Basmati rice is in high demand in the world market because of its fine, soft, long and distinctive aromatic grains. Bacterial leaf blight (BLB), caused by Xanthomonas oryzae pv. oryzae, is a destructive disease, resulting in enormous yield loss in basmati rice. Chemical management against BLB is effective but not the preferred approach because of its detrimental effects on humans, environment and ecological disruption. In the present study, seed treatment, seedling treatment and foliar application with microbial antagonists, such as Trichoderma harzianum and Pseudomonas fluorescens, and a consortium encompassing both microorganisms plus Trichoderma asperellum, were assessed against BLB in basmati rice. Among all the bio-formulation treatments, seed treatment with the microbial consortium revealed significant (p<0.05) results, achieving maximum inhibition of BLB incidence, intensity and lesion length of 48.56, 52.42 and 45.41 percent respectively, compared to the untreated control. Simultaneously, the same treatment also resulted in significant (p<0.05) enhancement of growth attributes, such as panicle length (55.83%), plant fresh weight (15.55%), grains per panicle (38.23%), test weight (55.49%) and yield (47.68%). A maximum increase in superoxide dismutase and hydrogen peroxide activities by 81.58 and 84.29 percent respectively over the control was also recorded. The results of the study suggest that the effectiveness of BLB management under field conditions applying the microbial consortium (T. harzianum+T. asperellum+P. fluorescens) was similar to that of the chemical control. Further, efforts are being made to evaluate the biocontrol activity of the microbial consortium in large-scale field experiments and its commercial potential.
El arroz basmati es sumamente valorado en el mercado global por sus granos largos, finos, suaves y aromáticos. El tizón bacteriano de las hojas (BLB), causado por Xanthomonas oryzae pv. oryzae, representa una seria amenaza en el cultivo del arroz basmati, generando pérdidas significativas en el rendimiento. Aunque el manejo químico de este patógeno ha demostrado eficacia, esta no es una práctica sostenible debido a sus efectos negativos sobre la salud humana, el ambiente y el equilibrio ecológico. En este estudio se evaluaron distintos antagonistas microbianos (Trichoderma harzianum, Pseudomonas fluorescens y un consorcio que incluyó dichos microorganismos más Trichoderma asperellum) y diferentes métodos de aplicación (tratamiento de semillas, de plántulas y aplicación foliar) para el manejo del BLB en el cultivo de arroz basmati. El tratamiento con el consorcio microbiano aplicado en semillas fue el más eficaz, logrando reducciones significativas (p<0,05) en la incidencia (48,56%), la intensidad (52,42%) y la longitud de la lesión (45,41%) respecto al control. Este tratamiento también promovió un aumento notable en indicadores agronómicos como la longitud de la panícula (55,83%), el peso fresco de la planta (15,55%), el número de granos por panícula (38,23%), el peso de la prueba (55,49%) y el rendimiento (47,68%). Además, se observaron incrementos en la actividad de superóxido dismutasa (81,58%) y peróxido de hidrógeno (84,29%), lo que indica una respuesta de defensa mejorada. Se concluye que el consorcio microbiano evaluado representa una alternativa eficaz y sostenible para reducir el impacto del BLB en el cultivo de arroz basmati. Se están llevando a cabo estudios para evaluar su aplicación a gran escala y el potencial comercial.
Basmati is an extraordinarily premium, long-grain rice variety characterized by a pleasant aroma32. Because of its fine, soft and nutrient-rich grains, basmati is highly demanded in international, national and regional markets. India is the leading producer of basmati rice, with 74 percent of the world's supply, followed by Pakistan, the Philippines and China27. Basmati rice is also well known for its health benefits, including good bioavailability of cystine, vitamin B1 and melatonin, its low glycemic index and its role in fighting diabetes2. However, the productivity of basmati rice is threatened due to the outbreak of several biotic stresses, such as bacterial leaf blight, blast and bakanae disease22.
Bacterial leaf blight (BLB), caused by Xanthomonas oryzae pv. oryzae (Xoo), is one of the major diseases affecting basmati rice. It is responsible for 20–80 percent of yield loss in India41, 30 percent in Japan44, 50 percent in Malaysia35, 80 percent in Indonesia43, 50 percent in the Philippines3, 20–40 percent in Bangladesh16 and up to 80 percent in Mali45. Diverse pathogenic variations among the Xoo populations have been reported in the basmati-growing regions of Jammu and Kashmir6. Xoo penetrates the host through hydathodes and wounds present on the surface of the plant, eventually affecting the parenchymatous cells of xylem, traveling vertically and clogging the xylem vessels. Subsequently, bacterial ooze is observed coming out from the hydathodes21. Xoo disease-causing ability is dependent on the type-3 secretion system (T3SS), which is important for infection of susceptible hosts and plant defense activation15.
Management of bacterial leaf blight can be achieved through chemical means, but the overuse of the antibiotic tetracycline causes several allergic and metabolic disorders in humans, potentially resulting in anti-microbial resistance4. The alternative to chemical pesticides is the biological management of plant diseases, which is efficient and effective through the production of antibiotics, hydrogen cyanide, siderophores, lytic enzymes and antimicrobial compounds against phytopathogens. Moreover, biocontrol improves the growth and development of the host plant34 by unveiling bio-stimulant traits, such as increasing the bioavailability and solubilization of various soil nutrients for plant growth, stimulating phytohormones such as auxins, cytokinins and gibberellins, and producing metabolites that improve tolerance to abiotic stress9. Biocontrol agents such as Trichoderma spp., Pseudomonas spp. and Bacillus spp. are known to exhibit several bioinsecticidal, bionematicidal and biopesticidal activities12, and are used individually or in combination as a consortium.
A microbial consortium is a combination of multiple strains or species of beneficial microbes gaining more importance than a single inoculant because of their multifaceted mechanisms of disease mitigation, enhancement of soil properties and improvement of growth and yield parameters of the host plant25. Further, the occasional inconsistent, ineffective and variable performance of single inoculants influenced by varying environmental conditions limit their competitiveness and effectiveness against plant pathogens46. The application of synthetic microbial communities (SynComs) to improve crop productivity by mitigating plant diseases, improving soil and plant health is a major area of research in this decade8. Therefore, this investigation was aimed at: (i) evaluating the microbial consortium against bacterial leaf blight in basmati rice and (ii) studying the effect of the consortium on plant growth and induced systemic resistance (ISR).
Materials and methodsExperimental detailsThe seed of Bamati-370 used in the experiment was obtained from the Mega Seed Unit of SKUAST-Jammu, Chatha. The biocontrol formulation of each inoculant along with the microbial consortium consisting of Trichoderma harzianum (GenBank accession no. MN721820), Trichoderma asperellum (GenBank accession no. MT395682), and Pseudomonas fluorescens (GenBank accession no. KJ194131), along with the pathogen X. oryzae pv. oryzae (GenBank accession no. MN718665) were obtained from the Biocontrol Laboratory, Advanced Centre for Horticulture Research, SKUAST-Jammu, Udheywala.
Artificial inoculation of X. oryzae pv. oryzaeX. oryzae pv. oryzae (Xoo) was mass-multiplied in a nutrient broth medium (Hi-media) and artificially inoculated onto the paddy crop using the leaf clipping method18. The top portion of fully developed leaves of Basmati-370, measuring between 2.5 and 6cm, were clipped off using sterilized scissors that had already been dipped in a suspension of Xoo inoculum (1×108CFU/ml) during the tillering stage.
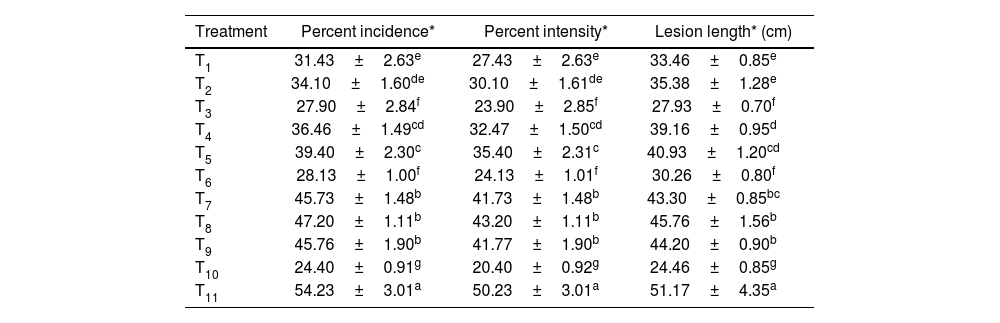
Effectiveness of the microbial consortium against bacterial leaf blight of rice under field conditionsExperimental plots were set up in the Research Field of the Division of Plant Pathology (32°39.1749′ N; 74°48.4268′ E) during the Kharif season of 2023–2024. Rice variety Basmati-370 was planted in plots (3×4m2) arranged in a randomized block design (RBD) with three replications. Each plot had rows spaced 20cm apart and plants spaced 15cm apart. Eleven treatments were applied to manage bacterial leaf blight of rice, whose details are mentioned in Table 1. Prior to sowing, the seeds were surface-sterilized with 0.1 percent of sodium hypochlorite. After sterilization, seeds were soaked separately in each inoculant and the microbial consortium consisting of T. harzianum, P. fluorescens and T. asperellum, then shade-dried and sown in plots on 24th June, 2023. Additionally, seedling treatment was carried out by soaking 30 day-old-seedlings of untreated plants for 20min in their respective treatments consisting of single inoculants and the microbial consortium. Similarly, foliar application of the respective bioagents and streptocycline+copper oxychloride was carried out using a knapsack sprayer pump after the 7th day of pathogen inoculation.
Different treatments tested for the management of bacterial leaf blight in rice.
| Treatment | Details |
|---|---|
| T1 | Seed treatment with Trichoderma harzianum @ 1×107spores/ml |
| T2 | Seed treatment with Pseudomonas fluorescens @ 1×108CFU/ml |
| T3 | Seed treatment using the microbial consortium [T. harzianum (1×107spores/ml)+T. asperellum (1×107spores/ml)+P. fluorescens (2×108CFU/ml)] |
| T4 | Seedling treatment with Trichoderma harzianum @ (1×107spores/ml) |
| T5 | Seedling treatment with Pseudomonas fluorescens @ (1×108CFU/ml) |
| T6 | Seedling treatment with the microbial consortium [T. harzianum (1×107spores/ml)+T. asperellum (1×107spores/ml)+P. fluorescens (2×108CFU/ml)] |
| T7 | Foliar application with Trichoderma harzianum at 0.1 percent (1×107spores/ml) |
| T8 | Foliar application with Pseudomonas fluorescens at 0.1 percent (1×108CFU/ml) |
| T9 | Foliar application using the microbial consortium at 0.1 percent [T. harzianum (1×107spores/ml)+T. asperellum (1×107spores/ml)+P. fluorescens (2×108CFU/ml)] |
| T10 | Foliar application with streptocycline (40g)+copper oxychloride (200g) |
| T11 | Control (untreated) |
Fresh leaf samples were collected from each plot after seven days of pathogen inoculation and assessed for the quantification of superoxide dismutase and hydrogen peroxide (H2O2)42.
Observations recordedThe disease attributes such as percent incidence, percent intensity,38 lesion length (cm) of BLB were recorded after 14 days of Xoo inoculation. Growth attributes such as fresh and dry root weight (g) were recorded at 30, 60 and 90 days after transplant (DAT), and panicle length (cm), plant fresh weight (g), grains per panicle, test weight (g), and yield (q/ha) were recorded at the time of harvesting.
Statistical analysisData were analyzed using ANOVA with Duncan multiple range tests in R software, and the correlation analysis was performed using the psych package of R software48. The graphical plots were made using Originlab software10. Additionally, the correlation analysis was performed among growth attributes (panicle length, plant fresh weight, fresh and dry root weight at 30, 60 and 90 DAT, number of grains per panicle, test weight and yield), biochemical attributes (SOD and H2O2) and disease attributes (percent intensity, incidence and lesion length) to determine the level of association of each attribute with the incidence of bacterial leaf blight.
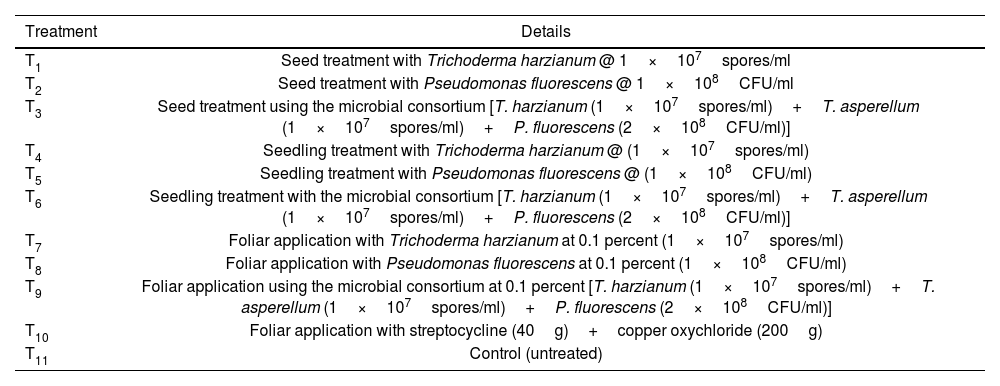
ResultsEffect of the microbial consortium on the incidence, intensity and lesion length of bacterial leaf blight of rice under field conditionsThe effectiveness of the microbial consortium (T. harzianum+T. asperellum+P. fluorescens), single inoculants (T. harzianum and P. fluorescens) and streptocycline+copper oxychloride were assessed against the bacterial leaf blight caused by X. oryzae pv. oryzae (Xoo). Streptocycline+copper oxychloride exhibited minimum percent disease incidence, percent intensity and lesion length (cm) of 24.40±0.91, 20.40±0.92 and 24.46±0.85, respectively, followed by seed treatment with the microbial consortium (T. harzianum+T. asperellum+P. fluorescens) resulting in 27.90±2.84, 23.90±2.85 and 27.93±0.70cm, respectively. Maximum percent incidence of 54.23±3.01, percent intensity of 50.23±3.01 and lesion length of 51.23±4.35cm was recorded in the control (Table 2 and Fig. 1A).
Effect of the microbial consortium on percent disease incidence, intensity and lesion length of bacterial leaf blight in rice.
| Treatment | Percent incidence* | Percent intensity* | Lesion length* (cm) |
|---|---|---|---|
| T1 | 31.43±2.63e | 27.43±2.63e | 33.46±0.85e |
| T2 | 34.10±1.60de | 30.10±1.61de | 35.38±1.28e |
| T3 | 27.90±2.84f | 23.90±2.85f | 27.93±0.70f |
| T4 | 36.46±1.49cd | 32.47±1.50cd | 39.16±0.95d |
| T5 | 39.40±2.30c | 35.40±2.31c | 40.93±1.20cd |
| T6 | 28.13±1.00f | 24.13±1.01f | 30.26±0.80f |
| T7 | 45.73±1.48b | 41.73±1.48b | 43.30±0.85bc |
| T8 | 47.20±1.11b | 43.20±1.11b | 45.76±1.56b |
| T9 | 45.76±1.90b | 41.77±1.90b | 44.20±0.90b |
| T10 | 24.40±0.91g | 20.40±0.92g | 24.46±0.85g |
| T11 | 54.23±3.01a | 50.23±3.01a | 51.17±4.35a |
Different alphabetical letters on the superscript within the columns indicate significant differences among the treatments according to Duncan's multiple range test at p≤0.05.
T1 to T11 in the table correspond to the treatment details mentioned in Table 1.
Effects of the microbial consortium on rice. (A) Percentage reduction in the incidence, intensity, and lesion length of bacterial leaf blight; (B) percentage increase in fresh and dry root weight after 30, 60, and 90 days of transplanting; (C) percentage increase in panicle length, fresh weight, grains per panicle, test weight, and yield; (D) superoxide dismutase activity (U/ml) and hydrogen peroxide levels (nMol H2O2/g FW).
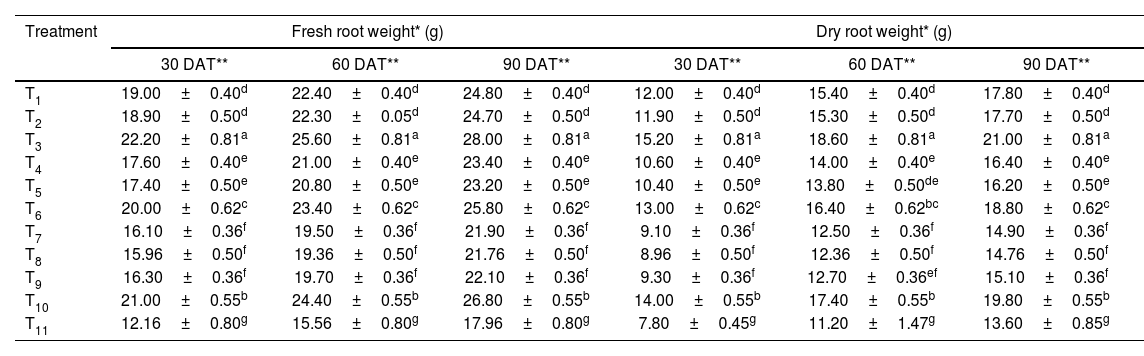
The maximum enhancement of fresh root weight was exhibited in the seed treatment involving the microbial consortium containing T. harzianum+T. asperellum+P. fluorescens, with values of 22.20±0.81, 25.60±0.81 and 28±0.81g at 30, 60 and 90 days after transplanting (DAT), respectively, followed by foliar treatment with streptocycline+copper oxychloride exhibiting 21±0.55, 24.40±0.55 and 26.80±0.55g at 30, 60 and 90 DAT, respectively. Additionally, minimum fresh root weight was observed in the control (12.160±0.80, 15.56±0.80, 17.96±0.80g, respectively). Seed treatment using the microbial consortium resulted in maximum dry root weight of 15.20±0.81, 18.60±0.81 and 21.00±0.81g after 30, 60 and 90 DAT respectively, followed by foliar application of streptocycline+copper oxychloride (14±0.55, 17.40±0.55 and 19.80±0.55g, respectively). Minimum dry root weight (7.80±0.45, 11.20±1.47 and 13.60±0.85g, respectively) was observed in the control (Table 3 and Fig. 1B).
Effect of the microbial consortium on fresh and dry root weight of rice plants after 30, 60 and 90 days of transplanting.
| Treatment | Fresh root weight* (g) | Dry root weight* (g) | ||||
|---|---|---|---|---|---|---|
| 30 DAT** | 60 DAT** | 90 DAT** | 30 DAT** | 60 DAT** | 90 DAT** | |
| T1 | 19.00±0.40d | 22.40±0.40d | 24.80±0.40d | 12.00±0.40d | 15.40±0.40d | 17.80±0.40d |
| T2 | 18.90±0.50d | 22.30±0.05d | 24.70±0.50d | 11.90±0.50d | 15.30±0.50d | 17.70±0.50d |
| T3 | 22.20±0.81a | 25.60±0.81a | 28.00±0.81a | 15.20±0.81a | 18.60±0.81a | 21.00±0.81a |
| T4 | 17.60±0.40e | 21.00±0.40e | 23.40±0.40e | 10.60±0.40e | 14.00±0.40e | 16.40±0.40e |
| T5 | 17.40±0.50e | 20.80±0.50e | 23.20±0.50e | 10.40±0.50e | 13.80±0.50de | 16.20±0.50e |
| T6 | 20.00±0.62c | 23.40±0.62c | 25.80±0.62c | 13.00±0.62c | 16.40±0.62bc | 18.80±0.62c |
| T7 | 16.10±0.36f | 19.50±0.36f | 21.90±0.36f | 9.10±0.36f | 12.50±0.36f | 14.90±0.36f |
| T8 | 15.96±0.50f | 19.36±0.50f | 21.76±0.50f | 8.96±0.50f | 12.36±0.50f | 14.76±0.50f |
| T9 | 16.30±0.36f | 19.70±0.36f | 22.10±0.36f | 9.30±0.36f | 12.70±0.36ef | 15.10±0.36f |
| T10 | 21.00±0.55b | 24.40±0.55b | 26.80±0.55b | 14.00±0.55b | 17.40±0.55b | 19.80±0.55b |
| T11 | 12.16±0.80g | 15.56±0.80g | 17.96±0.80g | 7.80±0.45g | 11.20±1.47g | 13.60±0.85g |
Different alphabetical letters on the superscript within the columns indicate significant differences among the treatments according to Duncan's multiple range test at p≤0.05.
T1 to T11 in table denote the treatment details mentioned in Table 1.
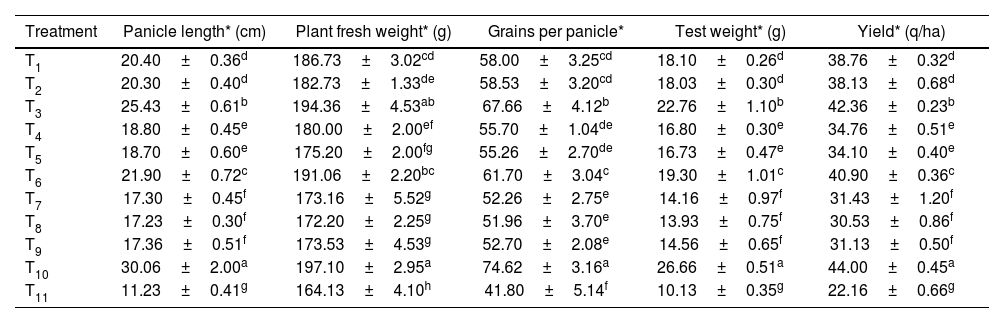
Among all the treatments, foliar application of streptocycline+copper oxychloride showed maximum enhancement of panicle length, plant fresh weight, grains per panicle, test weight and yield with 30.06±2cm, 197.10±2.95g, 74.62±3.16, 26.66±0.51g and 22.16±0.66q/ha, respectively, followed by seed treatment with microbial consortium, exhibiting 25.43±0.61cm, 194.36±4.53g, 67.66±4.12, 22.76±1.10g and 42.36±0.23q/ha, respectively. Furthermore, in the control, a panicle length of only 11.23±0.14cm, plant fresh weight of 164.13±4.10g, grains per panicle of 41.80±5.14, test weight of 10.13±0.35g and yield of 22.16±0.66q/ha were recorded (Table 4 and Fig. 1C).
Effect of the microbial consortium on plant growth promotion and yield attributes of rice.
| Treatment | Panicle length* (cm) | Plant fresh weight* (g) | Grains per panicle* | Test weight* (g) | Yield* (q/ha) |
|---|---|---|---|---|---|
| T1 | 20.40±0.36d | 186.73±3.02cd | 58.00±3.25cd | 18.10±0.26d | 38.76±0.32d |
| T2 | 20.30±0.40d | 182.73±1.33de | 58.53±3.20cd | 18.03±0.30d | 38.13±0.68d |
| T3 | 25.43±0.61b | 194.36±4.53ab | 67.66±4.12b | 22.76±1.10b | 42.36±0.23b |
| T4 | 18.80±0.45e | 180.00±2.00ef | 55.70±1.04de | 16.80±0.30e | 34.76±0.51e |
| T5 | 18.70±0.60e | 175.20±2.00fg | 55.26±2.70de | 16.73±0.47e | 34.10±0.40e |
| T6 | 21.90±0.72c | 191.06±2.20bc | 61.70±3.04c | 19.30±1.01c | 40.90±0.36c |
| T7 | 17.30±0.45f | 173.16±5.52g | 52.26±2.75e | 14.16±0.97f | 31.43±1.20f |
| T8 | 17.23±0.30f | 172.20±2.25g | 51.96±3.70e | 13.93±0.75f | 30.53±0.86f |
| T9 | 17.36±0.51f | 173.53±4.53g | 52.70±2.08e | 14.56±0.65f | 31.13±0.50f |
| T10 | 30.06±2.00a | 197.10±2.95a | 74.62±3.16a | 26.66±0.51a | 44.00±0.45a |
| T11 | 11.23±0.41g | 164.13±4.10h | 41.80±5.14f | 10.13±0.35g | 22.16±0.66g |
Different alphabetical letters on the superscript within the columns indicate significant differences among the treatments according to Duncan's multiple range test at p≤0.05.
T1 to T11 in the table correspond to the treatment details mentioned in Table 1.
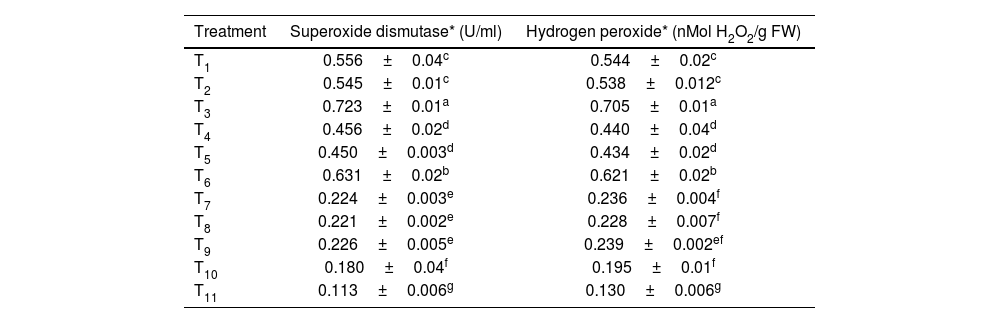
The seed treatment with the microbial consortium containing T. harzianum+T. asperellum+P. fluorescens, resulted in maximum superoxide dismutase and hydrogen peroxide with 0.723±0.01U/ml and 0.705±0.01nMol H2O2/g FW, respectively, followed by seedling treatment using the microbial consortium (0.631±0.02U/ml and 0.621±0.02nMol H2O2/g FW, respectively). Minimum activity of superoxide and hydrogen peroxide was recorded in the control with values of 0.113±0.006U/ml and 0.130±0.006nMol H2O2/g FW, respectively (Table 5 and Fig. 1D).
Effect of the microbial consortium on superoxide dismutase (U/ml) and hydrogen peroxide (nMol H2O2/g FW) of rice.
| Treatment | Superoxide dismutase* (U/ml) | Hydrogen peroxide* (nMol H2O2/g FW) |
|---|---|---|
| T1 | 0.556±0.04c | 0.544±0.02c |
| T2 | 0.545±0.01c | 0.538±0.012c |
| T3 | 0.723±0.01a | 0.705±0.01a |
| T4 | 0.456±0.02d | 0.440±0.04d |
| T5 | 0.450±0.003d | 0.434±0.02d |
| T6 | 0.631±0.02b | 0.621±0.02b |
| T7 | 0.224±0.003e | 0.236±0.004f |
| T8 | 0.221±0.002e | 0.228±0.007f |
| T9 | 0.226±0.005e | 0.239±0.002ef |
| T10 | 0.180±0.04f | 0.195±0.01f |
| T11 | 0.113±0.006g | 0.130±0.006g |
Different alphabetical letters on the superscript within the columns indicate significant differences among the treatments according to Duncan's multiple range test at p≤0.05.
T1 to T11 in the table correspond to the treatment details mentioned in Table 1.
The correlation analysis of several factors, such as percent disease intensity, fresh and dry root weights (30, 60 and 90 DAT), yield, test weight, panicle length, plant fresh weight and lesion length of bacterial leaf blight was conducted along with disease incidence, to determine the association between them. BLB intensity and disease lesion length were positively correlated with disease incidence, with a correlation coefficient (R) of 0.99 and 0.99, respectively. Moreover, fresh and dry root weight (30, 60, 90 DAT), yield, test weight, panicle length and plant fresh weight had a significantly negative correlation with disease incidence, with correlation coefficients (R) of −0.96, −0.96, −0.96, −0.95, −0.95, −0.95, −0.98, −0.93, −0.98, −0.93 and −0.91, respectively (Supplementary Figure 1).
Correlation analysis of superoxide dismutase (SOD) and hydrogen peroxide (H2O2) with percent incidence of BLB in rice plantsBiochemical factors, viz., superoxide dismutase (SOD) and hydrogen peroxide (H2O2) showed a significantly but moderately negative correlation with the incidence of BLB, with correlation coefficients (R) of −0.66 and −0.67, respectively (Supplementary Figure 2).
DiscussionBasmati is considered ‘the pearl of scent’, because of its texture, aroma, sweetness and long shelf life, having a distinct heritage and special geographical indication (GI). There has been increasing demand of basmati rice in local, national and international markets6. However, due to several biotic stresses, such as the bacterial leaf blight of rice caused by X. oryzae pv. oryzae, its production and productivity has been declining over time. Management of BLB with agro-chemicals has become inefficient and hazardous due to several consumer, environmental and sustainability-related issues. Resistance of bacteria against the antibiotics is also a concern in using such chemicals24. Fifty eight out of 107 isolates of Xanthomonas smithii subsp. citri have shown resistance against streptomycin, due to chromosomal mutations. Similarly, Clavibacter michiganensis has exhibited resistance against streptomycin because of the production of streptomycin-inactivating enzymes26. These negative effects of chemicals have led to the exploration of several other management strategies, such as biological management using various plant growth-promoting agents such as T. harzianum, T. asperellum and P. fluorescens29. Our research on exploring the effectiveness of the microbial consortium containing T. harzianum+T. asperellum+P. fluorescens, using biocontrol agents alone, along with chemicals, against bacterial leaf blight of rice have shown that, foliar application of streptocycline+copper oxychloride resulted in the maximum reduction of percent incidence, percent intensity and lesion length of bacterial leaf blight by 55.01, 59.39 and 52.19 percent, respectively, and exhibited maximum panicle length, plant fresh weight, number of grains per panicle, test weight and yield by 62.64, 16.73, 43.99, 62.00 and 49.62 percent, respectively. Cupric ions (Cu++), the active ingredient in copper oxychloride, caused denaturation of structural and enzymatic proteins along with alterations in membrane semipermeability, resulting in the death of phytopathogens14. Additionally, streptocycline produces several antibiotics and enhances host defense by generating inhibitors such as H2O2, siderophores and organic acids. The observed reduction in BLB incidence, intensity, and lesion length following the consortium application can be attributed to several synergistic mechanisms. First, both Trichoderma spp. and P. fluorescens are well-documented producers of antimicrobial compounds, such as antibiotics, siderophores, and lytic enzymes, which directly antagonize X. oryzae pv. oryzae (Xoo)31. Pseudomonas strains have been shown to produce siderophores and secondary metabolites, such as phenazines and rhamnolipids, which inhibit Xoo growth and disrupt pathogen colonization49. Similarly, Trichoderma spp. secretes chitinases and glucanases that degrade pathogen cell walls, further limiting disease progression30.
Due to several health hazards and environmental pollution, the current investigations to evaluate the effectiveness of biocontrol agents, either alone or in combination (T. harzianum+T. asperellum+P. fluorescens) were used to inhibit X. oryzae pv. oryzae causing bacterial leaf blight in basmati rice. The present study demonstrates that the application of a microbial consortium significantly suppressed bacterial leaf blight (BLB) and enhanced key agronomic parameters in Basmati rice under field conditions. These results are in agreement with recent literature, which highlights the multifaceted mechanisms through which beneficial microorganisms mediate plant disease suppression and growth promotion40.
This study revealed that seed treatment using the microbial consortium (T. harzianum+T. asperellum+P. fluorescens) reduced disease incidence by 48.56 percent, intensity by 52.42 percent and lesion length by 45.41 percent, and significantly enhanced panicle length, plant fresh weight, number of grains per panicle, test weight and yield by 55.83, 15.55, 38.23, 55.49 and 47.68 percent, respectively. The microbial consortium expressed multiple mechanisms, such as competition, hyperparasitism, secretion of volatile compounds, plant growth-promoting activity, and induction of defence mechanisms that led to increases in the growth of rice plants19. Maximum increase of fresh and dry root weight by 82.47 and 94.87 percent at 30 DAT, 64.45 and 66.07 percent at 60 DAT and 55.84 and 54.41 percent at 90 DAT were recorded with the seed treatment involving the microbial consortium. Beneficial microbes colonize the root of rice, helping in the proliferation of the roots and shoots of the host, which stimulates plant growth7. Applying T. harzianum to the rhizosphere is known to expand the root surface area, allowing the roots to explore a greater soil volume and absorb more nutrients. This enhanced nutrient uptake leads to substantial improvements in the growth and yield of rice50. Applying P. fluorescens (PDY7) has shown better results with a maximum number of grains per panicle of 18.28g as compared to the control (9.11g)47. Bacillus cereus has shown maximum increase in fresh root weight of rice by 31.4 percent and the application of the microbial consortium (T. harzianum+P. fluorescens+herbal kunapajala) resulted in the maximum dry root weight of rice36. These enhancements are likely mediated by the plant growth-promoting properties of the constituent microorganisms. Both Trichoderma and Pseudomonas spp. are known to solubilize inorganic phosphate, produce phytohormones, such as indole acetic acid, and facilitate nutrient uptake, leading to improved root and shoot development33. The increased root biomass observed in this study is consistent with reports that microbial inoculants enhance root architecture, enabling better water and nutrient acquisition and contributing to overall plant vigor40.
In addition to direct antagonism, the microbial consortium appears to induce systemic resistance in rice plants. This is evidenced by the significant increase in superoxide dismutase (SOD) activity and hydrogen peroxide (H2O2) concentration observed in consortium-treated plants23. Our study revealed that seed treatment using the microbial consortium enhanced the amount of superoxide dismutase and hydrogen peroxide by 81.58 and 84.29 percent, respectively. SOD is the reason for detoxification of intercellular O2− and secretion of H2O2, removing the dangerous reactive oxygen species (ROS) which results in reduced fitness and fungus virulence5,17. H2O2 is sensitive to pathogens but not to the host because H2O2 strengthens the cell wall by aiding in the process of lignification, contributing to the cross-linking of cell wall polymers and proline or hydroxyproline-rich proteins during pathogen invasion, suppressing the growth and development of the invading pathogen20. Previous studies have shown that inoculation with plant growth-promoting rhizobacteria (PGPR) such as Pseudomonas spp. can enhance the activity of defense-related enzymes, including peroxidase, catalase, and phenylalanine ammonia-lyase, thereby conferring increased resistance to BLB28. The use of a microbial consortium offers advantages over single-strain inoculants. The combination of multiple beneficial microbes ensures functional redundancy and complementary modes of action, which can buffer against environmental variability and enhance overall effectiveness. This is supported by field studies demonstrating that co-inoculation with multiple antagonistic bacteria or fungi results in greater disease suppression and yield improvement compared to individual strains11.
In the correlation analysis, disease intensity and lesion length exhibited a significantly positive correlation with the incidence of bacterial leaf blight (BLB) of rice. Our findings are in line with earlier studies showing similar results39. Conversely, panicle length, plant fresh weight, fresh root weight at 30, 60 and 90 days after transplanting (DAT), dry root weight at 30, 60, and 90 DAT, number of grains per panicle, test weight, and yield, all showed a negative correlation with BLB incidence, as reported by earlier studies13. The application of the microbial consortium increased the uptake of nutrients, the production of siderophores and phytohormones, such as auxins and IAA, resulting in disease mitigation and plant growth promotion37. The correlation analysis of hydrogen peroxide (H2O2) concentration and superoxide dismutase activity (SODs) with the incidence of bacterial leaf blight revealed a significantly negative correlation. Biocontrol agents are known to trigger induced systemic resistance by increasing the activity of several enzymes SOD and H2O2, PPO and CAT1.
ConclusionSeed treatment using the microbial consortium (T. harzianum+T. asperellum+P. fluorescens) proved its effectiveness by reducing percent disease incidence, percent intensity and lesion length of BLB, simultaneously enhancing shoot and root weights, yield and biochemical parameters of rice. These results encourage the use of the microbial consortium for increasing the production of basmati rice by organic means. There is an urgent need for further research to explore the effectiveness of the microbial consortium against several other Basmati rice diseases in large-scale field experiments.
ORCID IDFayaz Ahmad Mohiddin: 0000-0001-9720-4429
FundingThe researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University, for financial support (QU-APC-2024-9/1).
Conflict of interestThe authors declare no conflict of interest.