Coronary fistulas are congenital heart diseases of generally low incidence. The fistulous vessel drainage occurs in the cardiac cavities or large vessels of the right heart, like the pulmonary artery or vena cava. The authors report the case of an asymptomatic young woman with a large fistula of the left anterior descending artery draining into the right ventricular apex, with no communication between this segment and the remainder of the ventricular cavity. This unusual presentation of a coronary-cavitary fistula has not been previously described.
As fístulas coronárias são usualmente cardiopatias congênitas de baixa incidência. A drenagem do vaso fistuloso ocorre nas cavidades cardíacas ou nos grandes vasos do coração direito, como a artéria pulmonar ou as veias cavas. Relatamos o caso de uma paciente adulta jovem, assintomática, portadora de grande fístula da artéria descendente anterior drenando no ápice do ventrículo direito, sem comunicação deste segmento com o restante da cavidade ventricular. Esta forma incomum de apresentação de uma fístula coronário-cavitária não foi previamente descrita.
Coronary-cavitary fistulas are communications, usually of congenital origin, between one or more coronary arteries and the heart cavities or large vessels of the right heart. They have a low prevalence and mainly affect the right coronary artery, followed by the left anterior descending artery (LAD).1 Over 90% of the fistulas drain into the right atrium and right ventricle (RV), vena cava, coronary sinus or pulmonary artery.2–4
The hemodynamic repercussion and the clinical presentation are quite variable, depending on the magnitude of the fistula blood flow, and may or may not show manifestations of volume overload or myocardial ischemia due to coronary artery steal phenomenon.
The authors report the case of a patient with large LAD fistula draining into the apical portion of the RV, which was not connected to the remainder of the ventricular cavity.
Case reportA 26-year-old asymptomatic female patient, with a diagnosis of heart murmur made in the first month of life and a history of pregnancy with vaginal delivery 2 years before was referred to our Interventional Cardiology service with an echocardiographic diagnosis of coronary-cavitary fistula for hemodynamic evaluation and treatment. At physical examination, her heart rate and blood pressure were normal, and she had palpable pulses in all four limbs, with normal amplitude. Continuous fremitus and murmur were evident at the lower left sternal border, radiating to the right hemithorax. The electrocardiogram showed altered ventricular repolarization in the anterior wall, and chest X-ray showed no abnormalities. The echocardiogram showed a large-caliber vessel in the LAD trajectory that drained with continuous flow into the apical portion of the RV. No dilatations of the right or left chambers suggestive of high flow were observed. At the exercise stress test using Bruce protocol, there were no additional ECG changes. The myocardial perfusion scintigraphy showed a discrete and reversible perfusion defect in the left ventricular anterolateral, posterolateral, inferior, and apical walls.
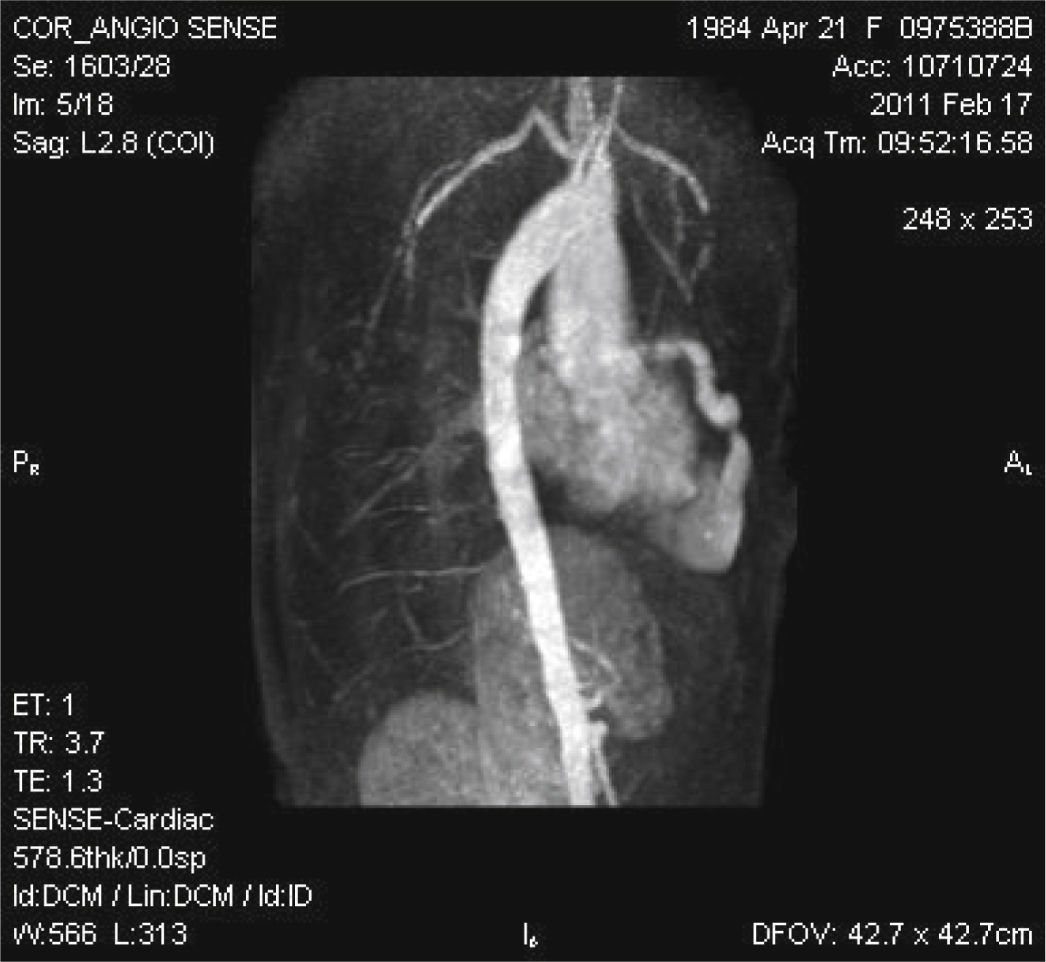
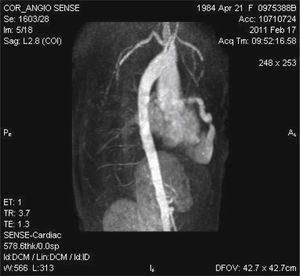
Magnetic resonance imaging showed significant LAD dilation with a maximum diameter of 14mm and bidirectional flow into the RV apex, but without communication between the apical region and the remainder of the ventricular cavity (Fig. 1).
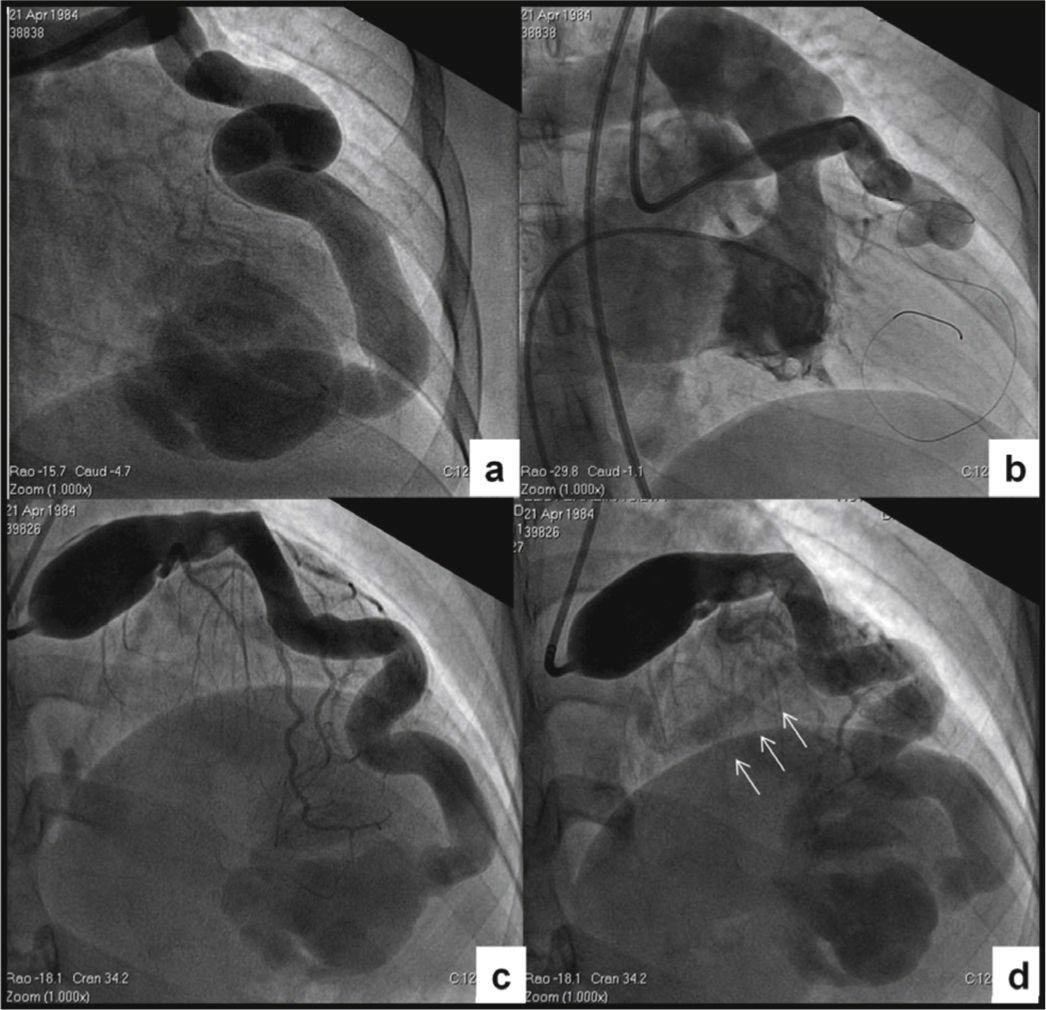
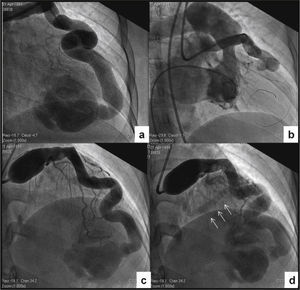
Cardiac catheterization was performed aiming to determine the hemodynamic repercussion and evaluate the possibility of percutaneous treatment. Intracavitary and large-vessel pressures were normal, and the pulmonary–to–systemic cardiac output ratio was 1, demonstrating the absence of left-right shunt. The left ventricular volumes and function were normal. In the angiographic study, the right coronary artery was normal, and all the branches of the left coronary artery had ≤ 2-mm caliber, except for the extremely dilated left main coronary artery and LAD. The LAD showed a tortuous trajectory towards the apex, with minimum and maximum diameters of 8.0 and 12.0mm, respectively, communicating distally with the RV apical segment through an 8.0-mm orifice. There was no communication between the apical segment and the other portions of the RV cavity, and the selectively injected contrast in the LAD showed a back and forth movement between the RV apical region and the LAD, remaining visible for a prolonged period of time (Fig. 2A). In order to characterize the fistula drainage site, a contrast injection was performed in the LAD with temporary balloon occlusion distal to the first diagonal branch. The contrast remained with an alternating anterograde and retrograde flow during diastole, and inversely during systole; the contrast washout was performed slowly and the drainage site was not evident (Figs. 2B to 2D). Due to the anatomical characteristics and the possibility of retrograde thrombosis of the entire left coronary artery if percutaneous occlusion was performed, even using anticoagulant therapy, and considering this was an asymptomatic patient, despite the presence of ischemia in the myocardial perfusion study, an expectant management was adopted, with periodic clinical examinations.
Cardiac catheterization. (A) Selective injection in the left coronary artery. A giant coronary artery fistula draining into the right ventricular apical region with no communication with the remainder of the ventricular cavity can be observed. (B) Right ventriculography showing absence of the apical segment. (C) Temporary balloon occlusion distal to the first diagonal branch and contrast injection through the distal opening of the balloon, allowing better visualization of the other branches of the left coronary artery. (D) Late phase of the post-occlusion injection, disclosing absence of contrast in the right ventricular sinus and infundibular regions, and coronary sinus opacification (arrows).
Similarly to most of these anomalies, in the described fistula the origin of the fistulous pathway was the LAD, which is the second most affected coronary artery and, in approximately 90% of cases, drains into the right heart.1–5 However, a peculiarity of this fistula is the absence of connection between the drainage site and the remainder of the RV cavity. This lack of connection could be congenital, due to the presence of anomalous muscle bands, constituting a type of “sequestration” of apical region, or acquired due to trabecular band hypertrophy, resulting in a complete bipartition of the RV. The emptying of this back and forth flow, observed by the slow disappearance of the injected contrast during the temporary occlusion of the proximal segment of the fistulous trajectory, suggests that it is performed through intramyocardial venous capillaries. The lack of communication between both ventricular portions determines a normal pulmonary–to–systemic cardiac output ratio, and the absence of volume overload of the heart cavities and symptoms of heart failure, even in situations of physiologic volume overload, such as pregnancy. The myocardial ischemia detected at the scintigraphy may be a consequence of the underdevelopment of the other branches of the left coronary artery, since, in the strict sense, there is no flow steal due to a left-right shunt.
Freund et al.6 published the case of a large fistulous vessel originating in the left coronary artery ending in a large aneurysm draining into the RV apex. Unlike the present case, the fistulous vessel was a branch of the left coronary artery after the LAD origin and the left circumflex artery, and the RV apex was not disconnected from the rest of the ventricular cavity.
In asymptomatic children with small-caliber fistulas and no hemodynamic repercussion, an expectant management should be adopted, due to the possibility, albeit remote, of spontaneous occlusion.7 Larger-caliber and higher-flow fistulas should be occluded, since they have been associated with symptoms of heart failure and/or myocardial ischemia and are subject to complications such as bacterial endocarditis, thrombosis, and rupture during their evolution. Currently, percutaneous occlusion is the recommended procedure, with the surgical indication being reserved for cases of impossibility or failure of the percutaneous approach. Depending on the anatomical characteristics and on the different coil types and sizes, vascular occlusion devices such as the AMPLATZER™ can be used for the occlusion of fistulous vessels and septal defects.2 In this patient, percutaneous occlusion presented several technical difficulties, such as long trajectory, extreme tortuosity, and large vessel caliber, which increase the risk of the procedure. The ideal occlusion device could be a vascular or a duct occluder, but its distal positioning would be difficult. Since the venous approach is impossible due to the lack of communication between the apical region and the rest of the RV cavity, the alternative is an arterial approach which, due to the total length of the fistulous trajectory and extreme tortuosity, would require the use of a special sheath.
The occlusion of fistulas with a distal drainage site and significant coronary artery dilation presents a significant risk of retrograde thrombosis, affecting the arterial branches that originate from it.8 Retrograde thrombosis, in this case, would result in the loss of the entire left coronary system, massive infarction, and risk of death.
Considering the technical difficulties, the absence of volume overload, the fact that the patient was asymptomatic (despite the presence of discrete ischemia at the scintigraphy), and especially the risk of left coronary artery thrombosis, even with anticoagulation therapy, the management comprised treatment with antiplatelet agents and periodic controls.
The authors did not find a description in the literature of a similar case of coronary fistula with obliteration of the drainage cavitary segment connection with the rest of the cavity, preventing an arteriovenous shunt with volume overload and heart failure symptoms.
Sources of fundingNone.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.