complications.7 New treatment options in this population of high-risk patients represent a medical necessity.
Sympathetic hyperactivity plays a critical role in the development, maintenance, and worsening of VAs.8 Percutaneous renal sympathetic denervation (RSD) has shown to reduce sympathetic activity9 and, therefore, decrease blood pressure in patients with resistant hypertension for up to 3 years of follow-up in some studies.10–12 The effects of RSD on the sympathetic nervous system activity suggest that this technique can be used in other diseases associated with increased sympathetic tone, such as chronic renal disease, heart failure, and cardiac arrhythmias.13 Particularly for the treatment of cardiac arrhythmias, RSD has a strong physiopathological rationale.14 Recently, some case reports have suggested RSD benefits in patients with electrical storm.15,16 However, data are scarce and any conclusive analysis regarding this context is compromised.
This study aimed to describe the results up to 30 days after RSD in patients with ICD and electrical storm refractory to optimal medical treatment.
MethodsSampleThis was a prospective study conducted at Instituto Dante Pazzanese de Cardiologia, a single tertiary hospital in São Paulo, Brazil. The study was approved by the local Ethics Committee and performed according to good standards of clinical practice. All patients read, understood, and signed the Informed Consent, which contained the most important information on the research protocol presented in an instructive summary.
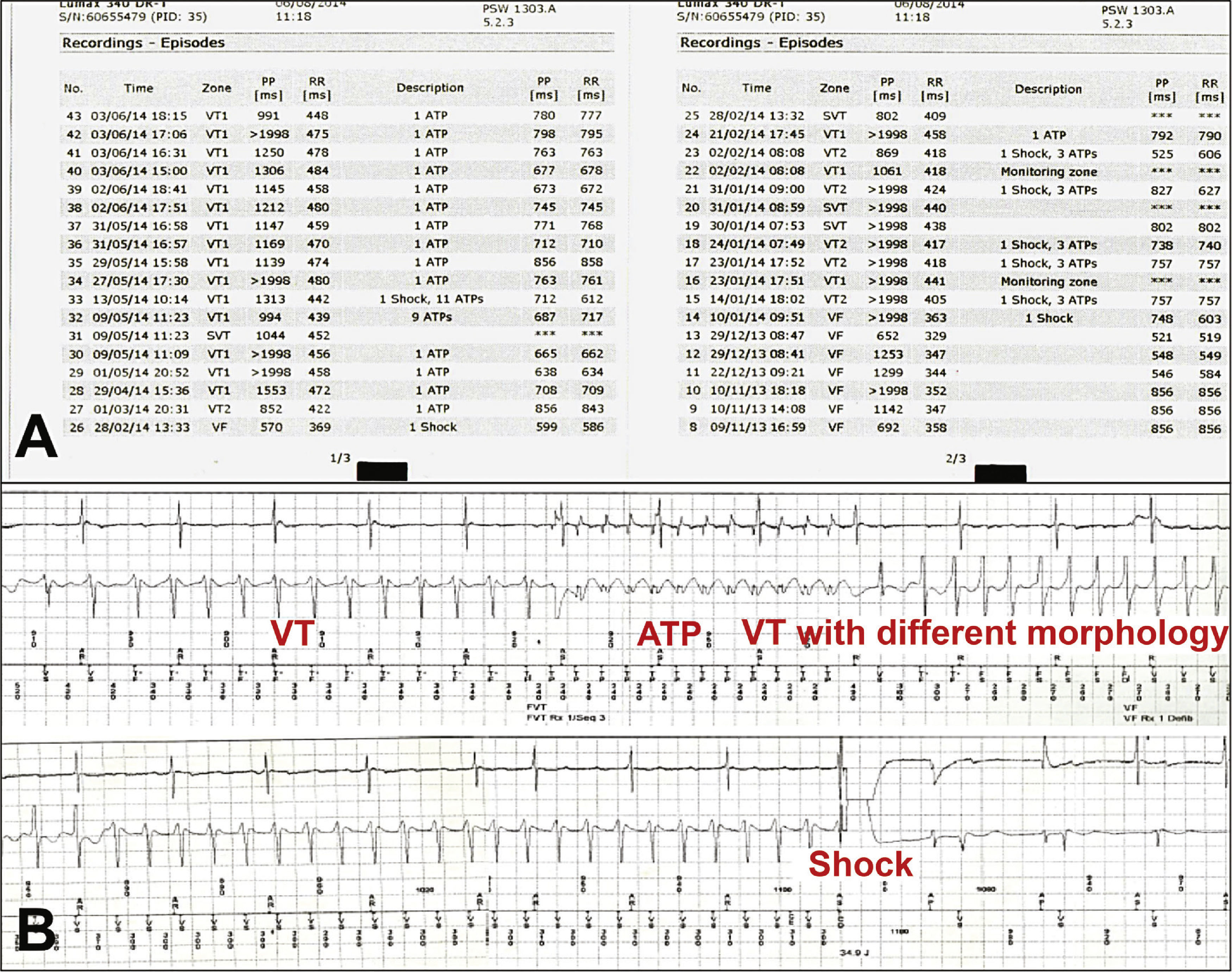
Thirteen consecutive patients admitted with VA refractory to optimal medical therapy and considered unsuitable for cardiac ablation were eligible for the study, between August 2013 and June 2014. Diagnostic confirmation of electrical storm was attained through device interrogation, by means of the analysis of the stored intracardiac electrograms and confirmation that the antitachycardia therapies were appropriate and effective (Fig. 1). Electrical storm was defined as the occurrence of three or more episodes of sustained ventricular tachycardia (VT)/ventricular fibrillation (VF) within a 24-hour period, whose reversion required ATP or shock. Duration longer than 30seconds and/or hemodynamic instability defined sustained ventricular tachycardia. Optimal medical treatment comprised the control of possible triggering causes of VA, such as electrolyte disturbances and the use of antiarrhythmic drugs. Cardiac ablation was qualified as inadequate in the presence of polymorphic VT, VF, unstable arrhythmias, non-mappable arrhythmias, intracardiac thrombus, or failure of previous ablation.
(A) Diagnostic confirmation of electrical storm, through implantable cardioverter-defibrillator interrogation and analysis of stored intracardiac electrograms, confirming that the antitachycardia therapies were appropriate and effective. (B) The unsuccessful attempt at ventricular tachycardia reversion by antitachycardia pacing (ATP), triggering the onset of another ventricular tachycardia with different morphology, which was reversed by shock. VT: sustained ventricular tachycardia.
Exclusion criteria included the following: active infection, significant hypotension (systolic blood pressure ≤ 90mmHg or need for vasopressor agents), renal failure (glomerular filtration rate - GFR < 45mL/min) and renal arteries anatomically inadequate for intervention (< 20mm long or < 3.5mm in diameter, presence of > 50% stenosis/fibrodysplasia/previous stent), or a solitary kidney.
The ICD monitoring zone was programmed between 120 and 130 bpm in all patients. Information on the number of VT/VF episodes and antitachycardia therapies (ATP and shocks) in the week before the procedure and 30 days post-treatment were obtained through ICD interrogation.
Renal sympathetic denervation procedureRSD was performed as previously described.17 After obtaining femoral artery access, unfractionated heparin was administered at a dose of 100 IU/kg. Aortography along the renal arteries was performed with a pigtail catheter, followed by catheterization and selective renal arteriography using a Judkins catheter, after nitroglycerin administration (50 to 200 mcg). In all cases, the procedure was conducted using an irrigated tip radiofrequency ablation catheter (Therapy Cool PathTM; St. Jude MedicalTM – Minneapolis, USA). At least four radiofrequency (RF) lesions were made along both renal arteries, from the distal segment toward the ostia. The catheter was retracted 5mm and turned after each radiofrequency application, thus producing a helical pattern of ablation. Due to the visceral pain caused using the ablation, analgesia was provided using fentanyl and morphine. At the end of the procedure, renal arteriography was performed to evaluate vascular integrity.
Post-procedure evaluation and clinical follow-upThe sheaths were removed when the activated clotting time reached values < 200seconds. Manual hemostatic compression was performed for at least 20minutes, followed by pressure dressing. Walking was allowed after 4hours of rest, in the absence of bleeding at the puncture site. During the resting period, special attention was paid to the occurrence of vascular complications at the femoral access, such as bleeding, hematomas, and pseudoaneurysms, as well as arrhythmia monitoring. Antiarrhythmic medications were maintained during follow-up. Patients were discharged after control of VA episodes.
Statistical analysisCategorical variables are shown as absolute and relative frequencies. Continuous variables are shown as mean±standard deviation or median (range), and were compared using Student's t-test or the Mann-Whitney test. Statistical analyses were performed using the IBM SPSS StatisticsTM program, version 22.0 (IBM – Armonk, USA). For all compared parameters, p-values < 0.05 were considered statistically significant.
ResultsBasal characteristicsOf the 13 patients admitted with refractory VA, electrical storm was confirmed in 11, as one had accelerated idioventricular rhythm below the ICD detection zone, which persisted for several days, and another had frequent VAs, but did not meet the criteria for electrical storm diagnosis. Three patients were excluded from the study due to renal failure/active infection, single kidney, and absence of ICD (which prevented the accurate qualification/quantification of VAs).
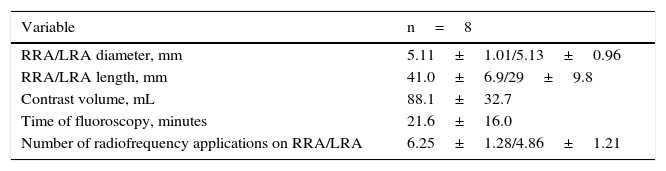
In the eight remaining patients with electrical storm refractory to optimal medical therapy, cardiac ablation was considered inadequate in four due to polymorphic VT (three with previous unsuccessful ablation), in three due to intracardiac thrombi, and in one with previous unsuccessful ablation/intracardiac thrombus. Table 1 summarizes the baseline characteristics of each of the eight patients (four males, aged 63.6±3.9 years) with ICD and electrical storm who were submitted to RSD and included in the analysis. Four were hypertensive; one was diabetic; four had dyslipidemia; one was a smoker; two had previous myocardial infarction; five had a previous stroke. Chagas disease was the most prevalent cardiomyopathy etiology (n=6), followed by one case of non-ischemic dilated cardiomyopathy and one case of ischemic cardiomyopathy. Mean ejection fraction was 0.28±0.04. The ICDs had been implanted for 5±5.4 years (range 1 to 17 years). The number of antiarrhythmic drugs ranged from 2 to 4 (3±0.53). Three patients had signs of skin hyperpigmentation due to amiodarone use.
Baseline characteristics of each patient with implantable cardioverter-defibrillator (ICD) and electrical storm, submitted to renal sympathetic denervation.
| Patient | Age (years) | Gender | Cardiomyopathy etiology | Ejection fraction | Time of ICD (years) | Previous cardiac ablation | Intracardiac thrombus | Medications |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | Chagas disease | 0.30 | 17 | Yes | No | A, BB, ACEI |
| 2 | 62 | M | Non-ischemic dilated cardiomyopathy | 0.34 | 3 | No | Yes | A, BB, ACEI, L |
| 3 | 62 | M | Ischemic cardiomyopathy | 0.22 | 1 | No | Yes | A, BB, ACEI |
| 4 | 69 | F | Chagas disease | 0.29 | 5 | No | No | A, BB, ACEI |
| 5 | 60 | F | Chagas disease | 0.30 | 5 | No | Yes | A, BB |
| 6 | 64 | F | Chagas disease | 0.22 | 1 | No | No | A, BB, ACEI |
| 7 | 62 | M | Chagas disease | 0.30 | 1 | Yes | No | A, BB, ACEI |
| 8 | 60 | F | Chagas disease | 0.28 | 7 | Yes | Yes | A, BB, ACEI |
M: male; A: amiodarone; BB: betablocker; ACEI: angiotensin-converting enzyme inhibitor; L: lidocaine; F: female.
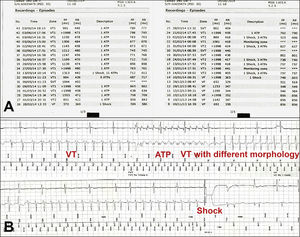
Mean diameters of the left and right renal arteries were 5.11±1.01mm and 5.13±0.96mm, respectively. The mean length of the right and left renal arteries were 41.0±6.9mm and 29±9.8mm, respectively. One patient had a polar artery on the right side, and two other cases had three polar arteries, being two on the right side and one on the left. Two patients had mild proximal stenosis in the renal artery.
All procedures were performed by transfemoral approach. On average, 88.1±32.7mL of contrast medium were used during 21.6±16.0minutes of fluoroscopy. The mean number of radiofrequency applications was 6.3±1.3 in the right and 4.9±1.2 in the left renal artery. The angiographic and procedure characteristics are shown in Table 2.
Angiographic and procedure characteristics.
| Variable | n=8 |
|---|---|
| RRA/LRA diameter, mm | 5.11±1.01/5.13±0.96 |
| RRA/LRA length, mm | 41.0±6.9/29±9.8 |
| Contrast volume, mL | 88.1±32.7 |
| Time of fluoroscopy, minutes | 21.6±16.0 |
| Number of radiofrequency applications on RRA/LRA | 6.25±1.28/4.86±1.21 |
RRA: right renal artery; LRA: left renal artery.
The procedure was conducted without complications in all patients. Focal and slight reductions in the renal artery lumen with no limitations in blood flow were observed in two cases (distally in the left renal artery and distally in both renal arteries) immediately after radiofrequency application, which were attributed to spasms and/or edema. There were no complications related to the femoral puncture during the periprocedural period. There was no increase in serum creatinine levels (1.1±0.3mg/dL initial vs. 1.1±0.2mg/ dL after 30 days; p=0.73), indicating maintenance of GFR during follow-up.
Blood pressure levels remained stable up to 30 days (systolic blood pressure of 109.6±20.7mmHg initial vs. 107.3±17.3mmHg after 30 days; p=0.81) and thus, adjustment in the chronic use of drugs due to ventricular dysfunction, present in all patients, was not required.
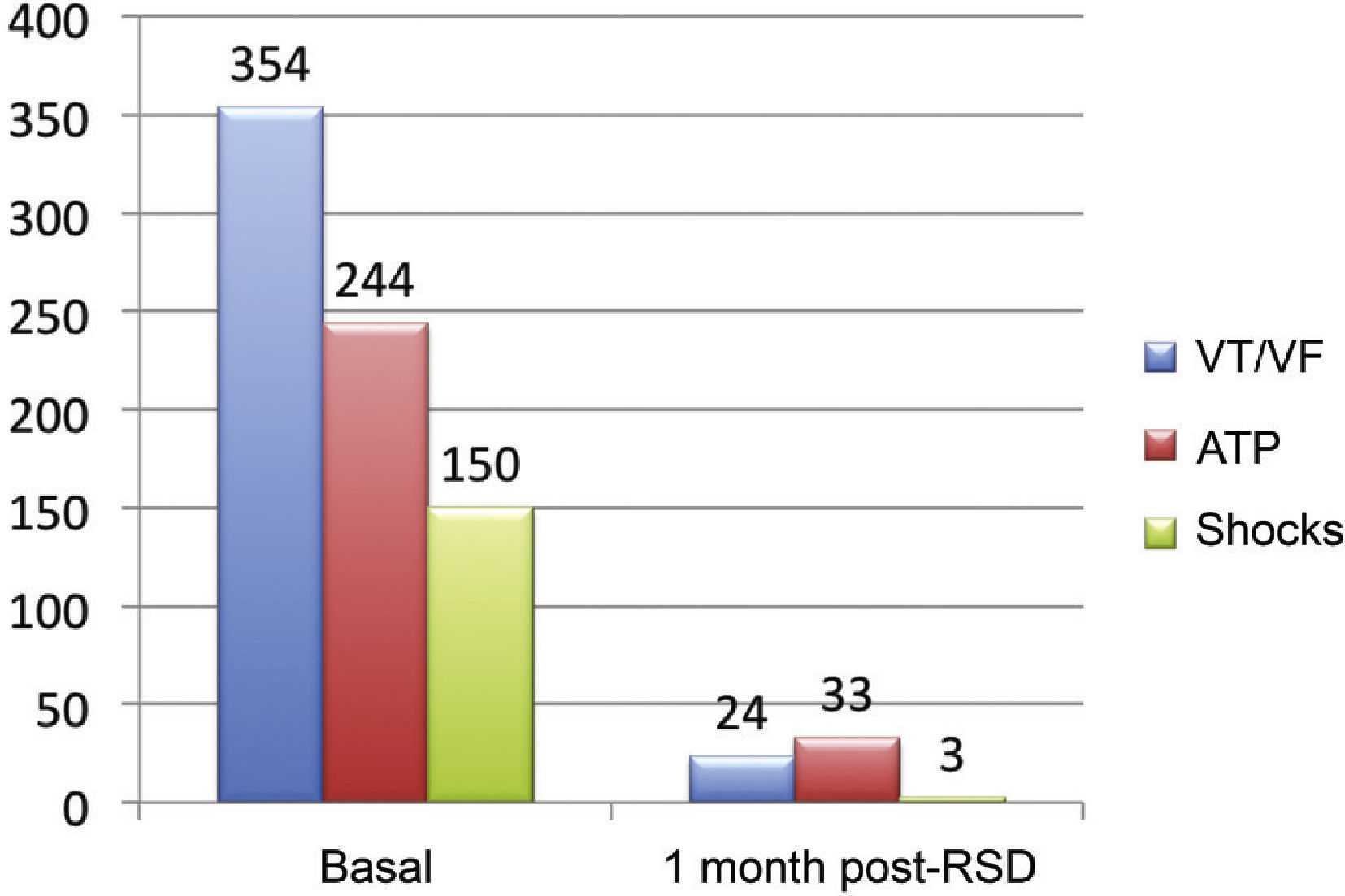
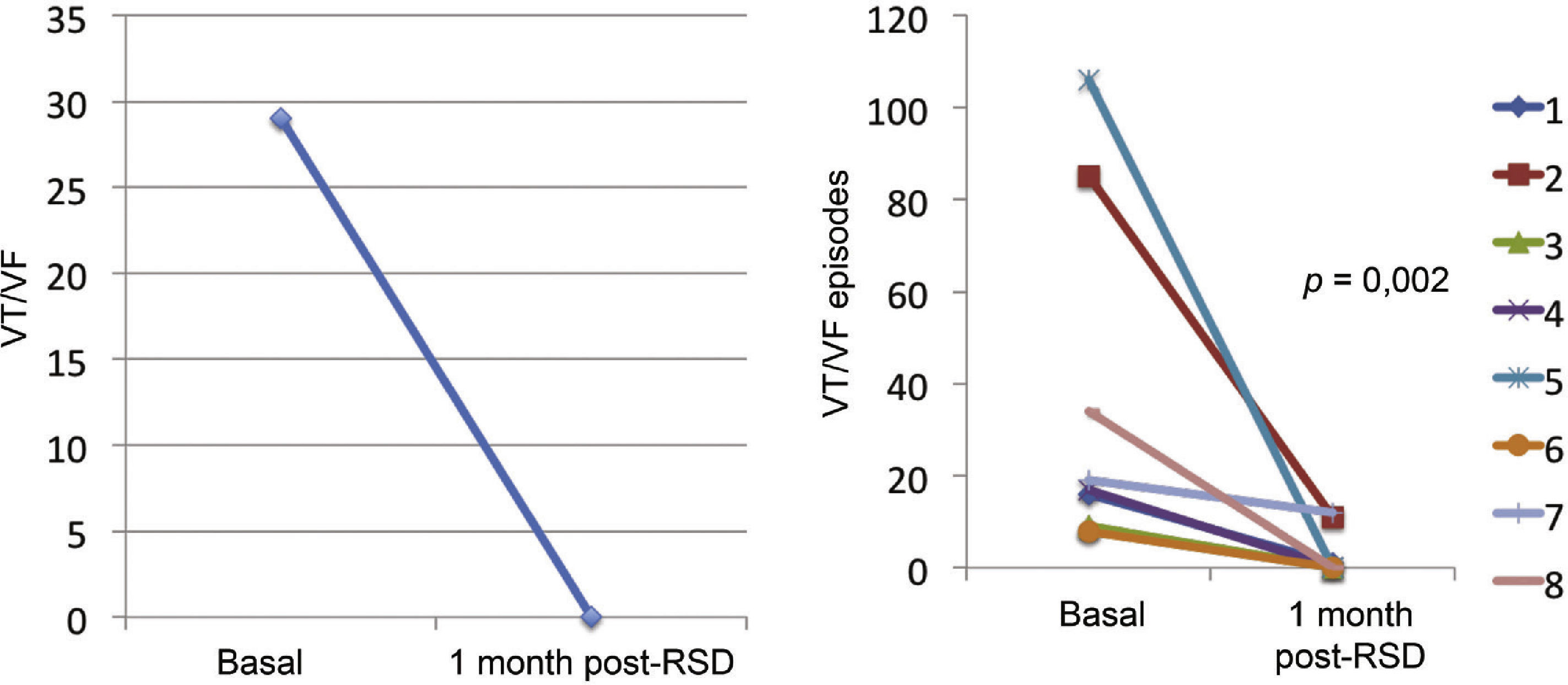
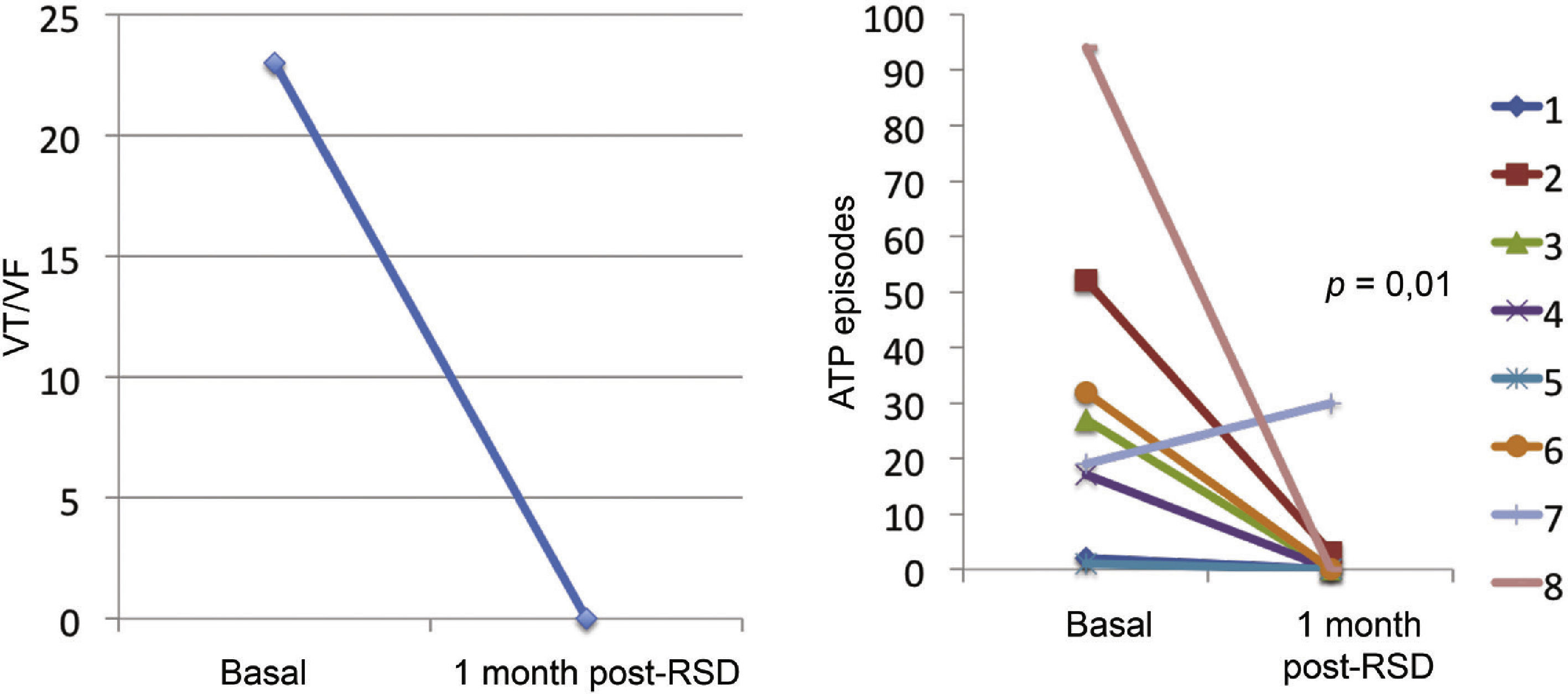
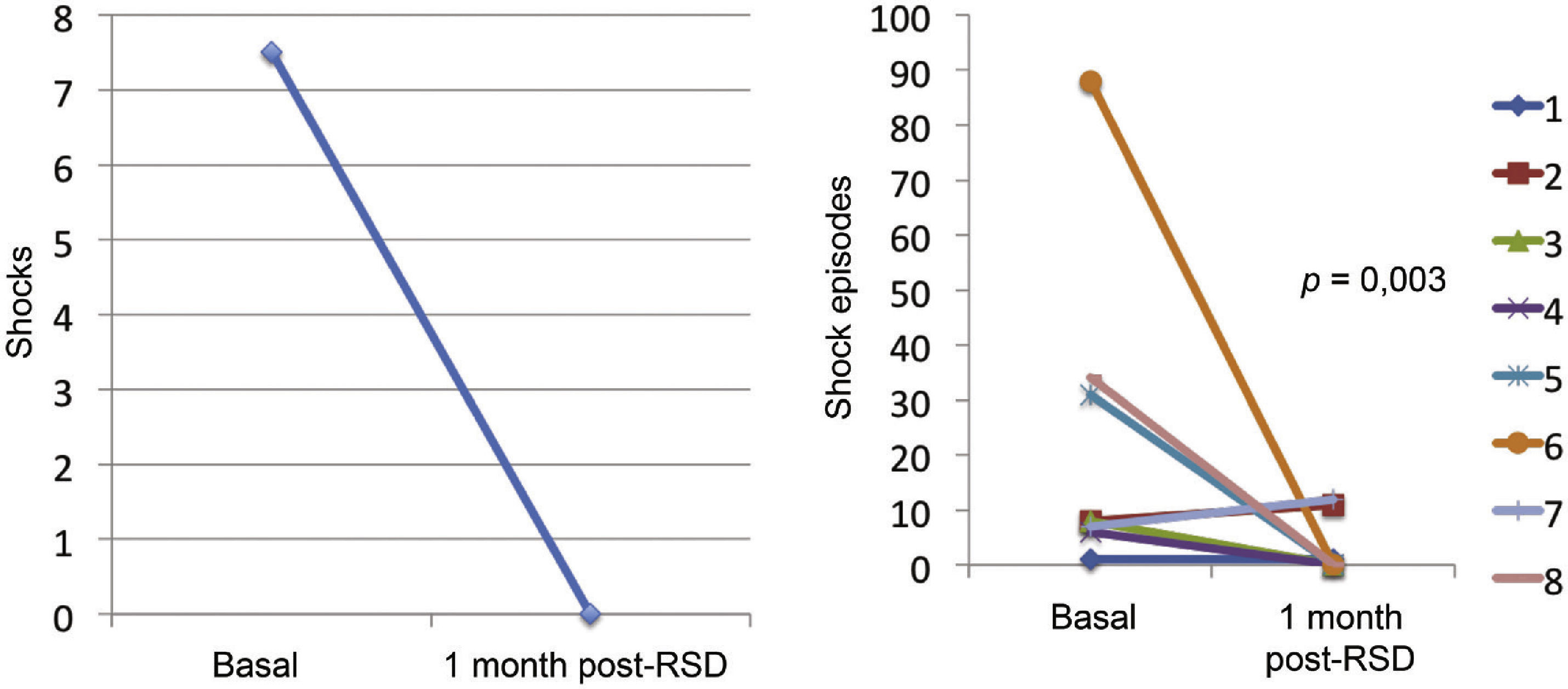
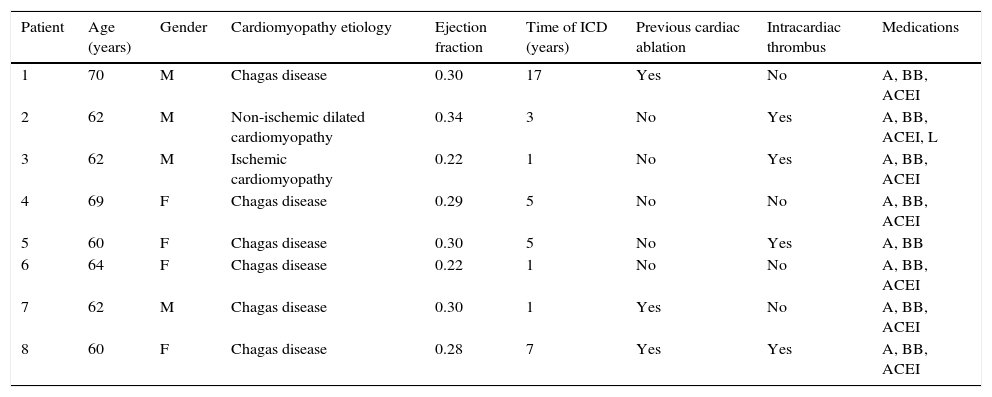
Effect of renal sympathetic denervation on ventricular arrhythmias and antitachycardia therapiesThe median number of episodes of VT/VF, ATP, and shocks in the week before the RSD was 29 (9 to 106), 23 (2 to 94), and 7.5 (1 to 88), respectively, which was significantly reduced to 0 (0 to 12), 0 (0 to 30), and 0 (0 to 1) 1 month after the procedure (p=0.002; p=0.01; p=0.003, respectively). Only three patients had VA after the RSD: the first had one VT episode in the first week, which required a shock; the second had ten VT episodes in the first week, with spontaneous reversion and a VT in the fourth week, which required three ATPs and one shock; the third had two VTs and ten ATPs in the first week, one VT/11 ATPs and one shock in the second week, and nine VTs/nine ATPs in the fourth week.
The total incidence of episodes of VT/VF and antitachycardia pacing (ATP and shocks) pre- and post-RSD is shown in Fig. 2. Figs. 3–5 show the median of episodes of VT/VF, ATP, and shocks, as well as the individual response of each of the eight patients submitted to RSD. There were no changes in anti-arrhythmic drug schemes from hospital discharge to the end of follow-up. No patients died during follow-up.
In patients with ICD and electrical storm refractory to optimal medical treatment, it was demonstrated that RSD significantly reduces the arrhythmic load without procedure-related complications. In spite of the small number of subjects included in the study, this has been the largest series in this context.
It is noteworthy that this study included patients with Chagas disease, non-ischemic dilated cardiomyopathy, and ischemic cardiomyopathy, although most had Chagas disease etiology. It is possible that, in addition to the effect on the autonomic tone, RSD may result in beneficial side effects by reducing the excess volume and hormonal activation observed in treated patients with heart failure; however, despite significant cardiac dysfunction in all patients, none showed significant signs/symptoms of pulmonary congestion/edema, which suggests that arrhythmia reduction was probably not related to heart failure improvement.
The heart is densely innervated by sympathetic fibers and it is well established that their activation increases heart rate and facilitates atrioventricular conduction.18 In ventricles, the increase in sympathetic tone reduces the ventricular effective refractory period, increases automaticity, and reduces the threshold for VA.19 Increasing clinical and preclinical evidence indicates that sympathetic modulation by surgical or catheter ablation may represent a new treatment option for cardiac arrhythmias.20 Vaseghi et al.21 recently demonstrated that surgical sympathetic denervation reduced ICD shock loads up to 90%; despite the promising results, the non-selective effects of surgical denervation may result in autonomic dysfunction, as demonstrated in this study with a small number of patients (change of 10% in the sweating pattern, 12% in skin sensitivity, and persistent ptosis in one patient).
Ukena et al.15 reported the first experiment in humans using percutaneous RSD in two patients with heart failure and electrical storm: one patient with hypertrophic cardiomyopathy, monomorphic VT despite the use of multiple antiarrhythmic drugs, and prior unsuccessful endocardial/epicardial cardiac ablation; another patient with dilated cardiomyopathy, and frequent episodes of VF and polymorphic VT, who refused cardiac ablation. In both cases, a significant reduction in VA was documented.
Our group16 described a significant reduction in VF/VT episodes and appropriate ICD therapies in one patient with dilated cardiomyopathy and contraindication for cardiac ablation due to the presence of thrombus in the left ventricle, submitted to RSD with irrigated-tip catheter. Remo et al.22 have recently shown the benefits of RSD as adjunctive therapy in refractory VT in four patients with underlying cardiomyopathy, with similar results in ischemic and non-ischemic patients. In an experimental model, Linz et al.23 were able to demonstrate that RSD by radiofrequency significantly reduced the occurrence of spontaneous VAs and attenuated the increase in left ventricular end-diastolic pressure during ischemic events.
Intriguingly, six of the eight patients in this study had Chagas disease. Despite the implementation of socioeconomic measures and the development of drugs that allow treatment in the acute phase of the disease, chronic Chagas cardiomyopathy remains a major public health problem in many Latin American countries, affecting approximately 15 to 16 million individuals, with a mortality rate of 20,000/year.24
Approximately two-thirds of individuals with chronic symptoms develop cardiac lesions, including severe ventricular dysfunction and dilation, tachyarrhythmias, bradyarrhythmias, and, not rarely, sudden death.25 Sudden death accounts for approximately 55-65% of overall mortality in patients with Chagas disease, which is higher than deaths from heart failure.26 The arrhythmogenic nature of Chagas disease is related to the presence of fibrotic tissue interspersed with areas of preserved infarction and dyskinetic regions, generating an area of high propensity to complex VAs.27
VA treatment includes antiarrhythmic drugs, correction of reversible causes, such as electrolyte disturbances, and cardiac ablation.28–30 Although amiodarone reduces the risk of sudden death (by 29%) and cardiovascular death (by 18%) in this population, antiarrhythmic therapy is neutral considering all-cause mortality and is associated with a two- and five-fold increase in the risk of lung and thyroid toxicity, respectively.31
Almost 50% of patients undergoing conventional endocardial ablation have arrhythmia recurrence.31 VT in chagasic patients frequently has multiple sites of origin, including the subepicardium, and is hemodynamically unstable/non-mappable, which greatly hinders its approach.32 Epicardial ablation in these cases is often a challenge, especially in individuals with severe ventricular dysfunction and clinical deterioration. Therefore, RSD may represent an alternative treatment for patients with Chagas disease with refractory VA.
There were no deaths in 30 days of follow-up. However, the follow-up period is short for drawing conclusions regarding mortality in this high-risk population included in the study, with advanced heart disease and severe ventricular dysfunction, which are poor prognostic predictors.
Study limitationsThe non-randomized design, the relatively small sample size, and the absence of a control group represent potential study limitations, although there is no other publication more significant on the subject. Although sympathetic activity was not directly measured, the significant association between RSD and VA load reduction was remarkable. Most patients had Chagas cardiomyopathy, and the response to RSD might be distinct in this population.
ConclusionsThis study showed the importance of sympathetic hyperactivity in patients with ventricular arrhythmia, and suggested the potential performance of catheter-based renal sympathetic denervation in this scenario. In patients with implantable cardioverter-defibrillator and electrical storm refractory to optimal medical treatment, renal sympathetic denervation significantly reduced the arrhythmia load and, consequently, antitachycardia pacing and shocks. Randomized controlled trials strictly designed in the context of renal sympathetic denervation to control refractory cardiac arrhythmias are needed to further support these findings. If proven to be safe and effective in this context, sympathetic denervation will become an important strategy for the treatment of ventricular arrhythmias.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.