The purpose of this study is to report the early experience with the use of the new CARDIA Ultrasept™ device for percutaneous occlusion of patent foramen ovale.
MethodsPatients with patent foramen ovale with previous embolic events, of any age or weight, or migraine with poor clinical control, were selected by transesophageal echocardiography. Efficacy criteria included lack of residual shunt at the transesophageal echocardiogram with microbubble testing after 6 months and no recurrence of neurologic events. No patients were excluded based on morphological patent foramen ovale criteria.
ResultsFrom April 2011 to May 2012, 22 patients (6 males and 16 females) with ages ranging from 16 to 68 years were submitted to the occlusion procedure. Only one patient had no history of stroke or transient ischemic attack, but migraine with poor clinical response. None of the patients had atrial septal aneurysms. The device was implanted in all cases, 23 devices were used and the 25mm device was used most often. Only one patient presented residual shunt at the 1-month follow-up echocardiogram and remains under observation. There have been no recurrences to date. There were two minor complications and no deaths
ConclusionsThe CARDIA Ultrasept™ device is safe, effective and easy to use. It must be further evaluated in more complex cases, such as larger patent foramen ovales and with atrial septal aneurysms.
Experiência Inicial com a Nova PróteseCARDIA UltraseptTM para Fechamento do Forame Oval Patente: Ainda uma Boa Opção
IntroduçãoNeste trabalho os autores visam a apresentar sua experiência inicial com o uso da nova prótese CARDIA UltraseptTM para fechamento percutâneo do forame oval
MétodosForam selecionados por ecocardiograma transesofágico pacientes portadores de forame oval com eventos embólicos prévios, de qualquer idade e peso, ou enxaqueca de difícil controle clínico. Os critérios de eficácia incluíram ausência de shunt residual pelo ecocardiograma transesofágico com teste de microbolhas após seis meses e ausência de recidiva de eventos neurológicos. Não foram excluídos pacientes com base em dados morfológicos do forame oval.
ResultadosDe abril de 2011 a maio de 2012 foram submetidos ao procedimento de oclusão 22 pacientes (6 do sexo masculino e 16 do sexo feminino) com idades entre 16 e 68 anos. Apenas uma paciente não tinha antecedente de acidente vascular cerebral ou ataque isquêmico transitório e sim enxaqueca de difícil controle clínico. Nenhum paciente apresentou aneurisma de septo atrial. Foi possível o implante em todos os casos, sendo utilizados 23 dispositivos. O dispositivo mais utilizado foi o de 25mm. Apenas um paciente ficou com shunt residual ao ecocardiograma de um mês e continua em seguimento. Não houve recidivas de eventos até o momento. Houve duas complicações menores e não houve óbitos.
ConclusõesO dispositivo CARDIA UltraseptTM é seguro, de fácil manuseio e eficaz. Ainda necessita ser mais bem avaliado em casos mais complexos, como os forames ovais maiores e com aneurisma do septo atrial.
The foramen ovale, which is a crucial structure for foetal circulation, remains patent in 25% to 30% of the adult population. 1,2 By allowing the passage of the interatrial flow from right to left, the patent foramen ovale is now recognized to be associated with cerebral thromboembolic events 3–9 and migraines with aura. 10–12 Several case series studies suggest that occlusion by the percutaneous technique may be indicated in these situations, 13–17 and may even be more effective than medical therapy in the secondary prevention of cerebrovascular event recurrence in patients with a patent foramen ovale-related ischemic stroke or transient ischemic attack (TIA). 16
Several devices have been developed for patent foramen ovale (PFO) occlusion. The CARDIA prostheses (Cardia Inc. – Eagan, MN, USA) have been available since 1998 and have been redesigned several times, 18–20 although the redesigned prostheses preserve the same basic structural concept of two Ivalon™ (polyvinyl alcohol) discs supported by nitinol struts.
The seventh-generation CARDIA prostheses, termed Ultrasept™, maintains the basic concepts described above, but now both discs have rounded edges to minimize the risk of erosion and/or perforations.
The objective of this study was to report an initial experience using this prosthetic device for PFO occlusion.
METHODSStudy ProjectThis prospective, single-center single-arm study included all patients who underwent foramen ovale occlusion with the CARDIA Ultrasept™ prosthesis.
Patients of any age and weight who had the following symptoms were selected: one or more neurological events with no definite causes (cryptogenic) and a diagnosis based on clinical and/or neurological imaging; and migraine with aura, with an unsatisfactory response to traditional medication or prophylactic clinical indication, as formalized by the attending physician in risk PFO with the passage of large bubbles during normal breathing.
The cases were selected by a transesophageal echocardiogram with a positive bubble test, with an agitated saline solution while breathing spontaneously or after the Valsalva manoeuver. No type of foramen was eliminated based on its morphological characteristics. All procedures were performed with the prior administration of 200mg of acetylsalicylic acid.
Device descriptionThe new version, as did the previous versions, consists of two round discs of Ivalon™ but is supported by six rounded nitinol loops instead of struts (Figure 1).
– Details of the new version of the CARDIA prosthesis. A, Left surface of the disc. Note the metal completely covered by the Ivalon™ sponges. B, Surface of the right disc, which is smaller than the left one, with the nitinol loops and the central capture pin clearly visible. C, Prosthesis profile view, held by the bioptome.
The prostheses are available in Brazil in sizes of 20mm, 25mm and 30mm, which correspond to the length of the nitinol loops. The device size was selected on a case-by-case basis, according to the length and opening of the produced tunnel, the stability and consistency of the septum primum, and the presence or absence of lipomatous degeneration of the septum secundum.
Along with the device, a flexible bioptome was offered, with a safety lock, to capture the contact pin in the center of the right disc. This bioptome allows for the introduction of the prosthesis into a transparent cylinder carrier, which has a distal end that can be dilated to enable better adaptation to the flexed disc carrier. The other end of the cylinder fits into a short sheath and is transparent, single-sized, and features a hemostatic valve (Figure 2). The prosthesis slides easily from the carrier to the short sheath and thus can be transferred to the long sheath (Mullins), which will lead it to the heart, pushed by the long bioptome cable. For the aforementioned prosthesis sizes, Mullins sheaths of 10-F to 11-F are required.
– Details of the carrier system. A, Carrier with a dilated distal extremity to better guide the device disks, thereby minimizing the trauma to the Ivalon™ sponges. The short sheath, also transparent, fits perfectly into the carrier, facilitating the prosthesis delivery. B, Detail of the steps of prosthesis delivery, from left to right: observe the prosthesis attached to the bioptome, the carrier and the short sheath.
The CARDIA Ultrasept™ prosthesis can be easily recaptured and repositioned while attached to the delivery system.
Implantation techniqueThe technique employed in all cases was the same used in previous versions and has already been described. 20
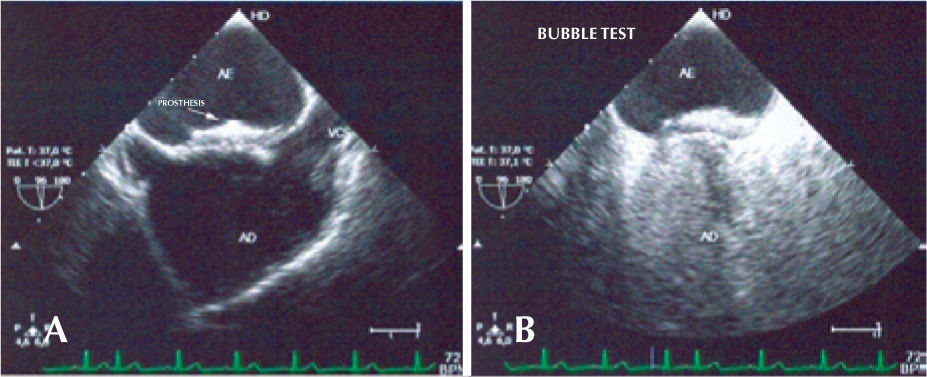
Follow-upAll patients were maintained on dual antiplatelet therapy with 200mg/day of acetylsalicylic acid and 75mg/day of clopidogrel for three months, followed by the isolated use of acetylsalicylic acid at the same dose between the third and sixth months after the procedure. The follow-up was conducted by clinical evaluations and transthoracic echocardiograms immediately after the procedure and after one and three months. A transesophageal echocardiography with the bubble test using an agitated saline solution was performed after six months.
Statistical AnalysisContinuous variables were expressed as means±standard deviations, and categorical variables were expressed as numbers and percentages.
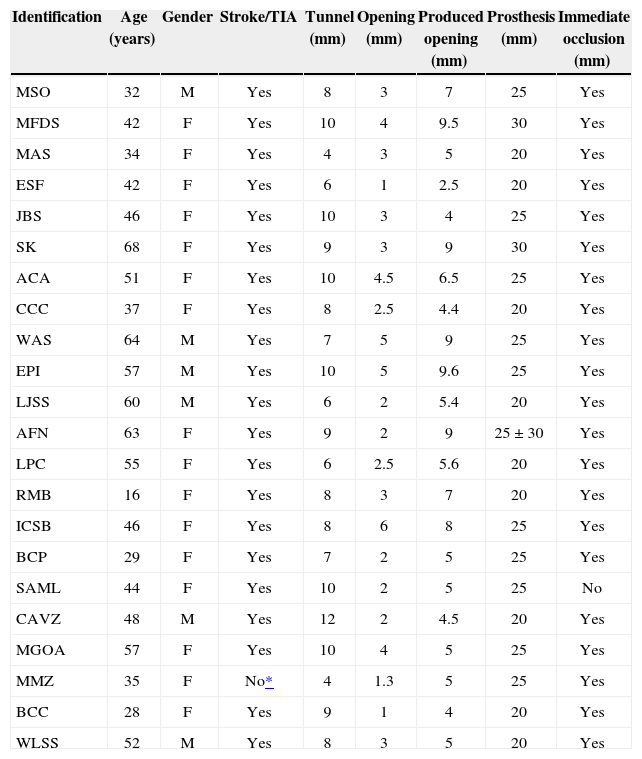
RESULTSFrom April of 2011 to May of 2012, 22 patients underwent the percutaneous occlusion of a patent foramen ovale with the CARDIA Ultrasept™ prosthesis. Only six patients were males. Their ages ranged from 16 to 68 years (45.7±13.1years). All patients had a prior clinical picture of ischemic cerebral episodes (CVA/TIA), except one, in whom the indication was the difficult clinical management of migraines (Table).
Patient characteristics
| Identification | Age (years) | Gender | Stroke/TIA | Tunnel (mm) | Opening (mm) | Produced opening (mm) | Prosthesis (mm) | Immediate occlusion (mm) |
|---|---|---|---|---|---|---|---|---|
| MSO | 32 | M | Yes | 8 | 3 | 7 | 25 | Yes |
| MFDS | 42 | F | Yes | 10 | 4 | 9.5 | 30 | Yes |
| MAS | 34 | F | Yes | 4 | 3 | 5 | 20 | Yes |
| ESF | 42 | F | Yes | 6 | 1 | 2.5 | 20 | Yes |
| JBS | 46 | F | Yes | 10 | 3 | 4 | 25 | Yes |
| SK | 68 | F | Yes | 9 | 3 | 9 | 30 | Yes |
| ACA | 51 | F | Yes | 10 | 4.5 | 6.5 | 25 | Yes |
| CCC | 37 | F | Yes | 8 | 2.5 | 4.4 | 20 | Yes |
| WAS | 64 | M | Yes | 7 | 5 | 9 | 25 | Yes |
| EPI | 57 | M | Yes | 10 | 5 | 9.6 | 25 | Yes |
| LJSS | 60 | M | Yes | 6 | 2 | 5.4 | 20 | Yes |
| AFN | 63 | F | Yes | 9 | 2 | 9 | 25±30 | Yes |
| LPC | 55 | F | Yes | 6 | 2.5 | 5.6 | 20 | Yes |
| RMB | 16 | F | Yes | 8 | 3 | 7 | 20 | Yes |
| ICSB | 46 | F | Yes | 8 | 6 | 8 | 25 | Yes |
| BCP | 29 | F | Yes | 7 | 2 | 5 | 25 | Yes |
| SAML | 44 | F | Yes | 10 | 2 | 5 | 25 | No |
| CAVZ | 48 | M | Yes | 12 | 2 | 4.5 | 20 | Yes |
| MGOA | 57 | F | Yes | 10 | 4 | 5 | 25 | Yes |
| MMZ | 35 | F | No* | 4 | 1.3 | 5 | 25 | Yes |
| BCC | 28 | F | Yes | 9 | 1 | 4 | 20 | Yes |
| WLSS | 52 | M | Yes | 8 | 3 | 5 | 20 | Yes |
The lengths of the tunnels ranged from 4–12mm (8.13±2mm) with non-induced openings of 1–6mm (2.94±1.30mm). The variation of the produced opening obtained with the rigid guidewire positioned behind the foramen was 4–9.5mm (6.13±2.01mm).
The implant was feasible in all cases. A total of 23 devices were used in 22 patients. The size used most often was 25mm (47.8% of patients), followed by the 20-mm size (39.1%) and the 30-mm size (Figure 3).
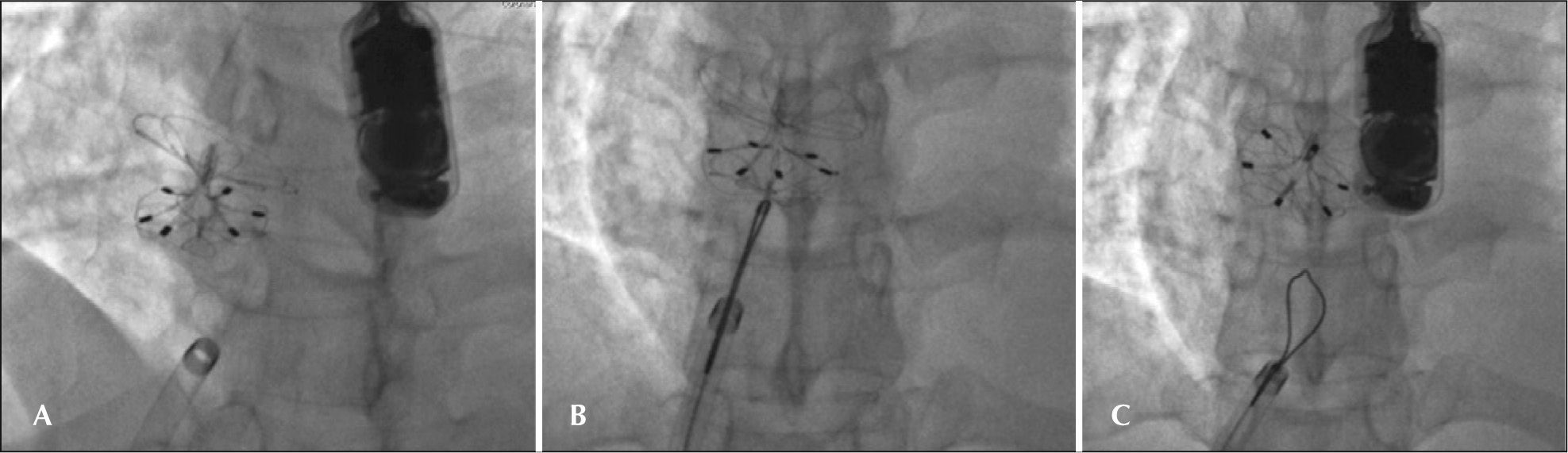
– Details of the implant. A, Prosthesis positioned in the atrial septum, completely configured but still attached to the delivery system. B, Prosthesis after release. Note that the prosthesis changes its spatial position and adapts perfectly to the plane of the septum, free of the weight of the delivery system. C, Released prosthesis, observed through the left atrium, on three-dimensional echocardiography.
The patient who required two prostheses (AFN) had a thickened septum secundum due to lipomatous degeneration and a hypermobile septum primum. A 25-mm device was implanted, which was fixed only in the septum primum, keeping the foramen open with the passage of large bubbles from right to left. In the same procedure, a second 30-mm device was implanted without difficulties; this device was able to capture both the lipomatous septum and the first device, completely occluding the residual defect. The bubble test conducted after implantation showed no passage of bubbles into the left atrium.
In one patient (JBS), the prosthesis right disk was trapped in the Eustachian valve, which was quite large, and could not be configured properly, keeping the prosthesis open with a large shunt inside it. After several unsuccessful attempts to loosen the right disc, the device was released from the delivery cable on the assumption that it would become free and then achieve the desired configuration, thus reducing the prosthesis profile. As that outcome did not occur, the right disc was recaptured with a 15-mm snare catheter and reintroduced into the long sheath. The set was advanced through the septum into the left atrium roof, and the prosthesis was repositioned with the right disk always configured near the extremity of the long sheath to prevent it from being arrested again in the large valve. The prosthesis was released, and the bubble test conducted after the release showed no passage of bubbles through the interatrial septum (Figure 4).
– Details of the implant in the patient JBS. A, Prosthesis released after the first implant, with the right disk fixed in the Eustachian valve, holding it open with a large residual shunt. B, Right disk captured by the loop catheter (Amplatz Goose Neck snare, 15mm) and retracted to be pulled again into the long sheath for a second implant. C, Repositioned prosthesis. After being released from the snare, it reached the correct position. In this profile, the residual shunt is no longer observed. The control bubble test was negative.
One patient (SAML) presented a minimal additional orifice near the inferior vena cava. The foramen was closed with a 25-mm prosthesis. The bubble test showed no subsequent passage of bubbles from right to left through the second orifice, which was allowed to remain open. There were two minor immediate complications. One patient (AFN) had bleeding in the endotracheal tube after implantation, presumably due to an entrance laceration of the left superior pulmonary vein caused by the long sheath. She was under observation in the Intensive Care Unit, but transfusion or any other surgical measure was not required. The patient was discharged the next day in good status. The second patient (BCP) had a fresh thrombus that was unorganized, adhered to the left prosthesis disc, and was visualized by transesophageal echocardiography during the procedure. The heparin dose was increased to 10,000 U. The prosthesis and the long sheath were withdrawn slowly and thoroughly washed with heparinized saline solution. Then, after careful ins pection, the sheath and prosthesis were reintroduced, and the new implant procedure was uneventful. The patient showed no signs of neurological involvement and was discharged in good status.
Over half of the patients (57.1%) reached the six-month follow-up. There has been no recurrence of neurological events in any of the cases to date. The patient with prior migraine (MMZ) had headache with aura in the first month after implantation, although less severe than the previous episodes. Another patient (CCC) showed an allergy to clopidogrel and was treated with aspirin only. One patient (SAML) showed a residual shunt through the interior of the prosthesis on the control transthoracic echocardiogram after one month. This patient is being monitored and has had no complications. The others were adequately occluded (Figure 5). There were no deaths in the present series.
DISCUSSIONThe percutaneous occlusion of patent foramen ovales is currently performed in most hemodynamics services worldwide. It has proved to be a safe, effective, and easily reproducible procedure with excellent results, especially with the new generations of prostheses developed specifically for this purpose. 1,12,16,21–28
There are few studies in the literature using the CARDIA prosthesis. In this new version, the round shape of the two discs has less risk of erosion or perforation. Notably, the left disc nitinol loops are located underneath the Ivalon™ sponge, reducing the risk of thrombosis and promoting more homogeneous endothelialisation.
The configuration of the prosthesis is facilitated by the use of the carrier and the short transparent sheath, which enables the easy detection of the presence of air within the system.
Despite the introduced changes, high-profile sheaths remain more necessary than desired. However, as they are introduced by venous access, the risk of complications is low, even in children. By always using the sheaths with the adequate gauge recommended by the manufacturer, there was no difficulty in loading the prosthesis, which slid easily through the interior of the sheath in all cases.
The implantation was performed without difficulty, and the technique does not differ from those described for other prostheses with the same purpose. Compared with previous models of the device, slightly more space might be needed for the right disk configuration, which could explain the case (JBS) in which it was trapped by the large Eustachian valve.
A point to be considered is the possibility of the bioptome becoming entrapped in the disk loops immediately after prosthesis release from the delivery system, as previously observed. It is important to always keep the bioptome open during withdrawal until its introduction into the long sheath. If any resistance or the movement of the prosthesis along with it is noticed, the right disc should not be pulled, which would increase the risk of prosthesis dislocation; instead, the open bioptome should be carefully rotated until it is released from the captured snare. Another suggestion is to keep the long sheath near the bioptome at the time of the opening, in order to prevent an excessive lateral displacement of the cable, which could increase the risk of the bioptome jaws becoming trapped in the right disc loops.
The visibility on the transesophageal echocardiography is quite satisfactory, although a less experienced echocardiographist might occasionally be confused regarding the contour of the left disc, which may appear to be maladapted to the septum, especially while the prosthesis is attached to the delivery system. Normally, after release, it adapts best to the plane of the septum and its correct positioning then becomes clear. A low device profile was observed in all cases and good adaptability to the defect, with good rates of immediate closure, either by color Doppler echocardiogram or through the bubble test performed after implantation.
CONCLUSIONSThe CARDIA Ultrasept™ device is durable, easy to use, fully repositionable and reimplantable, has a low profile and has good occlusion rates compared with similar prostheses.
The short time of the follow-up did not allow for drawing conclusions regarding the effectiveness of the endothe lialisation on the rates of event recurrence or long-term complications.
The initial results are encouraging in all cases. Although this new device was used in septa with great mobility, its use in more complex scenarios (e.g., cases with large septal aneurysms) has yet to be tested.
CONFLICTS OF INTERESTFrancisco Chamie is a technical consultant and proctor of Neomex Hospital Ltda. (representative in Brazil of Cardia Inc. – Minneapolis, MN, USA). The remaining authors declare no conflicts of interest.