Intravascular ultrasound (IVUS) is the most widely used ancillary method in Interventional Cardiology, and its analysis depends on standards for acquisition, measurement and interpretation of the images. By associating tissue characterization, the artifact caused by the guidewire may overestimate the percentage of necrotic core in certain lesions, leading to misclassification of fibroatheroma. In this paper we described quantitative and tissue analysis effects resulting from subtracting the effect of guidewire artifact on atherosclerotic lesions in patients with acute coronary syndrome.
MethodsTwenty-one patients with post-thrombolysis myocardial infarction were evaluated with grayscale IVUS and iMAPTM technology, totaling 76 lesions.
ResultsGrayscale IVUS showed that the lesions had a mean length of 21.01 ± 18.03mm and revealed high plaque burden (52.07 ± 7.56%). The analysis by iMAPTM demonstrated that, after subtracting the guidewire artifact, there was a reduction of all tissue (necrotic, calcific, lipid and fibrotic) components, but more markedly in necrotic core (mean difference: 3.59%). In addition, after artifact subtraction 12.4% of the lesions that initially exhibited a necrotic core ≥ 10% ceased to be classified as fibroatheroma.
ConclusionsAn atheroma analysis by iMAPTM technology showed that the guidewire artifact overestimated the tissue component of the necrotic core. This interference may change, in an erroneous and categorical way, the phenotypic characteristics of more stable and benign (fibrotic) lesions to potentially unstable lesions, for example, fibroatheromas, in a ratio of one out of ten patients.
O ultrassom intracoronário (USIC) é o método adjunto mais utilizado na Cardiologia Intervencionista, e sua análise depende da padronização para realização do procedimento e da interpretação das imagens. Ao associar a caracterização tecidual, o artefato causado pelo fio-guia pode hiperestimar o porcentual de núcleo necrótico em determinadas lesões, levando à classificação equivocada de fibroateroma. Descrevemos os efeitos quantitativos e na análise tecidual resultantes da subtração do efeito do artefato do fio-guia nas lesões ateroscleróticas em pacientes com síndrome coronária aguda.
MétodosForam avaliados 21 pacientes com infarto do miocárdio pós-trombólise com USIC em escala de cinzas e com a tecnologia iMAP®, totalizando 76 lesões.
ResultadosO USIC em escala de cinzas mostrou que as lesões tinham extensão média de 21,01 ± 18,03mm e apresentavam elevada carga de placa (52,07 ± 7,56%). A análise pelo iMAP® demonstrou que, após a subtração do artefato do fio-guia, houve redução de todos os componentes teciduais (necrótico, calcífico, lipídico e fibrótico), porém de maneira mais acentuada do núcleo necrótico (diferença média de 3,59%). Além disso, após a subtração do artefato, 12,4% das lesões que inicialmente apresentavam núcleo necrótico ≥ 10% passaram a não ser mais classificadas como fibroateroma.
ConclusõesA análise da placa de ateroma pela tecnologia iMAP® mostrou que o artefato do fio-guia superestimou o componente tecidual do núcleo necrótico. Essa interferência pode mudar errônea e categoricamente as características fenotípicas de lesões mais benignas e estáveis (fibróticas) para lesões potencialmente instáveis, como os fibroateromas, na relação de um em cada dez pacientes.
Intravascular ultrasound (IVUS) is one of the most often used adjunct methods in modern interventional cardiology. Although coronary angiography remains the most important imaging method in the diagnosis of coronary artery disease (CAD), in recent decades IVUS has developed considerably, and has assumed a relevant role in the hemodynamics laboratory.
Although initially its analysis was based on individual and non-uniform accounts,1,2 recently, several groups have been organized in an attempt to standardize the analysis and interpretation of ultrasound images, both by gray scale analysis3,4 and by tissue characterization with virtual histology (VHTM IVUS; Volcano Corporation, San Diego, USA) and iMAPTM technology (Boston Scientific, Santa Clara, USA).5 With this standardization, much progress has been made in the use of IVUS in everyday clinical practice, mainly as a guide in complex percutaneous coronary interventions (PCIs),6,7 such as in bifurcation lesions, long lesions, PCIs of the left main coronary artery,8,9 and studies of progression and regression of atherosclerosis.10–12
Adding tissue characterization to data obtained by grayscale analysis, by means of radio frequency analysis of ultrasound waves, it has become possible to identify the characteristics of fibroatheroma and its several subtypes,13 and of the vulnerable plaque, which is prone to rupture and responsible for acute coronary events.14–17 Conceptually, among other criteria, such as thin fibrous cap (< 65μm) and positive arterial remodeling, the vulnerable plaque, also known as thin-cap fibroatheroma, must have a large confluent necrotic core (≥ 10%), and maintain > 30% contact with the lumen, for at least three consecutive frames.
However, a major obstacle to the appropriate identification and especially the quantification of the necrotic core is the artifact caused by the guidewire, when iMAPTM technology is applied. The guidewire, consisting of material that is highly reflective of ultrasound waves (echo-reflector), generates an artifact similar to that generated by calcium, called an acoustic shadow.18 The iMAPTM technology, when detecting the signal resounded from the guidewire, interprets this signal as compatible with necrotic tissue and erroneously classifies the area of acoustic shadow as necrotic core. This fact ultimately causes an overestimation of the necrotic core quantity in a particular lesion.
This study aimed to describe the quantitative and tissue analysis effects resulting from subtraction of the guidewire artifact effect in atherosclerotic lesions of patients with acute coronary syndrome, by using IVUS with gray scale analysis and iMAPTM technology.
MethodsPatients and study designFrom September 2011 to February 2012, 21 patients with clinical and electrocardiographic diagnosis of ST-segment elevation acute myocardial infarction (STEMI), totaling 76 lesions, were prospectively included in the iWonder study. The study design has been previously described.19 In short, 100 patients with acute myocardial infarction were analyzed with IVUS of the three epicardial coronary arteries in grayscale and with iMAPTM regarding the phenotypic and tissue characteristics of the culprit and non-culprit lesions. The study was carried out at the Hemodynamics and Interventional Cardiology Department of Hospital São Paulo, São Paulo (SP), and was previously approved by the Research Ethics Committee of the institution (project 0889/11, August 5, 2011) and identified at ClinicalTrials.gov under number NCT01437553. All patients or their legal representatives were informed about the objectives and risks of the procedures related to the study and signed the informed consent before undergoing the diagnostic procedure.
IVUS procedureImages were obtained by IVUS of the epicardial coronary arteries, which was performed immediately after the diagnostic procedure, under full heparinization (unfractionated heparin 100 U/kg, aiming an activated clotting time between 250 and 350seconds) and intracoronary vasodilator administration (nitroglycerin 100-200μm). Initially, IVUS study of the artery related to the clinical event (culprit vessel) was performed, followed by angioplasty, if necessary. Next, the two other coronary arteries unrelated to the clinical event (non-culprit vessels) were analyzed through IVUS. A 40MHz IVUS catheter was used (AtlantisTM SR Pro; Boston Scientific, Santa Clara, USA), with grayscale analysis and morphological characterization using iMAP-Intravascular UltrasoundTM evaluation (iMAP-IVUSTM; Boston Scientific, Santa Clara, USA). Automatic pullbacks of the IVUS catheter were performed at a velocity of 0.5mm/s, beginning at a point 10mm distal from the culprit lesion, toward the ostium of the artery being evaluated. In non-culprit arteries, the same routine was performed to analyze plaques unrelated to the event.
Analysis of intravascular ultrasound imagesAll angiographic and IVUS images were stored in digital media and copied to an external hard drive for offline analysis in the Intravascular Imaging Core Laboratory of the Cardiovascular Research Foundation (New York, USA).
The analysis of IVUS was performed in three sequential steps, as follows.
Step 1 – quantitative analysisThe quantitative volumetric analysis was performed according to current guidelines.3,4 This offline step of IVUS analysis consisted of the definition of the segment to be analyzed in each pullback, including at least 10mm in length distally to the ostium of the respective vessel. Then, using QIvusTM version 2.1 software (Medis Medical Imaging Systems, Leiden, The Netherlands), the automatic contours of the vessel and lumen were obtained at each 1mm within the defined segment. Then, using the Simpson method, the volumes of the lumen, vessel, and plaque (vessel less lumen) were computed. The plaque burden was calculated as the cross-sectional area of the plaque divided by the cross-sectional area of the vessel, multiplied by 100. Minimum luminal area was defined as the smallest cross-sectional area of the lumen within the lesion.
Step 2 – tissue characterization by iMAPTMIMAP-IVUSTM is an imaging modality that uses radiofrequency spectral analysis to obtain an algorithm used to classify the atherosclerotic plaque into four components: fibrotic, lipidic, necrotic, and calcific. The development of this algorithm was based on histological analyses ex vivo20 and each component is assigned a color: fibrotic component as green, lipidic component as yellow, necrotic component as red, and calcific component as white.
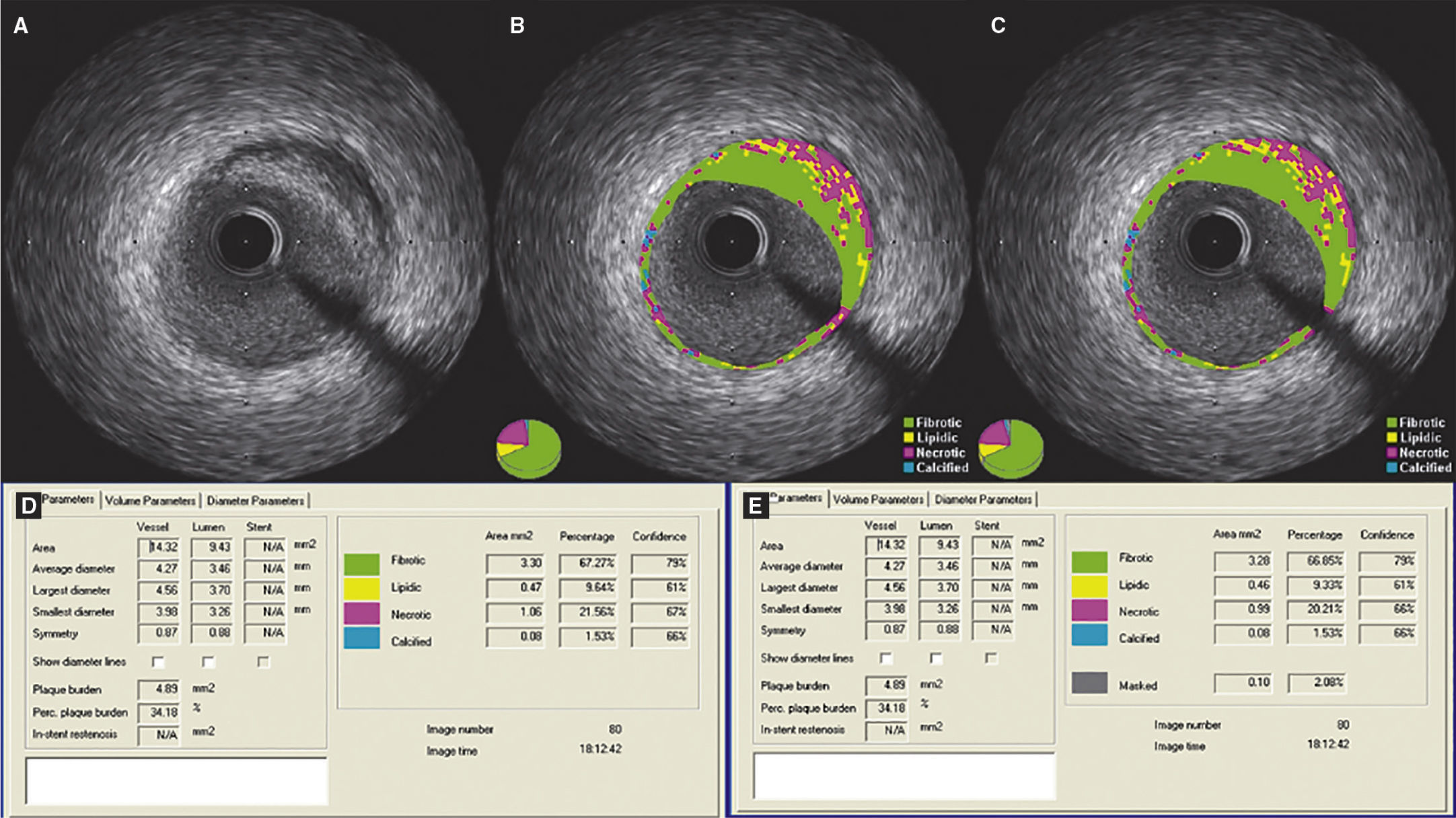
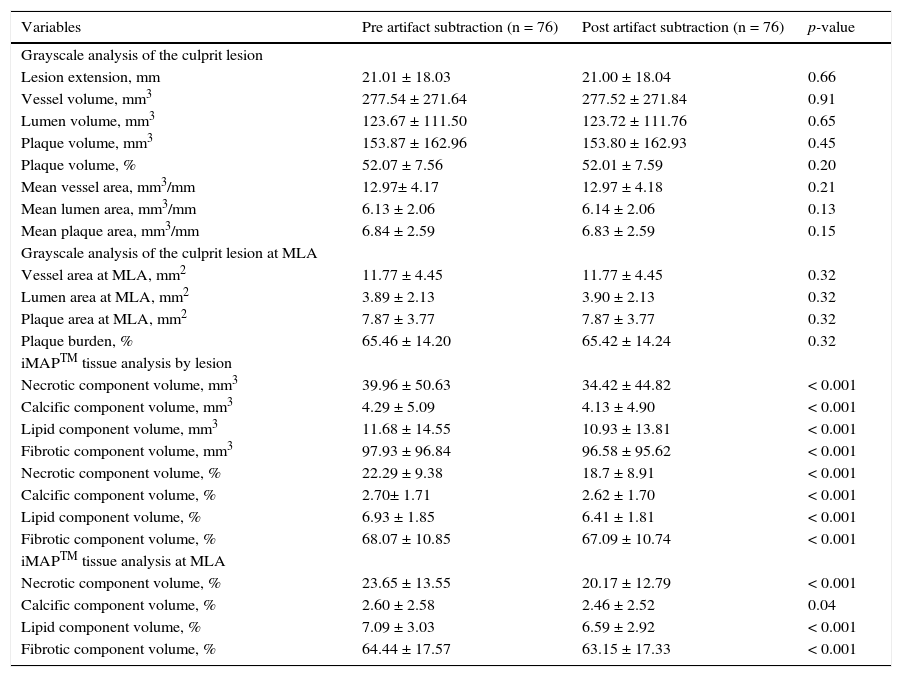
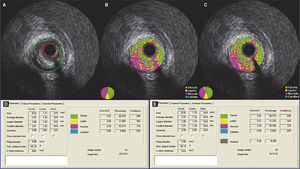
Step 3 – subtraction of the guidewire artifactAfter finishing the contour of the vessel and lumen cross-sectional areas, the measurements were exported to the appropriate database. Then, using the subtraction tool available in QIvus 2.1TM, the guidewire artifact was manually delimited by an experienced examiner (C.F.S.) in each frame of the segment defined as lesion (proximal and distal reference segments were excluded), as shown in Figure 1. The values obtained were then exported to the database for comparison with the initial measures.
Demonstration of the effect of guidewire artifact masking (A) in grayscale intravascular ultrasound; (B) tissue characterization with iMAPTM; observe the artifact caused by the guidewire at 4 o’clock, interpreted as necrotic core (red); (C) tissue characterization after masking of the guidewire artifact; (D and E) results by iMAPTM pre- and post-masking of the guidewire artifact, showing necrotic core variation from 21.56 to 20.21%.
In the descriptive statistical analysis, categorical variables were expressed as absolute and percentage frequency. Continuous variables were expressed as mean ± standard deviation. Paired t-test was used for comparison between the groups. The p-value was considered significant when < 0.05. To perform the analyses, SPSS version 13.0 (SPSS Inc., Chicago, USA) was used.
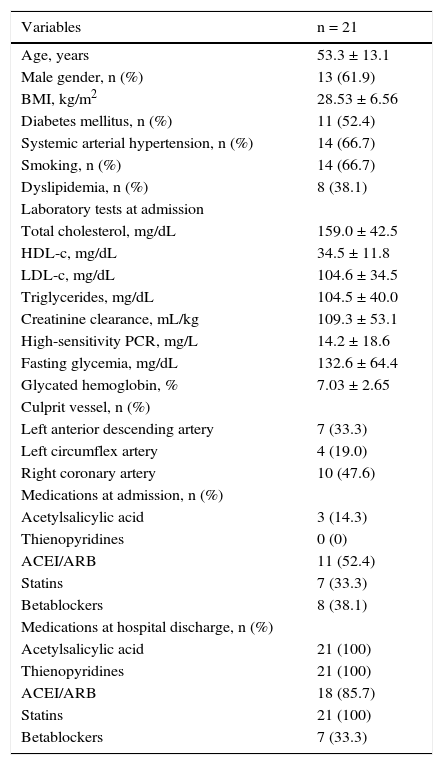
ResultsThe mean age of patients was 53.3 ± 13.1 years, with a predominance of males (61.9%). The mean time between the index event and the performance of IVUS was 6.1 ± 2.4 days. Among the risk factors for CAD, the following were observed: high prevalence of diabetes (52.4%), systemic arterial hypertension (66.7%), and smoking (66.7%). The other clinical and angiographic characteristics are summarized in Table 1.
Baseline clinical characteristics, laboratory tests, and drug therapy.
| Variables | n = 21 |
|---|---|
| Age, years | 53.3 ± 13.1 |
| Male gender, n (%) | 13 (61.9) |
| BMI, kg/m2 | 28.53 ± 6.56 |
| Diabetes mellitus, n (%) | 11 (52.4) |
| Systemic arterial hypertension, n (%) | 14 (66.7) |
| Smoking, n (%) | 14 (66.7) |
| Dyslipidemia, n (%) | 8 (38.1) |
| Laboratory tests at admission | |
| Total cholesterol, mg/dL | 159.0 ± 42.5 |
| HDL-c, mg/dL | 34.5 ± 11.8 |
| LDL-c, mg/dL | 104.6 ± 34.5 |
| Triglycerides, mg/dL | 104.5 ± 40.0 |
| Creatinine clearance, mL/kg | 109.3 ± 53.1 |
| High-sensitivity PCR, mg/L | 14.2 ± 18.6 |
| Fasting glycemia, mg/dL | 132.6 ± 64.4 |
| Glycated hemoglobin, % | 7.03 ± 2.65 |
| Culprit vessel, n (%) | |
| Left anterior descending artery | 7 (33.3) |
| Left circumflex artery | 4 (19.0) |
| Right coronary artery | 10 (47.6) |
| Medications at admission, n (%) | |
| Acetylsalicylic acid | 3 (14.3) |
| Thienopyridines | 0 (0) |
| ACEI/ARB | 11 (52.4) |
| Statins | 7 (33.3) |
| Betablockers | 8 (38.1) |
| Medications at hospital discharge, n (%) | |
| Acetylsalicylic acid | 21 (100) |
| Thienopyridines | 21 (100) |
| ACEI/ARB | 18 (85.7) |
| Statins | 21 (100) |
| Betablockers | 7 (33.3) |
BMI: body mass index; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; PCR: C-reactive protein; ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker.
Using the grayscale analysis by IVUS (Table 2), culprit lesions with a mean length of 21.01 ± 18.03mm and plaque volume of 52.07% ± 7.56% were observed. The minimum luminal area was 3.89 ± 2.13 mm2, and plaque burden was 65.46% ± 14.20%.
Data from the grayscale intravascular ultrasound of the culprit lesion.
| Variables | Pre artifact subtraction (n = 76) | Post artifact subtraction (n = 76) | p-value |
|---|---|---|---|
| Grayscale analysis of the culprit lesion | |||
| Lesion extension, mm | 21.01 ± 18.03 | 21.00 ± 18.04 | 0.66 |
| Vessel volume, mm3 | 277.54 ± 271.64 | 277.52 ± 271.84 | 0.91 |
| Lumen volume, mm3 | 123.67 ± 111.50 | 123.72 ± 111.76 | 0.65 |
| Plaque volume, mm3 | 153.87 ± 162.96 | 153.80 ± 162.93 | 0.45 |
| Plaque volume, % | 52.07 ± 7.56 | 52.01 ± 7.59 | 0.20 |
| Mean vessel area, mm3/mm | 12.97± 4.17 | 12.97 ± 4.18 | 0.21 |
| Mean lumen area, mm3/mm | 6.13 ± 2.06 | 6.14 ± 2.06 | 0.13 |
| Mean plaque area, mm3/mm | 6.84 ± 2.59 | 6.83 ± 2.59 | 0.15 |
| Grayscale analysis of the culprit lesion at MLA | |||
| Vessel area at MLA, mm2 | 11.77 ± 4.45 | 11.77 ± 4.45 | 0.32 |
| Lumen area at MLA, mm2 | 3.89 ± 2.13 | 3.90 ± 2.13 | 0.32 |
| Plaque area at MLA, mm2 | 7.87 ± 3.77 | 7.87 ± 3.77 | 0.32 |
| Plaque burden, % | 65.46 ± 14.20 | 65.42 ± 14.24 | 0.32 |
| iMAPTM tissue analysis by lesion | |||
| Necrotic component volume, mm3 | 39.96 ± 50.63 | 34.42 ± 44.82 | < 0.001 |
| Calcific component volume, mm3 | 4.29 ± 5.09 | 4.13 ± 4.90 | < 0.001 |
| Lipid component volume, mm3 | 11.68 ± 14.55 | 10.93 ± 13.81 | < 0.001 |
| Fibrotic component volume, mm3 | 97.93 ± 96.84 | 96.58 ± 95.62 | < 0.001 |
| Necrotic component volume, % | 22.29 ± 9.38 | 18.7 ± 8.91 | < 0.001 |
| Calcific component volume, % | 2.70± 1.71 | 2.62 ± 1.70 | < 0.001 |
| Lipid component volume, % | 6.93 ± 1.85 | 6.41 ± 1.81 | < 0.001 |
| Fibrotic component volume, % | 68.07 ± 10.85 | 67.09 ± 10.74 | < 0.001 |
| iMAPTM tissue analysis at MLA | |||
| Necrotic component volume, % | 23.65 ± 13.55 | 20.17 ± 12.79 | < 0.001 |
| Calcific component volume, % | 2.60 ± 2.58 | 2.46 ± 2.52 | 0.04 |
| Lipid component volume, % | 7.09 ± 3.03 | 6.59 ± 2.92 | < 0.001 |
| Fibrotic component volume, % | 64.44 ± 17.57 | 63.15 ± 17.33 | < 0.001 |
MLA: minimal luminal area.
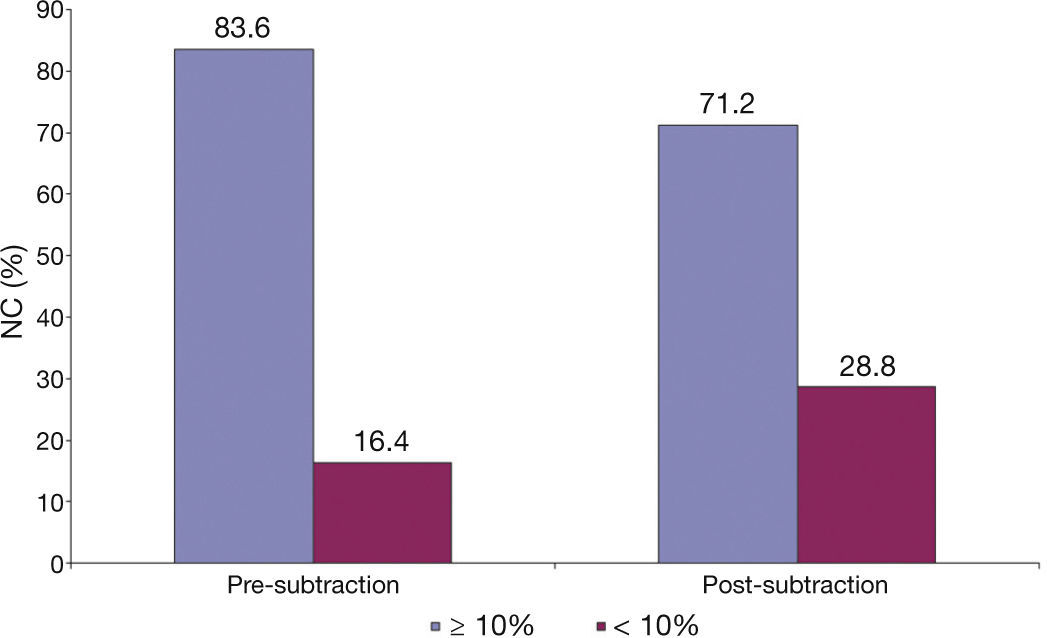
It is important to observe the differences related to tissue composition by iMAPTM analysis (Fig. 2). After the subtraction of the guidewire artifact, there was a statistically significant reduction in the percentage of all components (necrotic, calcific, lipidic, and fibrotic; p < 0.001 for all). Furthermore, the only tissue component that showed mean relevant difference in the percentage pre- and post-subtraction of the guidewire artifact was the necrotic core (difference of 3.59%), while the rest showed minimal variation (calcific component, 0.07%; lipidic, 0.52%; fibrotic, 0.98%).
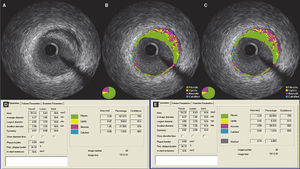
Finally, as shown in Figure 3, using 10% necrotic core as a diagnostic criterion for defining a lesion as a fibroatheroma, after the subtraction of the guidewire artifact, 12.4% of lesions that had showed necrotic core ≥ 10% ceased to be characterized as fibroatheromas.
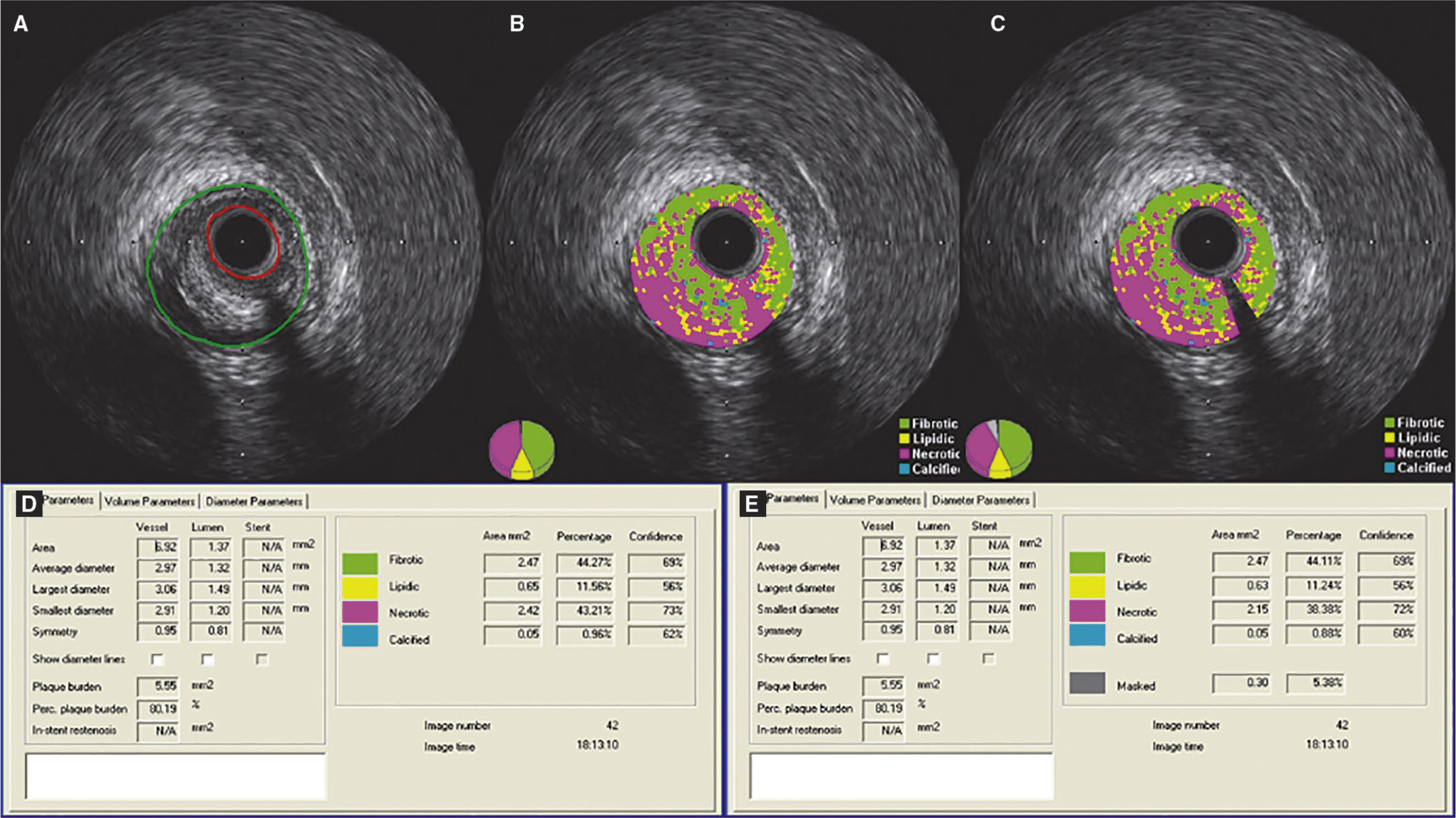
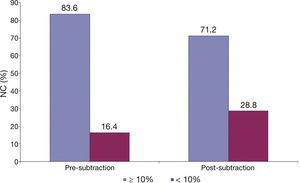
Demonstration of the effect of the subtraction of the guidewire artifact in a lesion classified as fibroatheroma. (A) Grayscale intravascular ultrasound; (B) tissue characterization with iMAPTM; observing the artifact caused by the guidewire at 4 o’clock, interpreted as necrotic core (red); (C) tissue characterization post-subtraction of the guidewire artifact; (D and E) results by iMAPTM pre- and post-subtraction of the guidewire artifact, showing variation of the necrotic core from 43.21 to 38.38%.
The present study evaluated 76 lesions in 21 patients with STEMI using IVUS with grayscale and tissue characterization with iMAPTM technology. The impact caused by the guidewire artifact on tissue composition of atherosclerotic plaques was defined. The main findings were as follows: after the subtraction of the guidewire artifact, there was a statistically significant reduction in all four tissue components identified by iMAPTM (necrotic, calcific, lipidic, and fibrotic); the tissue component that showed the most significant reduction was the necrotic component (difference 3.59%); due to subtraction of the guidewire artifact, 12.4% of the lesions that had previously shown a necrotic core ≥ 10% (meeting one of the criteria for fibroatheroma classification) were no longer classified as such.
The present study is the first to describe the effects of subtraction of the artifact caused by the guidewire in tissue characterization of CAD using iMAPTM technology. It is possible that the misinterpretation of the guidewire's acoustic shadow as a necrotic core may lead to misclassification of a lesion still in the early stage of atherosclerosis as a fibroatheroma. Furthermore, the possibility of the guidewire overestimating the necrotic content can be even more pronounced in larger vessels and lesions with higher plaque burden.
As the ultrasound definition of fibroatheroma involves the mandatory presence of ≥ 10% of confluent necrotic core,13 any artifact that leads to erroneous increase in this component amount can result in misclassification. In a similar study using VH-IVUSTM, Sales et al.21 demonstrated that the artifact caused by the metallic stent struts, by reflecting the IVUS waves, produced an effect similar to that of calcium. Therefore, with this type of technology, the artifact is interpreted as a calcific component. In 17 lesions treated with stent, these authors showed that, when comparing VH-IVUS before and after PCI, there was a statistically significant increase in the amount of the calcific component. However, they also showed that the necrotic component could be identified around the stent struts. As there was no proven association between PCI and the appearence of local necrotic tissue, the authors concluded that this finding was due to the artifact caused by the metallic stent struts.
Differently from VH-IVUSTM, iMAPTM technology identifies the guidewire artifact (acoustic shadow) as necrotic component (rather than calcific).22 In one of the present cases, a lesion that initially showed 43.21% necrotic component later showed 38.38%, after subtraction of the guidewire artifact. In this circumstance, there was no impact on the lesion phenotypic classification. However, it is possible that borderline lesions originally classified as fibroatheroma would be classified as more incipient forms of atherosclerosis after subtraction of the artifact, such as pathological intimal thickening or fibrotic plaque (both with necrotic core < 10%).
LimitationsThe analysis of guidewire artifact subtraction was performed manually and may have under- or over-estimated tissue components. The clinical applicability of iMAPTM technology and others that offer tissue diagnosis of the atheroma plaque is still a matter of debate, showing no support in clinical decision-making considering the current guidelines. The low spatial resolution of IVUS (approximately 200μm) may interfere with the design and calculation of vessel areas, mainly in the lumen area and, therefore, may have an impact on the percentage of each tissue component.
ConclusionsThe analysis of atheroma plaque using iMAPTM technology in patients with STEMI showed that the guidewire artifact overestimated the necrotic core tissue component of the atherosclerotic plaque. This interference can categorically and erroneously alter the phenotypic characteristics of more benign and stable lesions (fibrotic) to potentially unstable lesions, such as atheromas, at a proportion of one in ten patients.
Funding sourcesThe study received partial funding from Boston Scientific, which donated catheters.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.