Infections caused by the genus Candida have acquired considerable significance in recent years due to the enhanced susceptibility of immunocompromised hosts. There have been increasing reports of multidrug resistance (MDR) in several Candida species, posing a major hurdle to antifungal therapy. Accordingly, exploring and developing novel anti-Candida agents has become a priority. In this study, we assessed the antifungal activity of seven methanolic extracts from the Argentinian native plants Peltophorum dubium, Schinus areira, Parastrephia quadrangularis and Lantana balansae against clinical isolates of different wild-type and MDR isolates of Candida. Synergism with fluconazole was also evaluated. All plant extracts showed antifungal activities against different Candida species, including MDR isolates such as C. haemulonii. Highly active extracts from these native plants provide promising sources of compounds for potentiating the antifungal effect of fluconazole. Further investigation of the chemical constituents of the extracts and their cytotoxicity is needed to develop plant-derived anti-Candida agents.
Las infecciones causadas por el género Candida han adquirido una importancia considerable en los últimos años debido a la mayor susceptibilidad de los hospedadores inmunocomprometidos. Se reportan cada vez más casos de aislamientos multidrogorresistentes (MDR) en varias especies de Candida, lo que representa un gran desafío para la terapia antifúngica. En consecuencia, se ha vuelto fundamental explorar y desarrollar nuevos agentes anti-Candida. En este estudio, evaluamos la actividad antifúngica de siete extractos metanólicos de las plantas nativas argentinas Peltophorum dubium, Schinus areira, Parastrephia quadrangularis y Lantana balansae contra aislamientos clínicos de diferentes especies de Candida, tanto de tipo silvestre como MDR. También se evaluó el sinergismo con fluconazol. Todos los extractos vegetales mostraron actividad antifúngica contra diversas especies de Candida, incluyendo aislamientos MDR, como uno de Candida haemulonii. Los extractos con elevada actividad provenientes de estas plantas nativas representan fuentes prometedoras de compuestos para potenciar el efecto antifúngico del fluconazol. Es necesario investigar más a fondo los constituyentes químicos de los extractos y su citotoxicidad para desarrollar agentes anti-Candida derivados de las plantas.
.
Candida species are among the most common human fungal pathogens, causing both superficial (mucosal and cutaneous) and systemic infections10. Invasive Candida infections are an important cause of morbidity and mortality, especially in immunocompromised or critically ill patients in intensive care units1.
For fungal infections there are only a few classes of antimycotic drugs on the market, such as polyenes, azoles, echinocandins, and a few molecules are under trial11. Although multidrug resistance (MDR) is uncommon, there have been increasing reports of MDR to azoles, echinocandins, and polyenes in several Candida species, most notably C. glabrata and, more recently, in the emerging opportunistic pathogens C. auris and species of the C. haemulonii complex, indicating an insufficient choice of available medications5. This situation has led us to look for other alternative treatment options. The pharmacotherapeutic use of natural compounds derived from plants, capable of controlling microorganisms such as Candida, has become a feasible option for further study and use.
In this regard, the vast territory and diverse geographical characteristics of Argentina, along with its resulting climatic diversity make it an important source of biological resources suitable for the search of new compounds with potential utility in the pharmaceutical or medicinal industry2,8. The objective of this study was to evaluate the antifungal activity of seven methanolic extracts from four native plants from Argentina (Schinus areira, Peltophorum dubium, Parastrephia quadrangularis, and Lantana balansae) against clinical isolates of different wild-type and MDR isolates of Candida recovered from patients with candidemia. Their synergism with the azole antifungal fluconazole was also evaluated.
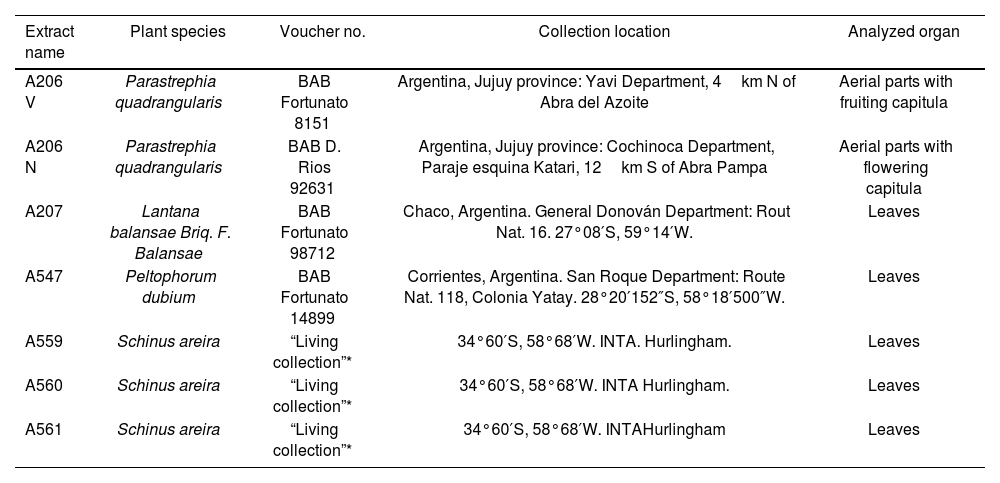
The plant specimens used were collected and identified by R.H Fortunato (Table 1). The voucher specimens were deposited in the BAB Herbarium: http://sciweb.nybg.org/Science2/IndexHe rbariorum.asp: BAB.
Plant species used, collected in Argentina.
| Extract name | Plant species | Voucher no. | Collection location | Analyzed organ |
|---|---|---|---|---|
| A206 V | Parastrephia quadrangularis | BAB Fortunato 8151 | Argentina, Jujuy province: Yavi Department, 4km N of Abra del Azoite | Aerial parts with fruiting capitula |
| A206 N | Parastrephia quadrangularis | BAB D. Rios 92631 | Argentina, Jujuy province: Cochinoca Department, Paraje esquina Katari, 12km S of Abra Pampa | Aerial parts with flowering capitula |
| A207 | Lantana balansae Briq. F. Balansae | BAB Fortunato 98712 | Chaco, Argentina. General Donován Department: Rout Nat. 16. 27°08′S, 59°14′W. | Leaves |
| A547 | Peltophorum dubium | BAB Fortunato 14899 | Corrientes, Argentina. San Roque Department: Route Nat. 118, Colonia Yatay. 28°20′152″S, 58°18′500″W. | Leaves |
| A559 | Schinus areira | “Living collection”* | 34°60′S, 58°68′W. INTA. Hurlingham. | Leaves |
| A560 | Schinus areira | “Living collection”* | 34°60′S, 58°68′W. INTA Hurlingham. | Leaves |
| A561 | Schinus areira | “Living collection”* | 34°60′S, 58°68′W. INTAHurlingham | Leaves |
Each plant material was dried, finely ground, and extracted with methanol (MeOH) (10g of dry plant material per 100ml) at room temperature in total darkness for 48h. The extracts were filtered, dried under reduced pressure at 40°C, and weighed. These crude methanolic extracts were redissolved in MeOH at a concentration of 80mg dry matter per ml. For extract conservation, 1ml of each extract was diluted with 9ml of dimethyl sulfoxide (DMSO) until a final concentration of 8000μg/ml. This solution was sterilized by passing it through a 0.45μm cellulose acetate membrane filter. All extracts were kept in cryovials at −35°C until analysis2.
All strains used in this study (1 C. albicans, 1 C. parapsilosis and 1 C. glabrata isolates susceptible to azole antifungals, echinocandins and polyenes; 1 C. albicans isolate resistant to fluconazole, 1 C. krusei isolate intrinsically resistant to fluconazole and 1 C. haemulonii isolate MDR to azoles, polyenes and echinocandins) are available in the culture collection of the Mycology Bank of the Center of Mycology of the School of Medicine (University of Buenos Aires), Buenos Aires, Argentina. These Candida isolates were collected from the bloodstream of critically ill patients with candidemia and identified at species level using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (VITEK® MS, bioMérieux, France). The in vitro susceptible profile to antifungal drugs was determined using the Sensititre method (Sensititre YeastOne™, Thermo Fisher Scientific, Argentina) at the medical institution where the Candida isolates were recovered. These isolates were immediately submitted to the Center of Mycology of the School of Medicine (University of Buenos Aires) for further studies. All yeast strains were stored at −20°C in Sabouraud dextrose broth (SDB; Difco Laboratories, USA) containing 10% glycerol. Prior to use, isolates were incubated on Sabouraud agar (Laboratorio Britania, Buenos Aires, Argentina), or in SDB, at 30°C for 24h. Cells from Sabouraud agar plates were used to prepare working suspensions in sterile saline.
Antifungal susceptibility testing was performed for all studied isolates against fluconazole and the seven methanolic extracts following the EUCAST antifungal MIC method for yeasts according to the Definitive Document E.Def 7.43.
Fluconazole was obtained as a standard powder from Merck-Sigma-Aldrich (Argentina). The final test concentration ranges for fluconazole and the seven methanolic extracts were 64μg/μl to 0.03μg/μl.
Briefly, the test was performed in sterile flat-bottom well microdilution plates using RPMI 1640 (Merck-Sigma-Aldrich) supplemented with 2% glucose and 0.165mol/l 3-(N-morpholino) propanesulfonic acid (MOPS), pH 7.0. The inoculum was prepared by suspending five representative colonies, obtained from an 18–24-h culture on SDA at 37°C, in sterile distilled water. The final inoculum was 0.5×105–2.5×105CFU/ml. Microdilution plates were incubated without agitation at 37°C in ambient air for 24–48h. Plates were read using a microdilution plate reader at a wavelength of 530nm to measure absorbance. The value of the blanks containing MeOH:DMSO (background) was subtracted from the readings of all other wells.
The MIC was defined as the lowest drug/extract concentration inhibiting ≥50% of growth compared to the drug/extract-free control. At least three separate replicates were performed for each assay. C. albicans ATCC 64548 and C. parapsilosis ATCC 22019 were used as quality control strains for susceptibility testing.
The interactions between methanolic extracts and fluconazole against fluconazole-susceptible Candida species were tested using the microdilution checkerboard technique, adapted from the EUCAST broth microdilution method, as previously described. The working concentration ranges of vegetal extracts and fluconazole were 0.03 to 4μg/ml and 0.03 to 16μg/ml, respectively. The interactions between the methanolic extracts and fluconazole were classified on the basis of the fractional inhibitory concentration index (FICI). The FICI was calculated applying the formula FICI=(Ac/Aa)+(Bc/Ba), where Ac and Bc are the MICs of antifungal drugs in combination, and Aa and Ba are the MICs of antifungal drugs A and B alone. The FICI results are classified as follows: FICI of ≤0.5, synergy; FICI of >0.5 to ≤4, no interaction (indifference); and FICI of >4, antagonism7. All experiments were conducted in triplicate.
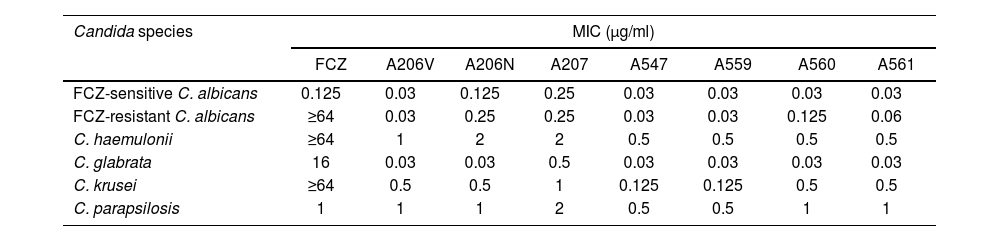
All evaluated extracts demonstrated an inhibitory effect on all evaluated Candida species (Table 2). The MIC ranges of individual tested agents against Candida isolates were 0.125 to ≥64μg/ml for fluconazole; 0.03 to 1μg/ml for A206V, A560 and A561; 0.03–2μg/ml for A206N; 0.25–2μg/ml for A207; and 0.03–0.5μg/ml for A547 and A559 (Table 2).
MIC results with fluconazole and the seven plant extracts against different Candida species.
| Candida species | MIC (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| FCZ | A206V | A206N | A207 | A547 | A559 | A560 | A561 | |
| FCZ-sensitive C. albicans | 0.125 | 0.03 | 0.125 | 0.25 | 0.03 | 0.03 | 0.03 | 0.03 |
| FCZ-resistant C. albicans | ≥64 | 0.03 | 0.25 | 0.25 | 0.03 | 0.03 | 0.125 | 0.06 |
| C. haemulonii | ≥64 | 1 | 2 | 2 | 0.5 | 0.5 | 0.5 | 0.5 |
| C. glabrata | 16 | 0.03 | 0.03 | 0.5 | 0.03 | 0.03 | 0.03 | 0.03 |
| C. krusei | ≥64 | 0.5 | 0.5 | 1 | 0.125 | 0.125 | 0.5 | 0.5 |
| C. parapsilosis | 1 | 1 | 1 | 2 | 0.5 | 0.5 | 1 | 1 |
FCZ: fluconazole; A206V (aerial parts of P. quadrangularis with fruiting capitula); A206N (aerial parts of P. quadrangularis with flowering capitula); A207 (L. balansae leaves); A547 (P. dubium leaves); A559 (S. areira leaves); A560 (S. areira leaves) and A561 (S. areira leaves).
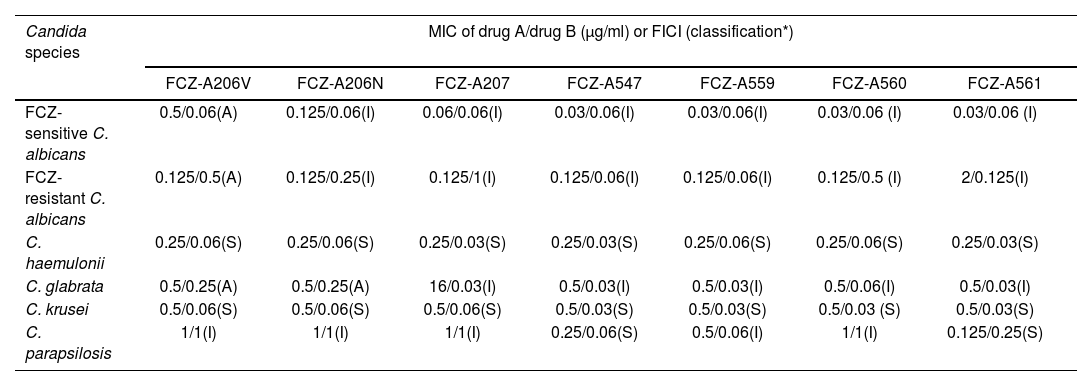
The combination of fluconazole with A206V, A206N, A207, A547, A559, A560 or A561 showed synergistic antifungal effect against C. krusei and C. haemulonii. Only the combination of fluconazole with A547 or A561 showed synergistic antifungal effects against C. parapsilosis. The combination of fluconazole with the remaining extracts showed antagonistic or indifferent antifungal effects against C. albicans (both fluconazole-sensitive and resistant) and in C. glabrata. The effective MIC ranges of fluconazole were mostly within the range of 0.03–0.5μg/ml (Table 3).
Fractional inhibitory concentration index results with combinations of fluconazole and the seven plant extracts against different Candida species.
| Candida species | MIC of drug A/drug B (μg/ml) or FICI (classification*) | ||||||
|---|---|---|---|---|---|---|---|
| FCZ-A206V | FCZ-A206N | FCZ-A207 | FCZ-A547 | FCZ-A559 | FCZ-A560 | FCZ-A561 | |
| FCZ-sensitive C. albicans | 0.5/0.06(A) | 0.125/0.06(I) | 0.06/0.06(I) | 0.03/0.06(I) | 0.03/0.06(I) | 0.03/0.06 (I) | 0.03/0.06 (I) |
| FCZ-resistant C. albicans | 0.125/0.5(A) | 0.125/0.25(I) | 0.125/1(I) | 0.125/0.06(I) | 0.125/0.06(I) | 0.125/0.5 (I) | 2/0.125(I) |
| C. haemulonii | 0.25/0.06(S) | 0.25/0.06(S) | 0.25/0.03(S) | 0.25/0.03(S) | 0.25/0.06(S) | 0.25/0.06(S) | 0.25/0.03(S) |
| C. glabrata | 0.5/0.25(A) | 0.5/0.25(A) | 16/0.03(I) | 0.5/0.03(I) | 0.5/0.03(I) | 0.5/0.06(I) | 0.5/0.03(I) |
| C. krusei | 0.5/0.06(S) | 0.5/0.06(S) | 0.5/0.06(S) | 0.5/0.03(S) | 0.5/0.03(S) | 0.5/0.03 (S) | 0.5/0.03(S) |
| C. parapsilosis | 1/1(I) | 1/1(I) | 1/1(I) | 0.25/0.06(S) | 0.5/0.06(I) | 1/1(I) | 0.125/0.25(S) |
FCZ: fluconazole; A206V (aerial parts of P. quadrangularis with fruiting capitula); A206N (aerial parts of P. quadrangularis with flowering capitula flowering); A207 (L. balansae leaves); A547 (P. dubium leaves); A559 (S. areira leaves); A560 (S. areira leaves) and A561 (S. areira leaves).
In the present study, the antifungal activity of seven methanolic extracts from four Argentinian native plants (S. areira, P. dubium, P. quadrangularis and L. balansae) were studied against six clinical Candida isolates with different susceptibility profiles to medical antifungal drugs, including MDR isolates such as C. hameulonii, which exhibited a MDR phenotype to azoles, echinocandins and polyenes.
All evaluated extracts demonstrated an inhibitory effect on all evaluated Candida species. Among the evaluated extracts, those from leaves of P. dubium and from root leaves of S. areira seedlings exhibited the highest antifungal activity, with median MIC values of 0.2μg/ml.
The combination of fluconazole with A206V, A206N, A207, A547, A559, A560 or A561 showed synergistic antifungal effect against C. krusei and C. haemulonii, although further studies are needed to elucidate such mechanism.
C. krusei is intrinsically resistant to fluconazole, though the precise mechanism is not completely understood. Several studies have attributed the innate azole resistance of C. krusei to efflux pump activity, mediated through the overexpression of the ATP-binding cassette transporter Abc1p, leading to reduced drug accumulation in combination with reduced azole affinity for Erg11p6. Similarly, efflux pump activity and mutations in ERG11p are implicated in high-level fluconazole resistance and pan-azole resistance in the C. haemulonii complex9.
All evaluated crude extracts likely contained several compounds with different bioactivities, including possible fungitoxic effects. Therefore, the purification of these secondary metabolites with antifungal activity might be used as alternative antifungal molecules or for the development of novel strategies to overcome the MDR phenotype conferred, for instance, by efflux transporters, such as in C. haemulonii. Interestingly, some of these secondary metabolites present in the crude extracts of other native plants from Argentina were reported to reverse fluconazole resistance in fluconazole-resistant Mdr1- and Cdr1-overexpressing clinical isolates of C. albicans by inhibiting the efflux transporters Mdr1 and Cdr14.
In this study, the absence of potentiation of fluconazole activity in the isolates of C. albicans resistant to this azole may be due to resistance mechanisms involving mutations in ERG11 or the increased expression of ERG11 due to activating mutations in the gene encoding the zinc-cluster transcriptional regulator Upc2p rather than the overexpression of the efflux pumps Mdr1p and Cdr1p/Cdr2p12. The results obtained so far encourage us to continue with these lines of research to characterize the compound(s) responsible for the antifungal activity present in the seven extracts studied. Since the evaluated plant extracts come from native Argentinian plants, this offers the possibility of widespread development and provides a further reason to work towards safeguarding the national heritage by contributing to the conservation and sustainable use of biological diversity. Our intention is to delve deeper into these studies to achieve the phytochemical characterization of active extracts, understand their site of action against fungi, thereby offering new therapeutic molecules in response to the challenge of antifungal resistance.
In conclusion, the observed antifungal activity of the studied extracts against different Candida species allowed S. areira, P. dubium, P. quadrangularis, and L. balansae to be included among the few species registered with this antifungal activity. Additionally, in C. krusei and C. haemulonii, all evaluated crude extracts potentiate the activity of fluconazole, although further studies are needed to elucidate this mechanism. Extracts of these native plants and their active compounds could serve as new antifungal candidates for future applications in medical settings.
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the authors do not used any generative AI and/or AI-assisted technology in the writing process.
FundingThis work was supported by grants from Agencia Nacional de Promoción Científica y Técnica (ANPCyT, Grant PICT 2021-I-A-00557), Proyectos INTA (2023-PE-L04-I120, 2023-PE-L04-I073, and 2023-PD-L06-I115), and Proyecto Unidad Ejecutora (INTA – CONICET) PUE22920180100066CO.
Conflicts of interestThe authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
RHF, ADN and MLC are members of CONICET. KHA thanks her doctoral scholarship to CONICET. ANE and LSDC thank the postdoctoral scholarships to CONICET.