Transcatheter aortic valve implantation (TAVI) is an alternative for patients with aortic stenosis at high surgical risk and for many of those considered inoperable. Despite its minimally invasive features, complications related to the procedure may occur. Coronary obstruction during TAVI is a rare (incidence rate of less than 1%) but potentially lethal complication. In Brazil, this complication was found in 0.72% of procedures – three of 418 cases from Brazilian Transcatheter Aortic Valve Implantation Registry – with an in-hospital mortality rate of 100%. This case report presents prevention and treatment measures for coronary occlusion after TAVI.
O implante de valva aórtica transcateter (TAVI) é uma alternativa para pacientes com estenose aórtica de alto risco cirúrgico e para muitos daqueles considerados inoperáveis. Apesar de sua característica minimamente invasiva, podem ocorrer complicações relacionadas ao procedimento. Obstrução coronária durante o TAVI é uma complicação rara, com incidência inferior a 1%, mas potencialmente letal. Em nosso país, essa complicação foi encontrada em 0,72% dos procedimentos − 3 de 418 casos do Registro Brasileiro de Implante de Bioprótese Aórtica por Cateter − com mortalidade hospitalar de 100%. Apresentamos, neste relato de caso, medidas de prevenção e tratamento de oclusão coronária após o TAVI.
Transcatheter aortic valve implantation (TAVI) is the treatment of choice for patients with inoperable aortic stenosis, provided that their life expectancy is greater than 12 months. TAVI is still an alternative to surgery for high-risk patients with a Society of Thoracic Surgeons (STS) score of > 8 and < 15.1 Although less invasive, TAVI is characterized by a substantial rate of serious (fatal and nonfatal) complications, especially coronary occlusion (CO). European guidelines consider that a patient is at high risk of CO when presenting asymmetric valve calcification, a low coronary ostium, and a small sinus of Valsalva. High risk of occlusion is a relative contraindication for TAVI.2
CO during TAVI is a rare event, occurring in less than 1% of cases in the registries and in more recent clinical trials. However, it represents a potentially lethal complication.3–6 In the Brazilian Transcatheter Aortic Valve Implantation Registry, CO occurred in 3 (0.72%) of 418 procedures, with an in-hospital mortality rate of 100%.4,5
Since the first CO report related to TAVI in humans, in 2006,7 there has been an effort by researchers to identify predictors of complication. However, while the use of TAVI is favored even in those cases of higher risk of CO, some measures can be taken to prevent and treat CO, such as those presented in this case report.
Case reportThis report describes the case of a 78-year old female, presenting heart failure (functional class III) caused by severe aortic valve stenosis. She had a medical history of coronary artery disease treated with percutaneous coronary intervention and implantation of bare-metal stents in 2010; permanent pacemaker implantation in 2002; hypertension; an asymptomatic 65% right internal carotid lesion; systemic lupus erythematosus; chronic hepatitis C; and total left hip prosthesis implanted in 2009.
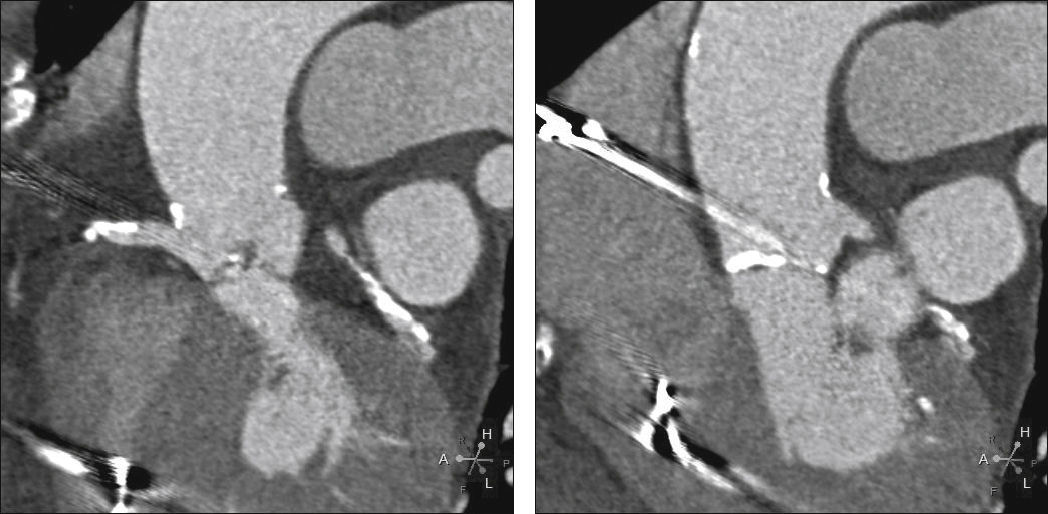
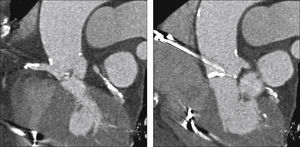
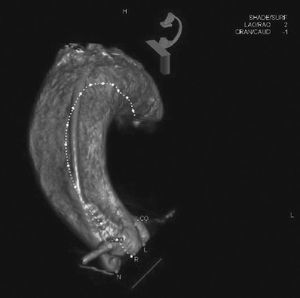
Echocardiography showed tricuspid aortic valve with significant calcification, reduced mobility, mild regurgitation, and an area of 0.55cm2; maximum and average transvalvular gradients of 70 and 43mmHg, respectively; and normal left ventricular (LV) systolic function. Coronary angiography showed intra-stent restenosis of 90% in the right coronary artery (RCA) and of 70% in the left anterior descending artery (LADA), as well as segmental stenosis of 90% in the left circumflex artery (LCx). Computed tomography (CT) angiography revealed aortic annulus diameter of 21mm, sinus of Valsalva diameter of 27mm, sinus of Valsalva/annulus diameter ratio of 1.29, RCA ostium height of 8.6mm, and left coronary artery (LCA) height of 10mm. A left coronary cusp length of 11mm was observed, while the right cusp measured 11.5mm (Fig. 1). Laboratory results were normal: creatinine of 1.1mg/dL and glomerular filtration rate estimated at 49mL/min. The estimated risk of death according to the STS score was 12.5%.
Computed tomography with electrocardiographic synchronization. The distance from the annulus to the right coronary artery was 8.6mm and the length of the right leaflet was 11.5mm. Extensive calcification of the aortic valve, particularly in non-coronary and right coronary leaflets, is observed.
Given the high risk of aortic valve replacement surgery as well as the preference of the patient, the therapeutic decision favored TAVI after percutaneous treatment of the coronary lesions.
TAVI was performed in an Artis Zeego (Siemens Healthcare Sector, Forchheim, Germany) hybrid room, with the patient under general anesthesia and monitored by transesophageal echocardiography. The right femoral arterial access was obtained by dissection. The left femoral venous access was percutaneously obtained for placement of a temporary pacemaker into the right ventricle (RV). The left femoral artery access with a 6 F sheath was percutaneously established for the placement of a guide catheter, and the right radial artery access with a 6 F sheath was established for the placement of a pigtail catheter.
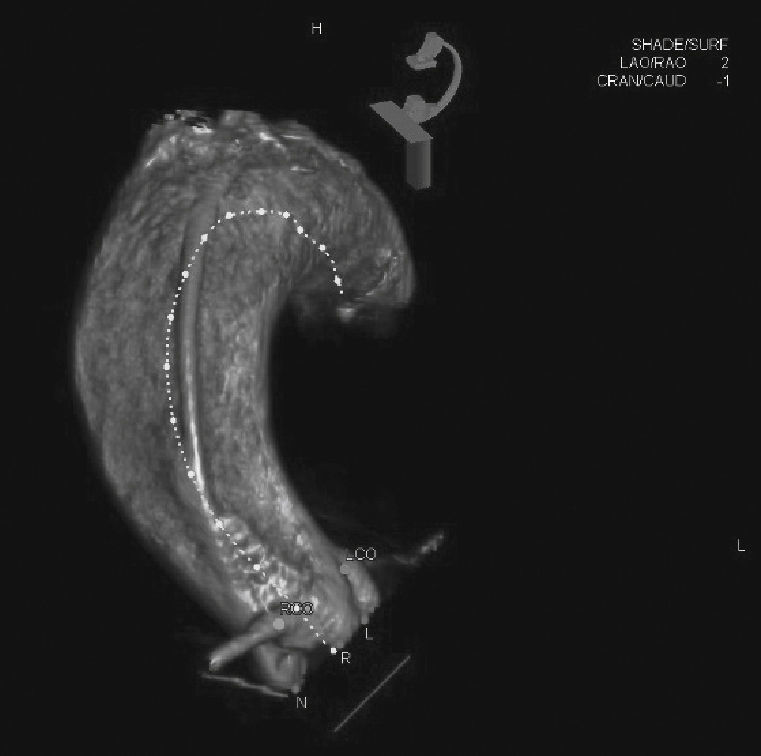
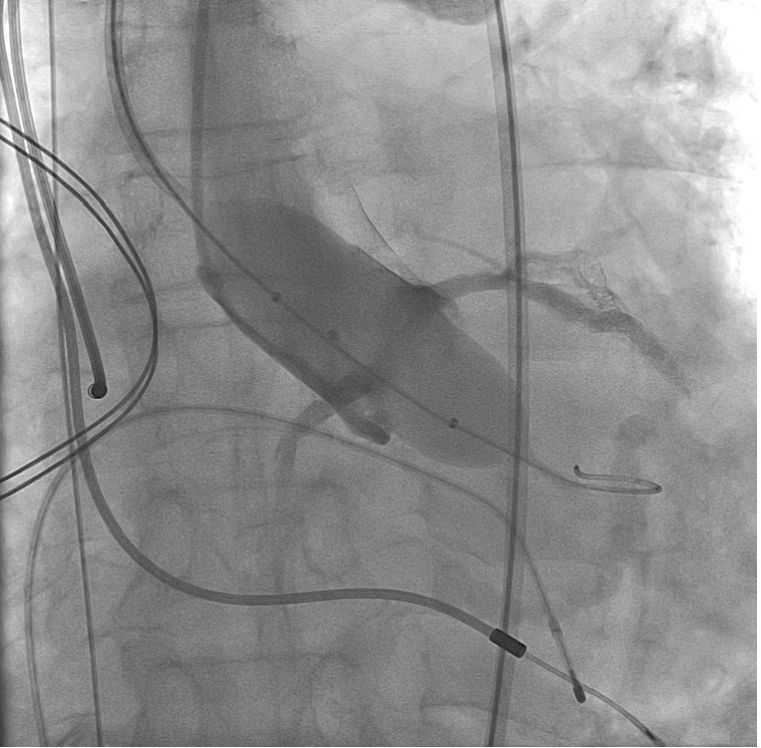
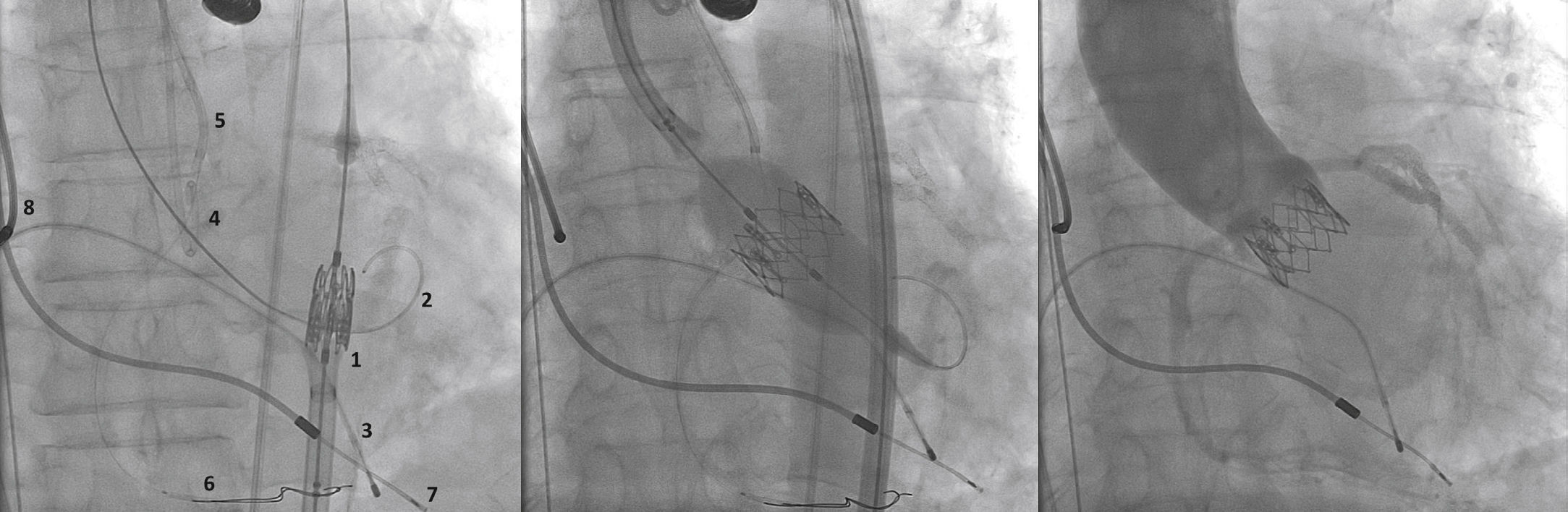
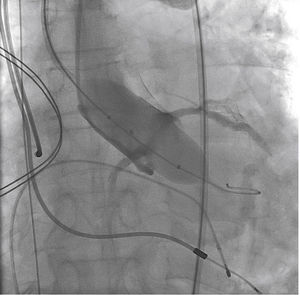
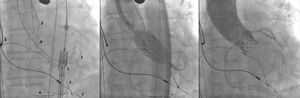
A rotational aortography with 3D reconstruction was obtained for identification of the projection with the alignment of the three cusps in the same plane (Fig. 2), which was used to release the prosthesis. The basal peak-to-peak transvalvular aortic gradient was 43mmHg. An Amplatz Super StiffTM 0.035-inch rigid guide wire (Boston Scientific Corporation, Natick, USA) was introduced into the LV and, over this guide wire, a 20mm/40mm Crystal valvuloplasty balloon (Balt, Montmorency, France) was advanced, positioned in the aortic valve and inflated to its nominal diameter, under pacemaker-induced tachycardia. At that moment, an aortography with the pigtail catheter positioned into right coronary sinus was performed. There was no impairment of the right or left coronary flow during maximum balloon inflation (Fig. 3). Despite this, it was decided to adopt an RCA protection strategy. The RCA was catheterized with a JR4 6F guide catheter (Medtronic, Minneapolis, USA), and two 0.014-inch BHW guide wires (Abbott Vascular, Santa Clara, USA) were positioned distally into the RCA. A 4.5mm/12mm Liberté stent (Boston Scientific Co., Natick, USA) was placed distally into the RCA, guaranteeing stability to the percutaneous coronary intervention system as well as immediate positioning and release into the ostium, if necessary. For TAVI, the guide catheter was withdrawn and positioned just above the sinus of Valsalva (Fig. 4A). Sapien XT 23mm valve (Edwards Lifesciences, California, USA) was advanced over the rigid guide wire and positioned in the aortic valve, in order to release the prosthesis lower (60% ventricular and 40% aortic) than usual (60% aortic and 40% ventricular). After angiographic studies for fine-tuning the positioning, the prosthesis was released under a heart rate of 180 beats per minute, with full balloon inflation (Fig. 4B). The control angiography showed minimum LV reflux and normal contrast of both coronary arteries (Fig. 4C). Subsequently, the percutaneous coronary intervention system was easily removed. The final manometry showed absence of transvalvular gradient. A control transesophageal echocardiogram showed mild periprosthetic aortic regurgitation and maximum gradient of 10mmHg. The patient was extubated in-room and transferred to the intensive care unit in a clinically and hemodynamically stable condition.
(A) On the left, demonstration of the therapeutic armamentarium: balloon expandable prosthesis release system into descending aorta (1) over Amplatz SuperStiffTM guide wire with its end into the left ventricle (2); a temporary pacemaker electrode into the right ventricle (3); pigtail catheter into the right coronary sinus (4); JR 6 F guide catheter (5) above the sinus of Valsalva; two 0.014-inch Extra Support guide wires and coronary stent placed distally into the right coronary artery (6); permanent pacemaker electrodes into the right ventricle and atrium (7 and 8) (B). Release of balloon expandable aortic valve prosthesis, with percutaneous coronary intervention system positioned into the right coronary artery. (C) Control aortography showing right and left coronary artery patency and minimum aortic regurgitation.
CO after TAVI is a rare event, with a mortality rate over 40%. This complication can be reasonably anticipated based on the anatomical details derived from CT angiography and from aortography during valve pre-dilation with balloon. Although the literature is still scarce, there is information indicating risk factors. The main predictors are female gender, balloon expandable prosthesis, sinus of Valsalva < 28mm, coronary ostium height < 10mm, and TAVI for treating bioprosthesis dysfunction (valve-in-valve).
Among the mechanisms for CO, the following should be mentioned: (1) ostium covering by a poorly positioned prosthesis, or by a well-placed prosthesis, but in a low-lying coronary ostium patient; (2) leaflet displacement with gross ostium calcification; (3) calcium debris; (4) aortic dissection; (5) leaflet migration towards the ostium through avulsion; and (6) displacement of a mobile, greater-length leaflet relative to the sinus of Valsalva.8 In the largest registry of coronary obstruction during TAVI, Ribeiro et al.6 analyzed 44 cases (0.66%) from 6,688 procedures in 81 centers across four continents. The LCA was involved in 88.6% of cases, with most patients showing acute signs of severe hypotension, ST-segment changes, and ventricular arrhythmias. In three patients (6.8%), death occurred within minutes, not allowing any coronary artery bypass graft procedure. Angioplasty was performed in 75% of cases, with an 81.8% success rate. The overall 30-day and 12-month mortality rates were 40.9% and 45.5%, respectively. The mortality of patients with successful angioplasty was 22.2%, and 100% in those whose angioplasty failed. For those patients who opted for coronary artery bypass graft (14%), mortality was 50%. Circulatory support was required in 36% of patients. There were no cases of stent thrombosis or need for coronary artery bypass graft of the target lesion in a mean follow-up of 12 months.
In the same study by Ribeiro et al.,6 the following predictors were identified for coronary obstruction: female gender, valve-in-valve procedure, balloon expandable prosthesis implantation (0.81% vs. 0.31% occurrence with auto-expandable prostheses), sinus of Valsalva diameter < 30mm, and left coronary ostium height < 12mm. Regarding LCA ostium height, the mean was 11mm among those patients with post-TAVI coronary obstruction, and 13mm in those without this complication. In women, the mean LCA ostium height was even lower (10mm); and in 96% of those with obstruction, their LCA ostium height was < 12mm. LCA obstruction was uncommon (only 11% of cases), a fact probably related to its often higher origin; however, it was not possible to estimate a safe cut-off point due to the small number of cases.
Other possible predictors include coronary obstruction observed during aortic balloon valvuloplasty with simultaneous aortography,9 and a leaflet length/curve length of sinus of Valsalva ratio > 1, as shown by Okuyama et al.8
Although the present report does not describe a case of coronary obstruction treated by the safety technique utilized, the risk of occurrence of this complication justifies the action taken, as the patient was a female with RCA ostium height < 10mm and with sinus of Valsalva diameter of 27mm. A valve pre-dilation balloon with 1:1 ratio for the valve annulus was used to assess coronary flow during inflation. There was no right or left coronary flow impairment. Aortography performed during pre-dilation with a balloon with a smaller diameter than that of the annulus may underestimate the risk of CO, because the leaflets are not fully displaced, as occurs after implantation of the prosthesis. The two 0.014-inch Extra Support guide wires and the stent distally positioned into RCA maintained perfect stability of the system, with guide catheter retrocession during positioning and release of the prosthesis. One of the recommendations, based on the expert opinion of the management of these high risk patients for occurrence of CO in TAVI, is to leave a percutaneous coronary intervention guide wire into the coronary artery;6,9 however, the authors had no access to any publication with a recommendation of distal stent placement into the coronary artery of interest, except for valve-in-valve.10 This maneuver provides a rapid reversal of any possible CO. Another measure was to release the prosthesis placed in a lower position (60% ventricular and 40% aortic) than usual in order to avoid covering the coronary ostia with bioprosthesis’ metallic struts.
The authors also believe that, given the need to start the circulatory support, the placement and positioning of 0.035-inch teflonized guide wires into the superior vena cava and into the contralateral femoral artery are justifiable in patients at higher risk of hemodynamic instability. Finally, the choice of repositionable or recapturable prostheses can provide greater safety for these patients.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.