Stroke secondary to atrial fibrillation has been associated to a high risk of permanent, severe disability, and high early mortality, and therefore its effective prevention is of paramount importance. Warfarin therapy reduces the risk of stroke by 60%, however, half of the patients with atrial fibrillation do not receive anticoagulation. Left atrial appendage closure has emerged as an alternative strategy for stroke prevention.
MethodsPatients with atrial fibrillation and CHADSVASc score≥2, not eligible for anticoagulation, were submitted to left atrial appendage closure using the WatchmanTM device. The procedure was performed under general anesthesia and was guided by transesophageal echocardiography.
ResultsOf the 11 selected patients, 2 were not treated due to thrombi presented prior or during the procedure and before device implantation. Mean age was 74±5.1years, 66% were male, CHA2DS2-VASc score was 4±1.4, HASBLED score was 3.4±1.1, 77% had contraindications or had unfavorable social conditions for anticoagulation. Technical success was 100% and complete occlusion was obtained in all of the cases, with a mean fluoroscopic time of 22.1±10.8minutes, and no hospital complications. At a follow-up of 78.3±41.5days, there were no clinical events but one patient had thrombus formation on the device and received anticoagulation for 3 months.
ConclusionsLeft atrial appendage closure with the WatchmanTM device is feasible and may be a good alternative therapy for stroke prevention in patients with atrial fibrillation and restrictions for anticoagulation.
Oclusão Percutânea do ApêndiceAtrial Esquerdo com Prótese Watchman®
IntroduçãoO acidente vascular cerebral secundário à fibrilação atrial tem sido associado a taxas de mortalidade e de incapacidade permanente elevada, porquanto sua prevenção eficaz é importante. O tratamento com varfarina diminui em 60% o risco de acidente vascular cerebral; todavia, até metade dos pacientes com fibrilação atrial não faz uso da anticoagulação. A oclusão do apêndice atrial esquerdo surgiu como estratégia alternativa para prevenção do acidente vascular cerebral.
MétodosForam selecionados pacientes com fibrilação atrial, escore de CHA2DS2-VASc≥2, não elegíveis para anticoagulação, para se submeterem ao fechamento percutâneo do apêndice atrial esquerdo com a prótese WatchmanTM. O procedimento foi realizado sob anestesia geral e guiado por ecocardiografia transesofágica.
ResultadosDos 11 pacientes selecionados, 2 não foram tratados por apresentarem trombo pré ou durante o procedimento, antes do implante do dispositivo. A idade foi de 74±5,1 anos, 66,6% eram do sexo masculino, com escores CHA2DS2-VASc de 4±1,4 e HAS-BLED de 3,4±1,1, 77% tinham contraindicação ou condições sociais desfavoráveis para utilizarem a anticoagulação. O sucesso técnico foi de 100%, sendo alcançada a oclusão completa em todos os casos, com tempo médio de fluoroscopia de 22,1±10,8 minutos e ausência de complicações hospitalares. No seguimento de 78,3±41,5 dias, não ocorreram desfechos clínicos, mas um paciente apresentou trombo no dispositivo e recebeu anticoagulação por 3 meses.
ConclusõesA oclusão percutânea do apêndice atrial esquerdo com dispositivo WatchmanTM é factível e pode ser uma alternativa atrativa na prevenção de acidente vascular cerebral nos pacientes com fibrilação atrial e limitação para anticoagulação.
Stroke is the leading cause of cardiovascular mortality and morbidity, affecting nearly 800,000 individuals annually in the United States. Its incidence increases substantially with age, attributable to atrial fibrillation (AF) in approximately 1.5% of patients aged<60 years and in more than 20% of patients aged>80 years.1 The absolute risk of systemic embolism in nonvalvular AF depends on the presence of associated factors such as hypertension, heart failure, diabetes mellitus, female gender, and a history of thromboembolic events, measured in clinical practice through the CHA2DS2-VASc score.2
Vitamin K antagonists are the most commonly used therapy for the prevention of thromboembolic events in AF, as they have proven efficacy, with a 60% reduction in the risk of stroke.3 However, approximately 50% of eligible individuals do not use this class of drugs, due to limitations related to risk of hemorrhage, previous hemorrhage, treatment interruption or withdrawal, interaction with other drugs and foods, the strict therapeutic window, and the need for careful monitoring of prothrombin time.4 Recent clinical trials have demonstrated the efficacy and safety of new anticoagulant agents when compared with warfarin, but with an annual risk of bleeding ranging from 1.4% to 3% throughout life, they have excluded patients at high risk of bleeding.5,6
In Latin America, unfavorable social conditions, low educational levels, and little access to health care make the use of anticoagulants more difficult, even in patients without contraindications. The development of percutaneous interventional strategies, such as occlusion of the left atrial appendage (LAA), appears to be an attractive alternative for the prevention of thromboembolism in nonvalvular AF. The aim of this study was to describe cases of the LAA closure with the WatchmanTM prosthesis in individuals with difficulty and/or contraindication to oral anticoagulation.
METHODSStudy populationThe Hemodynamics Services of hospitals José Carrasco Arteaga and Santa Inés, both located in Cuenca, Ecuador, started their training programs for percutaneous occlusion of the LAA in August 2013, in association with physicians of Hospital San Vicente de Paul of Medellin, Colombia. The criteria for patient selection included presence of chronic or nonvalvular paroxysmal AF, CHA2DS2-VASc score≥2, previous thromboembolic events, presence of thrombus in the LAA in spite of adequate anticoagulation (but with resolution before the intervention), and limitations to anticoagulation due to clinical contraindications or due to social, cultural, or educational factors that prevented the prescription.
DeviceThe WatchmanTM (Boston Scientific Corporation, Natick, MA) is a parachute-shaped device, percutaneously deployed in the LAA. It consists of a self-expanding nitinol metal frame, which is covered by a polyester mesh. The physical properties of nitinol allow the device to adapt to the contours of the LAA after implantation. The structure has ten anchors that help it to attach inside the LAA. The polyester membrane covering the device on the atrial side prevents the escape of blood clots to the left atrium. The WatchmanTM device is currently available in five sizes (21, 24, 27, 30, and 33mm) and allows the occlusion of LAAs measuring up to 31mm in diameter. The delivery system has three components: the 14F access sheath (WatchmanTM Access System), the delivery catheter preloaded with the device (WatchmanTM Delivery System), and a transeptal puncture sheath.
PROCEDUREBefore the procedure, patients received acetylsalicylic acid (ASA) at a dose of 100mg/day and clopidogrel at a dose of 75mg/day. However, patients with image of thrombus in the LAA received warfarin (international normalized ratio [INR] of 2 to 3) and ASA for at least 15 days before the procedure and were then submitted to transesophageal echocardiography (TEE) one day prior to implantation, to verify the presence of thrombi. Under general endotracheal anesthesia and TEE monitoring, the LAA was measured, as well as its inlet orifice and depth.
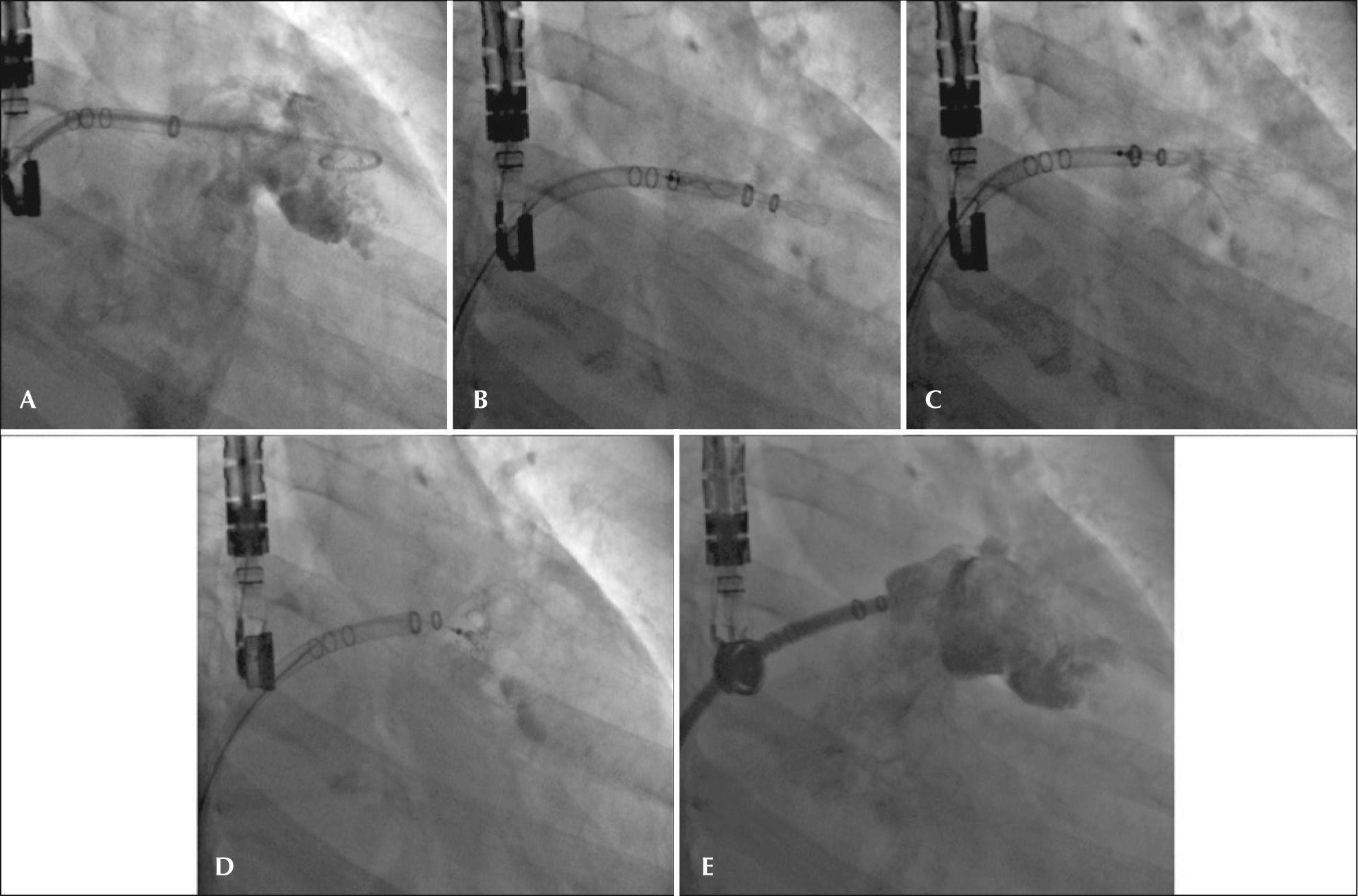
Vascular access was obtained with 7F and 5F introducers in the right femoral vein and right femoral artery, respectively. The atrial septum was punctured at a low and posterior position, followed by systemic intravenous anticoagulation. With the help of a pigtail catheter, manual angiograms were obtained in the right anterior oblique view with cranial and caudal angulation for anatomic delineation and measurement of structures. The 14F double-curve sheath was advanced into the dominant lobe of the LAA under the pigtail catheter and a new angiography was performed to establish the relative depth of radiopaque markers, which helped in prosthesis size selection.
Simultaneously, guided by TEE, the inlet orifice of the LAA and its depth were evaluated at cuts of 0°, 45°, 90°, and 135°. The device was selected in accordance with the table provided by the manufacturer and based on the inlet orifice and the depth of the LAA primary lobe.
Once the desired depth was achieved with the double-curve sheath, the device, which was preloaded in the 12F delivery catheter, was advanced up to its extremity and the cable was pushed to align the radiopaque markers of the delivery catheter and of the 14F sheath. Then, the 14F catheter (WatchmanTM Access System) was slowly retracted, keeping the delivery system fixed inside the LAA. While holding the cable, the delivery system was slowly withdrawn along the 14F sheath, thus exposing and configuring the prosthesis in the LAA.
After the device was deployed, adequate positioning of the prosthesis was confirmed by TEE and measurements were performed of the shoulders of the largest prosthesis diameter, at cuts of 0°, 45°, 90°, and 135° to establish the optimum percentage of compression (8% to 20% of the original diameter). Proper anchorage was tested with gentle push-and-pull movements of the delivery wire (tug test). Control angiography with color Doppler was performed to confirm the absence of flow within the LAA; counterclockwise movements were also performed to release the device.
Post-procedurePatients were extubated in the hemodinamic laboratory and transferred to the intermediate care unit. They were discharged from the hospital 48 hours later than the intervention, after being assessed through TEE and receiving ASA 100mg/day indefinitely and clopidogrel 75mg/day, maintained for six months. In patients with evidence of LAA thrombus before the procedure, warfarin associated with ASA (81mg) was maintained for 45 days, followed by dual antiplatelet therapy. A clinical follow-up was scheduled at the end of the first month and then every three months after the procedure. Control with TEE was scheduled to be performed at 45 days, six months, and one year after the intervention.
Clinical outcomesThe events of interest for this analysis were divided into safety and efficacy outcomes. Regarding safety, the following were considered: pericardial effusion with tamponade or need for intervention; stroke associated with the procedure; device embolization; and severe bleeding (gastrointestinal or intracranial). Regarding efficiency, the incidence of cardiac death, stroke, or thromboembolic events during follow-up were evaluated.
Continuous variables were described as maximum and minimum values, as well as means and standard deviations. Categorical variables were described as percentages.
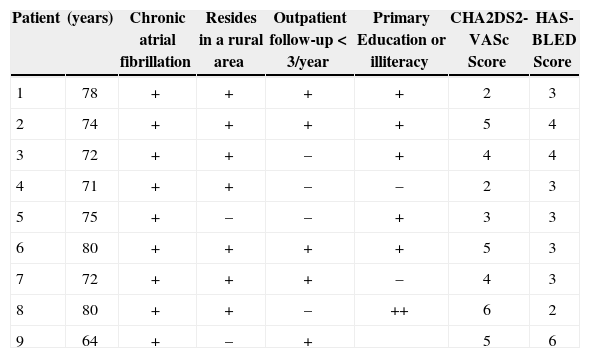
RESULTSEleven patients were selected for LAA closure after consultation with the heart team. In the analysis using the initial TEE, LAA thrombi were detected in three patients who had their procedures postponed for two to three months in order to receive warfarin associated with ASA. Two patients responded to treatment and were maintained in the series; the third was definitely excluded due to the persistence of the thrombus in the LAA. Another patient had thrombus formation immediately after the atrial septum puncture, and thus the procedure was interrupted. Thus, nine patients underwent device implantation, of which 66.6% were males, with mean age of 74±5.1years. The social status of the study group was unfavorable, as 77.7% lived in rural areas, 77.7% had only elementary school education or were illiterate, and 55.5% had been attended to at a medical institution less than three times a year – factors that were considered when indicating device implantation. The mean CHA2DS2-VASc score was 4±1.4, and the mean HAS-BLED score was 3.4±1.1 (Table 1).
Clinical characteristics
| Patient | (years) | Chronic atrial fibrillation | Resides in a rural area | Outpatient follow-up<3/year | Primary Education or illiteracy | CHA2DS2-VASc Score | HAS-BLED Score |
|---|---|---|---|---|---|---|---|
| 1 | 78 | + | + | + | + | 2 | 3 |
| 2 | 74 | + | + | + | + | 5 | 4 |
| 3 | 72 | + | + | – | + | 4 | 4 |
| 4 | 71 | + | + | – | – | 2 | 3 |
| 5 | 75 | + | – | – | + | 3 | 3 |
| 6 | 80 | + | + | + | + | 5 | 3 |
| 7 | 72 | + | + | + | – | 4 | 3 |
| 8 | 80 | + | + | – | ++ | 6 | 2 |
| 9 | 64 | + | – | + | 5 | 6 |
Except for one case, all others had arterial hypertension; 77% of patients had previous bleeding, 55% had difficult-to-control INR, and 44% were taking medications that increased the risk of bleeding (Table 2).
Risk factors for thromboembolic and hemorrhagic phenomena
| Patient | Arterial hypertension | Diabetes mellitus | HF | Previous stroke, TIA, or thromboembolic phenomenon | Coronary or vascular disease | Severe previous hemorrhage | Difficult-to-control INR | Use of medications that predispose to bleeding |
|---|---|---|---|---|---|---|---|---|
| 1 | – | – | – | – | – | – | + | – |
| 2 | + | – | – | + | – | + | + | – |
| 3 | + | – | + | + | – | + | – | + |
| 4 | + | – | – | – | + | + | + | + |
| 5 | + | – | – | – | – | + | – | – |
| 6 | + | – | + | + | – | – | + | – |
| 7 | + | + | – | – | + | + | – | + |
| 8 | + | – | + | + | – | + | – | – |
| 9 | + | + | + | + | – | + | + | + |
HF, heart failure; TIA, transient ischemic attack; INR, international normalized ratio.
The success rate for this procedure was 100%, as complete occlusion was attained, as well as absence of residual flux in all cases. The mean procedure time was 42.8±13.2minutes and mean fluoroscopy time was 22.1±10.8minutes (Table 3).
Characteristics of the procedure
| Patient | Ostium (mm) | Depth of left atrial appendage (mm) | Device size (mm) | Percentage of compression (%) | Residual shunt>2mm | Time of procedure (minutes) | Time of fluoroscopy (minutes) |
|---|---|---|---|---|---|---|---|
| 1 | 21 | 26.4 | 24 | 4.8 | – | 58 | 35.2 |
| 2 | 17 | 19 | 21 | 23.8 | – | 36 | 9.7 |
| 3 | 18 | 26 | 24 | 15.8 | – | 39 | 14.1 |
| 4 | 16.7 | 21 | 21 | 18.1 | – | 33 | 18.2 |
| 5 | 28 | 33.7 | 30 | 9 | – | 35 | 24.1 |
| 6 | 20 | 28.8 | 27 | 9.2 | – | 39 | 14.7 |
| 7 | 19 | 26.3 | 24 | 9.1 | – | 48 | 32.3 |
| 8 | 25 | 21 | 30 | 10 | – | 69 | 38.5 |
| 9 | 24 | 29.2 | 27 | 17.7 | – | 28 | 12.5 |
All patients showed a favorable evolution after the procedure, with no immediate clinical events, nor during the mean follow-up of 78.3±41.5days. Two patients received warfarin after device implantation, which was maintained for 45 days until the TEE was performed, when dual antiplatelet therapy was prescribed for another six months. The remaining patients received dual antiplatelet therapy with clopidogrel 75mg per day, for six months, and acetylsalicylic acid 100mg per day indefinitely.
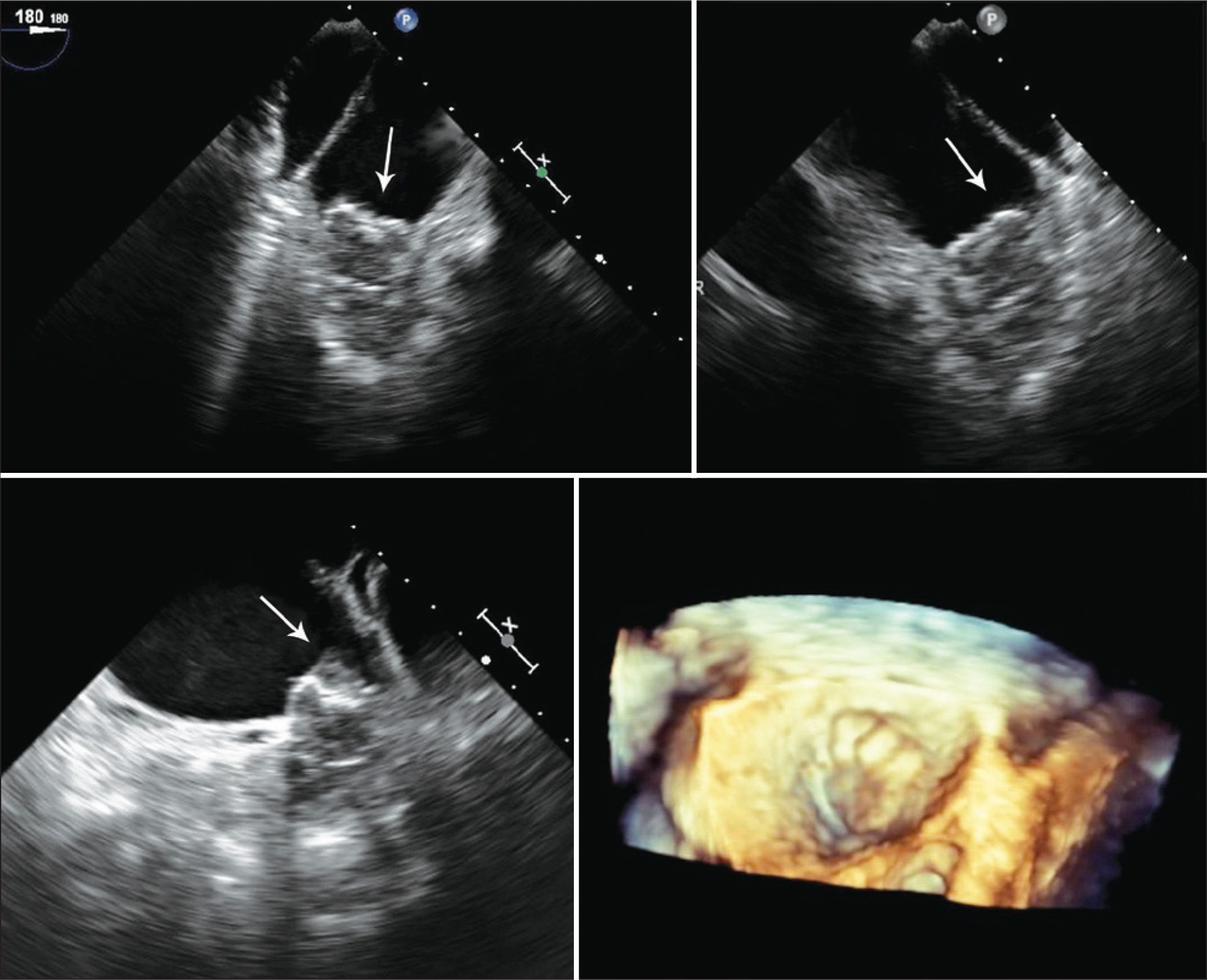
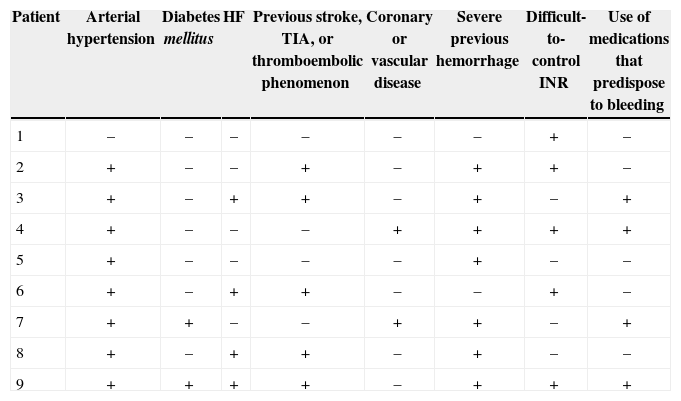
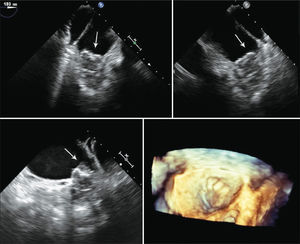
One patient had a giant thrombus in the LAA before the procedure, which resolved with anticoagulation. The patient then underwent a successful intervention, but developed a thrombus above the device 55 days after implantation, identified at the control TEE. The patient remained asymptomatic and received enoxaparin 80mg every 12 hours and ASA 81mg for one month, and was then resubmitted to TEE, showing a large decrease (> 70%) in thrombotic burden (Figures 1-3).
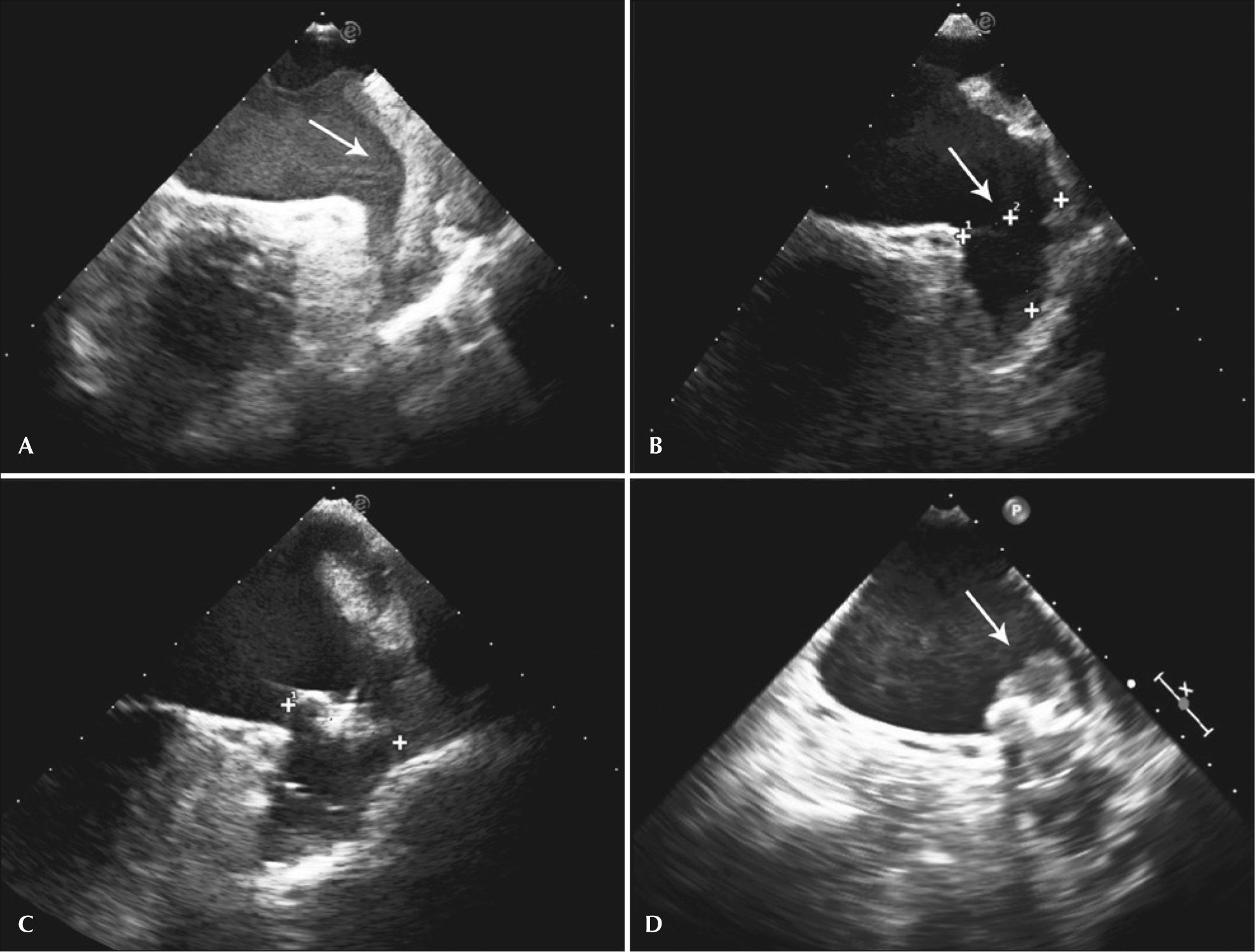
– Transesophageal echocardiography images. (A) Left atrial appendage with large thrombus inside, affecting the edge of the left superior pulmonary vein (arrow). (B) Left atrial appendage after 55 days of anticoagulation, showing thrombus resolution and measurement of the inlet orifice. (C) Device adequately deployed without residual shunt. (D) Presence of thrombus located in the prosthesis, above the site of the release system connection, visualized 72 days after the procedure (arrow).
The implantation of the WatchmanTM device for LAA occlusion in patients at high risk of thromboembolic events and difficulty or contraindication to the use of anticoagulants has shown to be feasible and safe. These outcomes are the result of the initial experience in Ecuador with the use of this device, and are part of a program of training and accreditation for percutaneous LAA occlusion of the Hemodynamics Services of hospitals José Carrasco Arteaga and Santa Inés.
International guidelines for the management of nonvalvular AF recommend anticoagulation as the treatment of choice in the prevention of stroke in the case of CHA2DS2-VASc score≥2, which represents two-thirds of the population with AF.7 However, 50% of patients with anticoagulation indication, even in developed countries, receive no treatment due to absolute or relative contraindications, concerning the patient’s or the physician’s fear of causing iatrogenic hemorrhage.4 This group of patients is unprotected from future occurrence of stroke, with a probability of development of permanent disability or death in up to 70% of cases. Additionally, a Swedish registry showed that the rate of permanent anticoagulation with warfarin, as secondary prevention for individuals who had a stroke, was 45% in up to two years.8
The present group of patients had elevated calculated risk of thromboembolic events (mean CHA2DS2-VASc score of 4), associated with low social status and level of health education, which led to the consideration of the procedure as a suitable alternative, although there were no contraindications for anticoagulation in some cases.
The efficacy and safety of percutaneous LAA occlusion with the WatchmanTM prosthesis as an alternative to anticoagulation in patients without contraindications were demonstrated in two clinical trials and several observational studies, which included over 2,000 patients. The emblematic trial was the PROTECT-AF, which included 707 patients with nonvalvular AF and CHA2DS2-VASc score≥1, randomly divided into a 2:1 non-inferiority model – device vs. warfarin with target INR of 2-3. The study demonstrated that the device was not inferior to warfarin and was more effective in preventing ischemic events at the four-year follow-up. However, in the safety analysis, the WatchmanTM prosthesis showed a higher number of early complications related to the procedure, especially pericardial effusion requiring intervention.9 The CAP Registry study was started later, aiming to evaluate the safety the procedure and factors related to the surgeon’s experience and the prosthesis. When comparing the PROTECT-AF study (542 patients) and the CAP Registry (460 patients) in relation to safety outcomes (which included bleeding, pericardial effusion, stroke, and device migration), there was a significant decrease in the procedure-related rate of complications, from 7.7% to 3.7 % (p=0.007). In addition, the incidence of pericardial effusion decreased during the first seven days after implantation, from 5.0% to 2.2% (p=0.018), and the rate of procedure-related stroke of 0.9% was reduced to zero (p=0.039).10
A second prospective randomized clinical trial, similar to the PROTECT-AF, was the PREVAIL study, which focused on the analysis of early events related to the procedure and the surgeons’ experience, which showed a high procedure success rate and easily reached the limit of non-inferiority concerning safety events.11 As occurred with other interventional procedures, there was a significant improvement in the safety of LAA occlusion with the WatchmanTM device concomitant with increasing experience of surgeons.10−12
The present study showed satisfactory initial results of the clinical outcomes regarding safety of the prosthesis and intervention in procedures performed in a hospital familiarized with structural procedures and under the supervision of certified physicians. At the follow-up, one patient had a thrombus in the device, which raises questions with regard to its incidence, its clinical implication, and its management. The PROTECTAF study reported an incidence of thrombus adhered to the prosthesis of 4.2%. However, the calculated incidence of thromboembolic events was 0.3 events per 100 patients/year – a fact that did not negatively affect the overall results of the WatchmanTM group.9,13 However, long-term clinical outcomes associated with the presence of thrombus in the device are yet to be clarified. It has been hypothesized that the site of insertion of the release cable screw has thrombogenic potential. A second hypothesis refers to the triangle not covered by the WatchmanTM prosthesis between the proximal portion of the device and the upper edge of the pulmonary vein, which could contain LAA trabecular tissue and generate thrombi. A registry showed three cases of thrombus formation in the WatchmanTM device, just above the screw insertion site, respecting the edge of the pulmonary vein. There were no thromboembolic phenomena in this group of patients.12 In the present patient, the thrombus was similarly located, above and at the center of the device, without involving the triangle on the upper edge of the pulmonary vein. There were no important events, but it was necessary to initiate and maintain anticoagulation for three months, even though there was no clinical evidence for it.
In order to establish the safety and efficacy of the WatchmanTM device in patients with anticoagulation contraindications and/or difficulty, the ASAP study was developed, which included 150 patients with nonvalvular AF and mean CHA2DS2-VASc score of 2.8. After the procedure, patients received ASA and clopidogrel continuously for six months. After a follow-up period of 176.9 patient-years, the observed rate of ischemic stroke was 1.7% per year and the presence of thrombus in the device was 4.0%, despite not receiving anticoagulation in the first 45 days.13
New oral anticoagulants have recently been evaluated in large scale clinical trials showing similar or even superior effectiveness when compared to warfarin, but with incidence of bleeding identical to that of warfarin (2.1% to 3.6%), in addition to risk of hemorrhagic events in the long term.5,6
The situation of the healthcare system in the present study’s region is a challenge to appropriate treatment and strict control of anticoagulation, with either warfarin or new oral anticoagulants, due to limitations arising from access to medical care, health education, high cost, and the availability of new drugs. Therefore, the use of devices for LAA occlusion is a more expensive, albeit valid alternative for patients at high risk of bleeding and thromboembolic events. There is no doubt that it is essential to emphasize the need to redouble efforts to improve medical treatment based on the use of anticoagulation, and to reserve percutaneous intervention only for cases in whom this strategy fails.
Study limitationsThe sample size was small, which limits the evaluation of clinical outcomes, but the safety of the procedure was demonstrated, as no short-term complications were observed.
CONCLUSIONSInitial experience with percutaneous occlusion of the LAA has shown to be a safe and effective alternative therapy in the prevention of thromboembolic events in patients with nonvalvular atrial fibrillation in the short-term follow-up.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.