During the COVID-19 epidemic, the prevalence of the disease means that practically any lung opacity on an X-ray could represent pneumonia due to infection with SARS-CoV-2. Nevertheless, atypical radiologic findings add weight to negative microbiological or serological tests. Likewise, outside the epidemic wave and with the return of other respiratory diseases, radiologists can play an important role in decision making about diagnoses, treatment, or preventive measures (isolation), provided they know the key findings for entities that can simulate COVID-19 pneumonia. Unifocal opacities or opacities located in upper lung fields and predominant airway involvement, in addition to other key radiologic and clinical findings detailed in this paper, make it necessary to widen the spectrum of possible diagnoses.
La prevalencia en fase epidémica de la COVID-19 hace que prácticamente cualquier opacidad pulmonar en la radiografía de tórax pueda ser una neumonía por SARS-CoV-2. Sin embargo, hallazgos radiológicos atípicos aumentarán la credibilidad de un resultado microbiológico o serológico negativo. Asimismo, fuera de la ola epidémica y con el retorno de otras entidades respiratorias, el radiólogo puede tener gran relevancia en la toma de decisiones diagnósticas, terapéuticas o preventivas (aislamiento) si conoce las claves diagnósticas de las entidades simuladoras de neumonía COVID-19. La distribución unifocal o en campos pulmonares superiores de las opacidades y la afectación predominante de vía aérea, entre otras claves radiológicas y clínicas detalladas en este capítulo, implican necesariamente ampliar el abanico de posibilidades diagnósticas.
As has been discussed in this journal,1,2 during the epidemic phase of COVID-19, numerous studies have given a key role to imaging techniques in the initial diagnostic management of the disease, be they chest X-ray as a first-line technique to confirm an initial diagnosis of pneumonia, or computed tomography (CT) as a second step, considered a “definitive” diagnostic test due to its high sensitivity and specificity. However, the characteristics of the disease mean that, at some hospitals, COVID-19 has completely edged out most respiratory diseases and other medical emergencies.2,3 This situation, together with the variable severity of the signs and symptoms and the duration of the clinical course, represents a significant bias when assessing the effectiveness of a diagnostic test. Without downplaying the importance of CT in detecting lung disease, grading its severity or suggesting its viral aetiology in the epidemic phase or with a scarcity of microbiological diagnostic tests, some reviews4 have referred to a lesser advantage in sensitivity and specificity values than that initially accorded to it5 versus detecting the virus using the polymerase chain reaction (PCR) technique.
This update will review the situations in which the radiologist must suggest alternative diagnoses to COVID-19 in patients in whom this disease is suspected based on signs and symptoms, laboratory findings, radiological findings or a combination thereof, with a focus on the acute phase of the disease. It must be remembered that, in chest radiology, lung disease patterns are rarely pathognomonic, so correlative signs and symptoms and laboratory findings are often essential (Fig. 1). Some data in the presentation of different diseases, both infectious and non-infectious, may be key to distinguishing them from COVID-19, while others are non-specific. Among others, the following stand out:
- •
Timeline. A sudden onset (e.g. alveolar haemorrhage) or a gradual onset over weeks (e.g. interstitial disease) differs from the onset over the course of hours or days seen in the clinical course of COVID-19, and suggests other non-infectious diseases or other infections such as tuberculosis or Pneumocystis jirovecii pneumonia.
- •
Fever characterises lung infections, but is present in acute diseases such as alveolar haemorrhage6 and even in some interstitial diseases.7 Therefore, it does not allow other diseases to be ruled out, although its absence will be uncommon in COVID-19.8
- •
Many of the laboratory abnormalities (elevation of C-reactive protein, lactate dehydrogenase, D-dimer, etc.) present in severe cases of COVID-198 result from the acute inflammatory process common also to infectious diseases and other causes.
- •
Knowledge of the patient’s personal history and clinical picture enables identification of precipitating factors for certain complications that constitute clinical and radiological alternatives to SARS-CoV-2 infection, although it cannot be ruled out (Fig. 2).
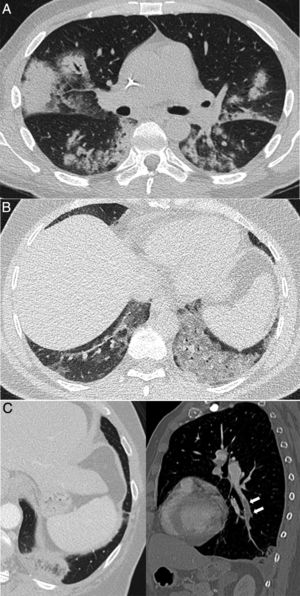
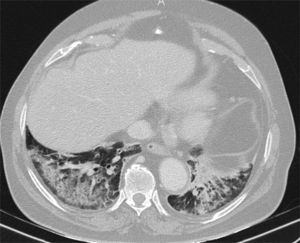
Figure 2.Pulmonary diseases with clinical and pathological confirmation that occur in characteristic clinical contexts, but are similar in appearance to COVID-19 pneumonia. A) Differentiation syndrome secondary to treatment with tretinoin in a patient with acute promyelocytic leukaemia showing bilateral consolidations associated with some areas of ground-glass attenuation and nodular opacities. B) Exogenous lipoid pneumonia manifesting as dense ground-glass areas of attenuation at both lung bases. C) Patient with suspected COVID-19 ultimately diagnosed with pulmonary thromboembolism with pulmonary infarction, which exhibited an inverted halo and a filling defect in the left lower lobe branch, shown in the sagittal reconstruction (arrows).
(0.3MB).
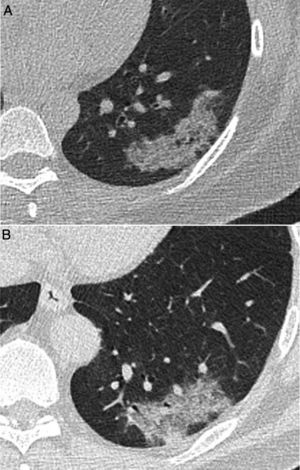
Computed tomography (CT) images corresponding to 2 patients with lesions featuring a similar radiological appearance: A) A 40-year-old woman with limited systemic sclerosis and cough, with persistent lung consolidation in the left lower lobe for more than 9 months and a pathology diagnosis concordant with inflammatory pseudotumour. B) A 52-year-old man who presented during the COVID-19 pandemic with a persistent high fever and dyspnoea for 4 days. As his symptoms persisted following a negative PCR test, a CT scan was performed that showed a consolidation similar in appearance to image A, finally diagnosed with SARS-CoV-2 pneumonia after confirmation by another PCR test.
To address the possible situations systematically, we will put forward two scenarios:
- 1
Patients with suspected respiratory infection, but with an alternative radiological pattern different from COVID-19.
- 2
Patients with dyspnoea and pulmonary opacities with a diagnosis other than infection.
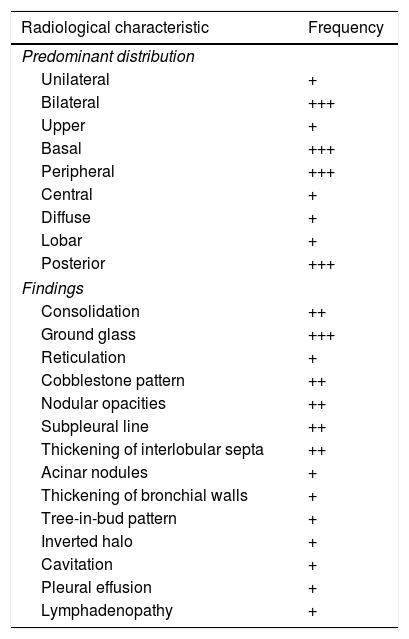
The typical clinical presentation of COVID-19, with cough, dyspnoea and fever, is a non-specific condition, common to most infectious processes of the respiratory tract. A diagnosis of pneumonia of any aetiology requires the presence of radiological abnormalities in addition to these symptoms.9 With the exception of the most characteristic patterns of the disease, reported in this same journal by Martínez et al.,1 outside the epidemic phase, the radiological presentation of COVID-19 can be completely non-specific and indistinguishable from non-infectious and infectious diseases of another aetiology. Negative PCR test results in a significant percentage of patients with COVID-191,10 mean that imaging tests, along with epidemiological, clinical and laboratory data, can play an important role in making decisions about isolation and treatment. To help manage the disease, radiologists must be alert to signs that suggest an infectious nature yet mandate consideration of an alternative diagnosis to COVID-19 as well as a bacterial co-infection or superinfection.1,11 To do this, evaluation of imaging in suspected SARS-CoV-2 pneumonia must be done with two considerations: the findings themselves and their distribution. Their relative frequency on CT is reflected in Table 1.
Relative frequency of the different image characteristics of computed tomography in acute SARS-CoV-2 infection.
| Radiological characteristic | Frequency |
|---|---|
| Predominant distribution | |
| Unilateral | + |
| Bilateral | +++ |
| Upper | + |
| Basal | +++ |
| Peripheral | +++ |
| Central | + |
| Diffuse | + |
| Lobar | + |
| Posterior | +++ |
| Findings | |
| Consolidation | ++ |
| Ground glass | +++ |
| Reticulation | + |
| Cobblestone pattern | ++ |
| Nodular opacities | ++ |
| Subpleural line | ++ |
| Thickening of interlobular septa | ++ |
| Acinar nodules | + |
| Thickening of bronchial walls | + |
| Tree-in-bud pattern | + |
| Inverted halo | + |
| Cavitation | + |
| Pleural effusion | + |
| Lymphadenopathy | + |
+: rare (<10%); ++: common (10–50%); +++: characteristic (>50%).
Based on the frequency in the description and presence of the findings obtained from the references that appear in Supplementary material.
Signs on chest X-ray in COVID-19 faithfully follow the patterns seen on CT,1,12,13 with a bilateral and peripheral distribution as well as a tendency towards a basal predominance (the most characteristic), in the form of frank consolidation, fainter opacities (which can be labelled “ground-glass” opacities, as in CT semiology) or more linear or reticular lesions.1,12,14–16 However, it must always be borne in mind that, in the epidemic phase, almost any pulmonary opacity could correspond to SARS-CoV-2 pneumonia.
Solitary pulmonary lesions (nodules, masses, cavitated lesions, etc.), pleural effusion, hilar lesions and mediastinal lesions as dominant findings are rare in COVID-19,1 and lie beyond the scope of this review.
In patients with respiratory infection, the presence of one or more pulmonary opacities suggests a diagnosis of pneumonia and, as mentioned, the distribution and characteristics thereof will be key to suggesting an alternative diagnosis to COVID-19. The characteristic bilateral distribution of COVID-19 can be influenced by the duration of the clinical course. In one series, only 25% of cases presented as bilateral or multiple lesions,14 so unilaterality and the presence of a single lesion do not rule out the disease. In addition, in many of these apparently unique lesions, CT reveals other lesions not identified on X-ray.
In the epidemic phase, severe SARS-CoV-2 pneumonia must be ruled out should a patient clinically and radiologically present dyspnoea and diffuse bilateral lesions. However, if the distribution of the abnormalities is predominantly in the upper lobes or central areas of the lung (Figs. 3 and 4), it is not characteristic of COVID-19, and can suggest alternatives. In patients with severe disease and equivocal radiographic findings, an early CT scan will help to characterise the distribution, as those figures show.
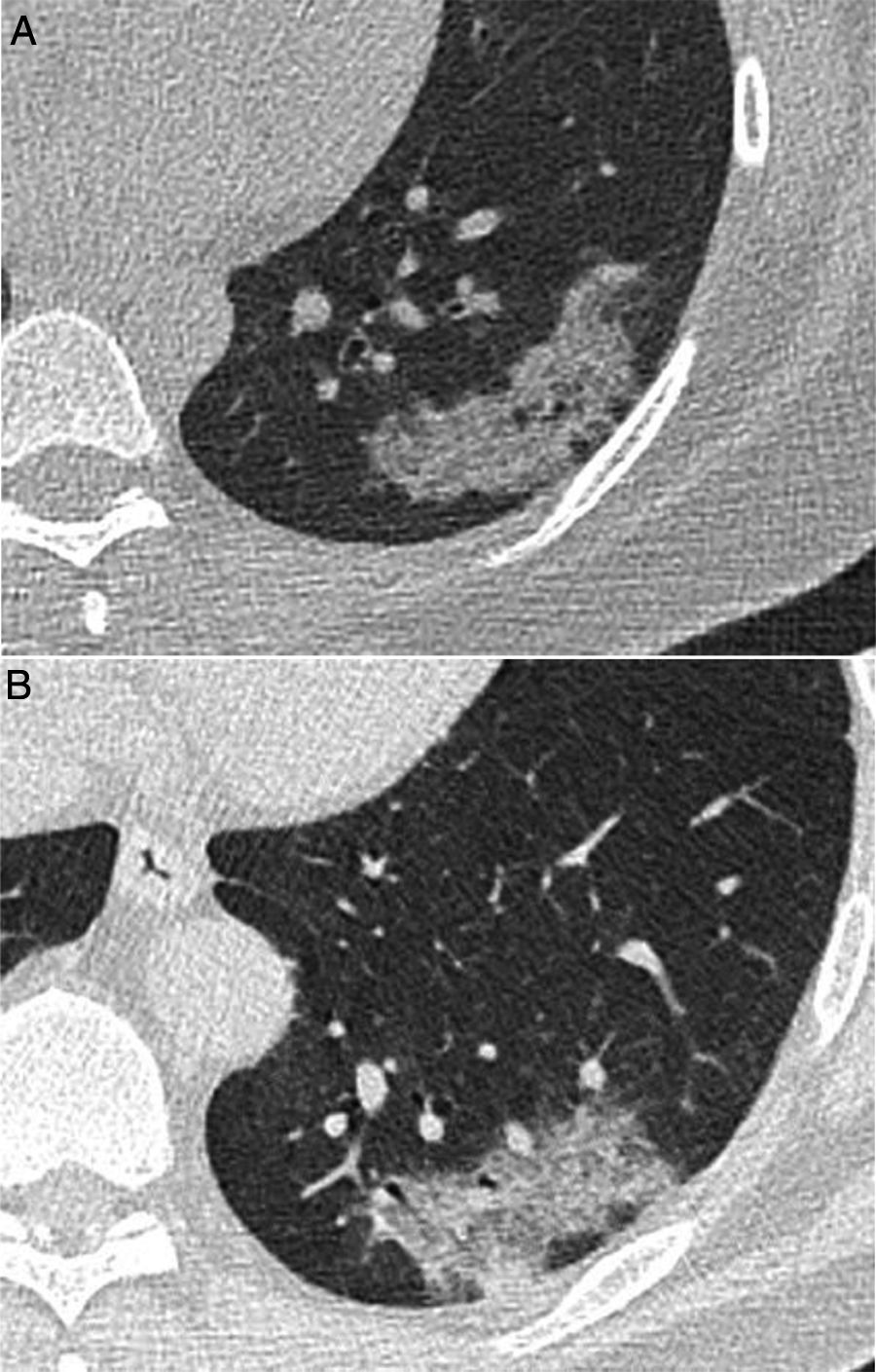
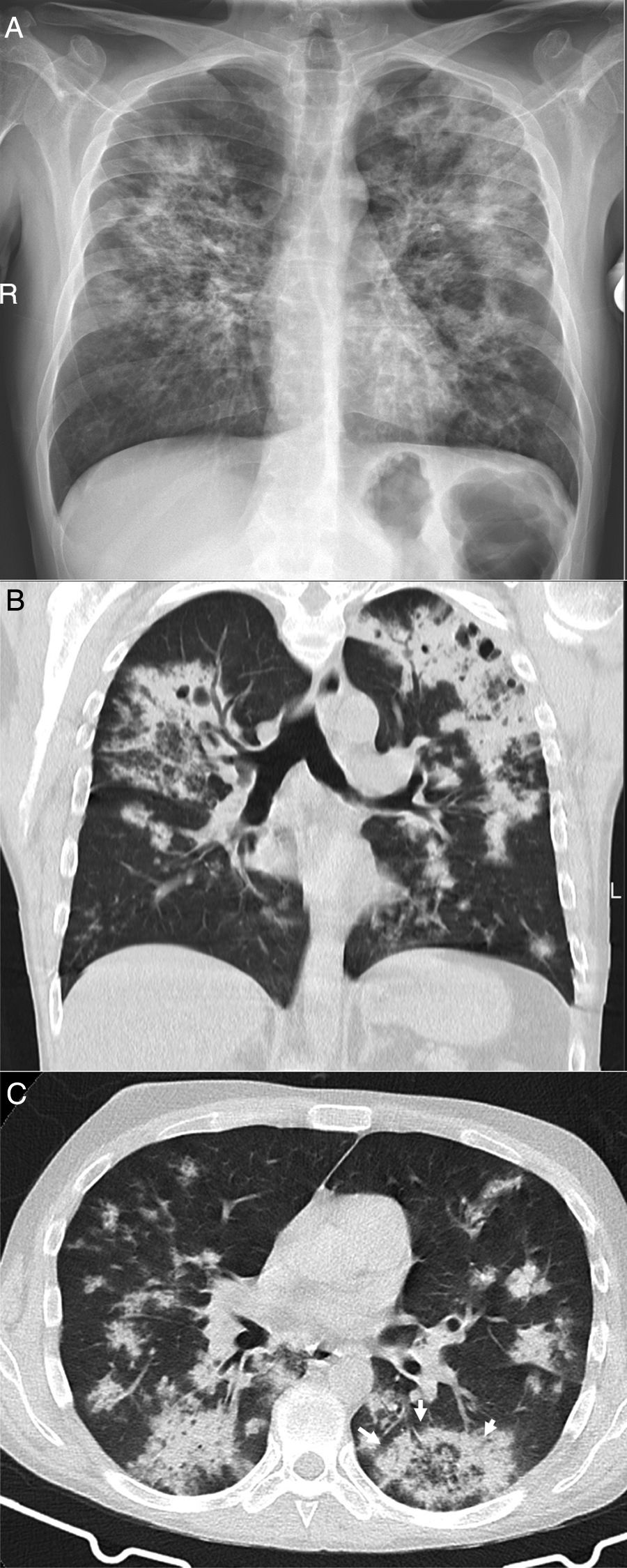
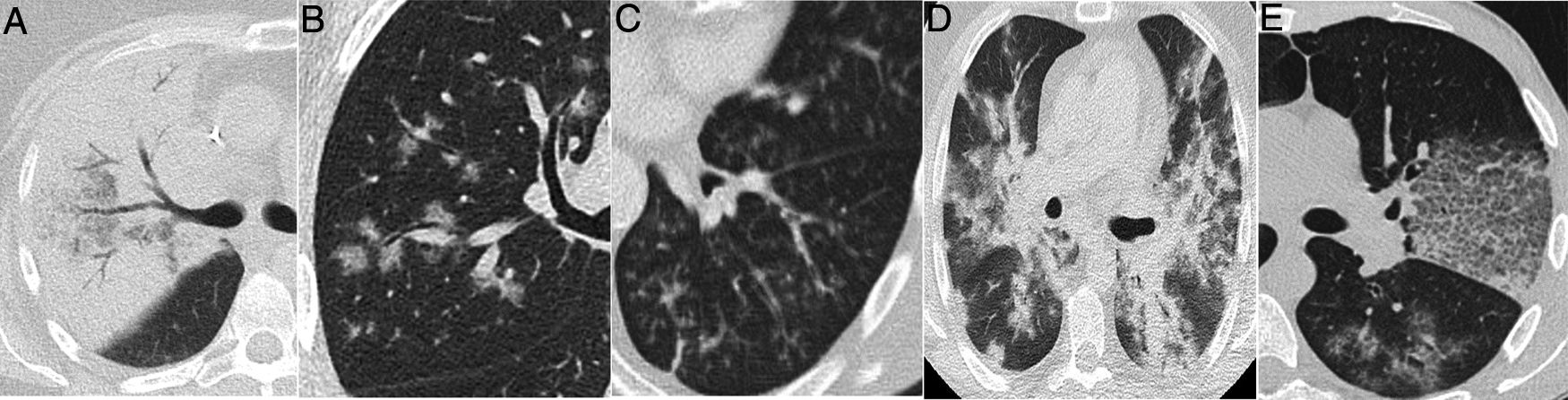
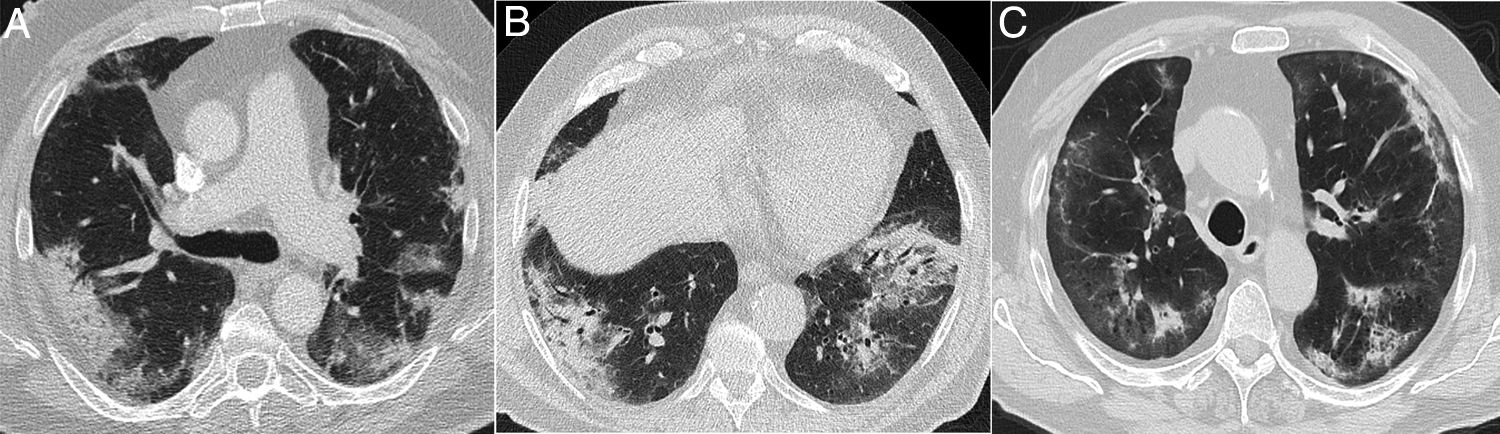
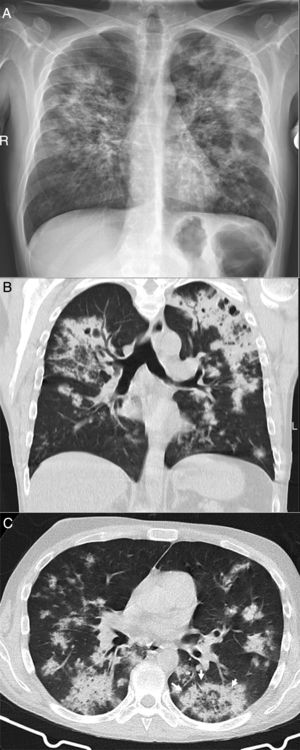
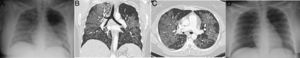
A 39-year-old man who presented during the pandemic with asthenia, myalgia and odynophagia, with a final diagnosis of pulmonary tuberculosis. A chest X-ray (A) showed extensive bilateral consolidations, predominantly upper and central on the right side and in upper and middle fields on the left side, with possible cavitations confirmed in the coronal CT reconstruction (B). Opacities with a tree-in-bud morphology and some lesions with an inverted halo appearance are also visible; their micronodular edges are a distinctive characteristic of the disease (arrows in C).
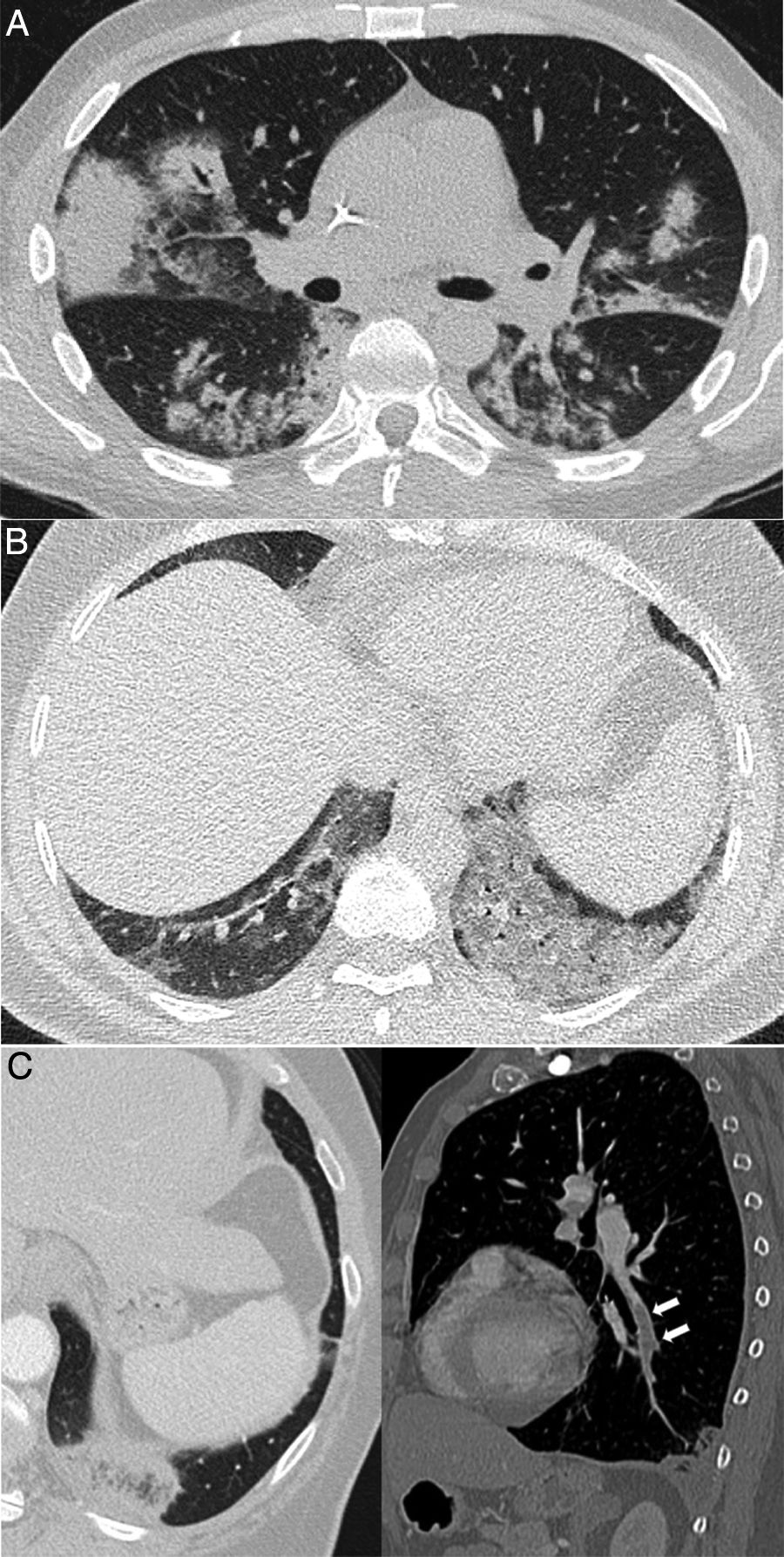
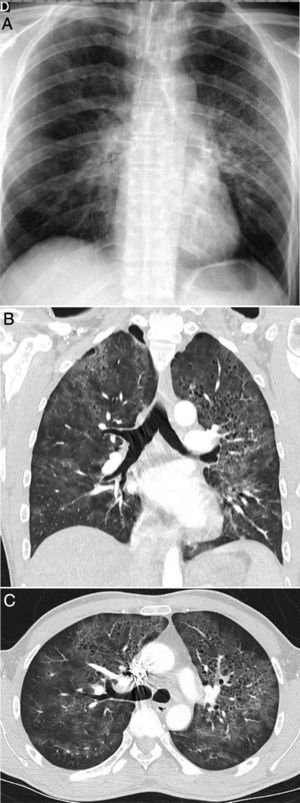
A 42-year-old man with no history of note who presented during the pandemic with fever and dyspnoea lasting 3 weeks accompanied by hypoxaemia, lymphopenia and elevated D-dimer and LDH. A chest X-ray (A) showed pulmonary opacities, predominantly perihilar and in the upper two-thirds of the lungs, with small cystic images on the left side. CT angiography to rule out pulmonary thromboembolism (B and C) showed the presence of ground-glass attenuation opacities with a slightly greater predominance and a tendency to spare the lung periphery, accompanied by thin-walled air-cyst lesions. Pneumocystis jirovecii pneumonia was suspected; it was confirmed by bronchoalveolar lavage. Later, the patient tested positive for human immunodeficiency virus.
Lobar involvement in the form of dense consolidation, of segmental or lobar extension, sometimes involving several lobes, is characteristic of community-acquired bacterial pneumonia (Fig. 5 and 5A). Despite occurring in the epidemic phase, these findings, along with some characteristics such as the presence of purulent sputum or different laboratory results, aid in diagnosis from the beginning.9,17 In our experience, the presence of air bronchogram on X-ray is uncommon in COVID-19. It is more common on CT,12,13,18 though it is seen in less than 20% of cases in some series.17,18 Regarding the presentation of COVID-19 in the form of interstitial involvement or as a reticular pattern, there must be a differential diagnosis with atypical forms of pneumonia6,15 and non-infectious causes (interstitial oedema, fibrosis, etc.).
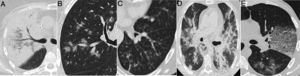
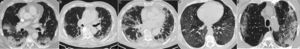
Radiological findings in cases of pneumonia with an aetiology other than COVID-19. A) Lobar consolidation in Streptococcus pneumoniae pneumonia. B) Peribronchovascular “clustered” opacities caused by respiratory syncytial virus. C) Branched opacities with a tree-in-bud morphology in influenza A virus infection D) Peribronchovascular consolidation in bilateral influenza A virus pneumonia E) Cobblestone pattern in Streptococcus pneumoniae pneumonia.
CT enables better identification and characterisation of abnormalities and their distribution, and in this sense it has higher specificity for suggesting alternative diagnoses.19,20 Studies having assessed the diagnostic cost-effectiveness of CT in series including similar numbers of cases of SARS-CoV-2 pneumonia and pneumonia of another aetiology21,22 have shown some limitations of CT in identifying the germ, given the overlapping of findings. These studies have yielded very disparate results, and have presented limitations in terms of population analysed and significant variability among radiologists.4,21,22 The intense epidemiological presentation of this pandemic precludes the availability of real data on the efficacy of CT in routine clinical practice, when SARS-CoV-2 coexists with other micro-organisms and only accounts for a certain proportion of cases of community-acquired pneumonia. Under these circumstances, perhaps only a very typical presentation on CT1 or a combination of a characteristic clinical picture with certain CT findings may be really useful.
COVID-19 mainly causes alveolar damage, both in initial stages23 and in its progression, in which the pattern of diffuse alveolar damage24,25 and vascular abnormalities26 predominate. This purely alveolar involvement would explain the manifestation in the form of ground-glass opacities and peripheral areas of consolidation as the predominant findings. Both types of lesions by themselves have little specificity, since they indicate an occupation of the air spaces that is common to many lung disease processes.27 However, unlike other types of pneumonia (bacterial or viral), in which pathological changes are concentrated in the airways,17,28–30 radiological findings that indicate airway or peribronchiolar disease (thickening of the bronchial walls or a tree-in-bud pattern) (Fig. 5) are not to be expected as dominant radiological signs of COVID-19. In fact, they do not appear in descriptions of CT findings in the disease (Table 1), or they do so in a small percentage,13 such that they should suggest an alternative diagnosis.11
There are other secondary findings that appear less commonly in COVID-19, but have sparked interest in the scientific literature, such as thickening of the intralobular and interlobular interstitium, the cobblestone pattern or the presence of an inverted halo.13 These signs lack specificity. The cobblestone pattern, for example, appears in many lung disease processes of varied aetiology, having been reported in Pneumocystis jirovecii,31–33 but also in other viral and bacterial types of pneumonia33,34 (Fig. 5E). Similarly, the inverted halo sign was initially considered characteristic of organising pneumonia, which may be of infectious aetiology. However, it was also considered typical of mucormycosis in immunosuppressed patients (and has been associated with other fungal and bacterial infections in that patient type35), septic embolism36 and tuberculosis,37 among other diagnoses.
An important distinguishing element is the distribution of the abnormalities, both along the longitudinal axis of the lung and, in particular, on the axial plane.
As mentioned, a distribution predominantly in the upper lobes should suggest other diagnoses (Figs. 3 and 4).12,13
A lobar or segmental consolidation from the periphery to the hilar slope, with air bronchogram, suggests a bacterial origin.17,30Clustered grouped lesions suggest disease spreading through the airway, whether resulting from aspiration,38,39 of tuberculous origin40 or due to viruses other than SARS-CoV-2, such as influenza41 (Fig. 5C and D). However, lesions with this distribution may occur in the initial stages of COVID-19, as corresponds to airborne spread of the disease.42 Similarly, the central predominance of the lesions should also raise suspicion of other diagnoses (Fig. 5D),12 although pneumonic lesions with a peribronchovascular distribution that do not reach the periphery are not rare in COVID-19.43
Except in severe cases of COVID-19, consolidation or diffuse ground-glass attenuation should suggest an alternative diagnosis. For example, in patients with lymphopenia, Pneumocystis jirovecii pneumonia may have an identical clinical and laboratory presentation (Fig. 4), although the clinical context and radiological findings will vary with diffuse and extensive pure ground glass, occasionally with an upper predominance and air cysts.31,32
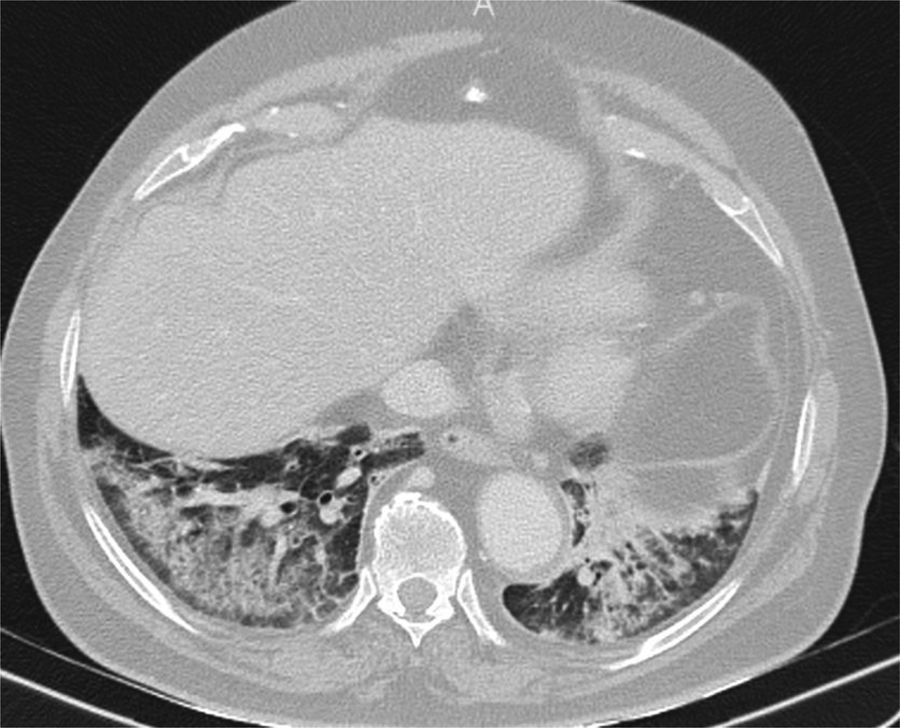
Finally, some specific infectious disease that can prompt a differential diagnosis with COVID-19 in our setting, such as aspiration and tuberculosis, will be addressed. Aspiration signs and symptoms and aspiration pneumonia are a relatively common cause of community- and hospital-acquired pneumonia,44 and should be considered in the differential diagnosis of bilateral lung lesions, fever and dyspnoea in patients with risk factors, especially the elderly.44,45 Radiologically, they are characterised by airspace lesions and predominant consolidations in the posterior regions of both lungs.38,39,43,44 The distribution of abnormalities, along with the presence of risk factors or aspiration, enable diagnosis (Fig. 6). Post-primary pulmonary tuberculosis is another common cause of bilateral lung infection that could raise doubts about COVID-19. However, both on chest X-ray and CT, the characteristic distribution, predominant in posterior regions of the upper lobes and apical segments of the lower lobes, and radiological manifestations (such as cavitation, medium-grade dilations of bronchi and dense centrilobular opacities with a tree-in-bud morphology40), and occasionally pleural effusion or extrapulmonary lesions, will enable it to be differentiated from SARS-CoV-2 infection (Fig. 3). Miliary tuberculosis, characterised by dense, randomly distributed, millimetric nodules, can also be distinguished from COVID-19.
Computed tomography, performed during the pandemic in an elderly man with fever and a fluctuating level of consciousness, showing bilateral posterobasal consolidations with subpleural sparing. Aspiration was suspected. Clinical and radiological signs and symptoms resolved with antibiotics, and COVID-19 was ruled out during admission.
From a clinical and radiological point of view, numerous non-infectious diseases can manifest as acute respiratory processes46 and overlap both with COVID-19 and with other infectious diseases.
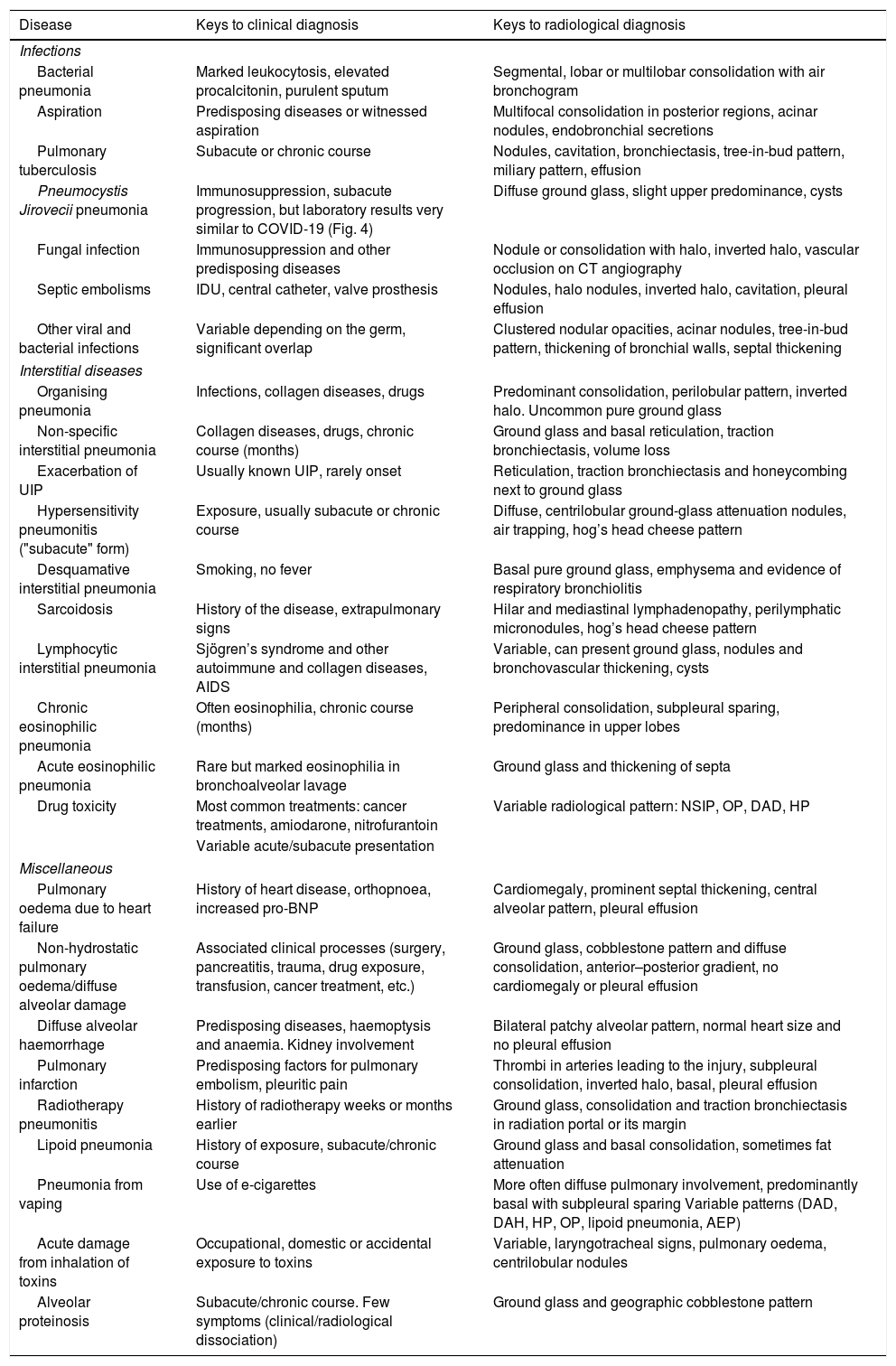
Due to space limitations, we will focus on the differential diagnosis of diseases with bilateral pulmonary involvement and a subacute or acute course that are more common or more similar to the typical radiological presentation of COVID-19. Table 2 shows some of them and the keys to their diagnosis.
Some diseases that may call for a differential diagnosis with COVID-19 of multiple pulmonary opacities in patients with acute/subacute dyspnea.
| Disease | Keys to clinical diagnosis | Keys to radiological diagnosis |
|---|---|---|
| Infections | ||
| Bacterial pneumonia | Marked leukocytosis, elevated procalcitonin, purulent sputum | Segmental, lobar or multilobar consolidation with air bronchogram |
| Aspiration | Predisposing diseases or witnessed aspiration | Multifocal consolidation in posterior regions, acinar nodules, endobronchial secretions |
| Pulmonary tuberculosis | Subacute or chronic course | Nodules, cavitation, bronchiectasis, tree-in-bud pattern, miliary pattern, effusion |
| Pneumocystis Jirovecii pneumonia | Immunosuppression, subacute progression, but laboratory results very similar to COVID-19 (Fig. 4) | Diffuse ground glass, slight upper predominance, cysts |
| Fungal infection | Immunosuppression and other predisposing diseases | Nodule or consolidation with halo, inverted halo, vascular occlusion on CT angiography |
| Septic embolisms | IDU, central catheter, valve prosthesis | Nodules, halo nodules, inverted halo, cavitation, pleural effusion |
| Other viral and bacterial infections | Variable depending on the germ, significant overlap | Clustered nodular opacities, acinar nodules, tree-in-bud pattern, thickening of bronchial walls, septal thickening |
| Interstitial diseases | ||
| Organising pneumonia | Infections, collagen diseases, drugs | Predominant consolidation, perilobular pattern, inverted halo. Uncommon pure ground glass |
| Non-specific interstitial pneumonia | Collagen diseases, drugs, chronic course (months) | Ground glass and basal reticulation, traction bronchiectasis, volume loss |
| Exacerbation of UIP | Usually known UIP, rarely onset | Reticulation, traction bronchiectasis and honeycombing next to ground glass |
| Hypersensitivity pneumonitis ("subacute" form) | Exposure, usually subacute or chronic course | Diffuse, centrilobular ground-glass attenuation nodules, air trapping, hog’s head cheese pattern |
| Desquamative interstitial pneumonia | Smoking, no fever | Basal pure ground glass, emphysema and evidence of respiratory bronchiolitis |
| Sarcoidosis | History of the disease, extrapulmonary signs | Hilar and mediastinal lymphadenopathy, perilymphatic micronodules, hog’s head cheese pattern |
| Lymphocytic interstitial pneumonia | Sjögren’s syndrome and other autoimmune and collagen diseases, AIDS | Variable, can present ground glass, nodules and bronchovascular thickening, cysts |
| Chronic eosinophilic pneumonia | Often eosinophilia, chronic course (months) | Peripheral consolidation, subpleural sparing, predominance in upper lobes |
| Acute eosinophilic pneumonia | Rare but marked eosinophilia in bronchoalveolar lavage | Ground glass and thickening of septa |
| Drug toxicity | Most common treatments: cancer treatments, amiodarone, nitrofurantoin | Variable radiological pattern: NSIP, OP, DAD, HP |
| Variable acute/subacute presentation | ||
| Miscellaneous | ||
| Pulmonary oedema due to heart failure | History of heart disease, orthopnoea, increased pro-BNP | Cardiomegaly, prominent septal thickening, central alveolar pattern, pleural effusion |
| Non-hydrostatic pulmonary oedema/diffuse alveolar damage | Associated clinical processes (surgery, pancreatitis, trauma, drug exposure, transfusion, cancer treatment, etc.) | Ground glass, cobblestone pattern and diffuse consolidation, anterior–posterior gradient, no cardiomegaly or pleural effusion |
| Diffuse alveolar haemorrhage | Predisposing diseases, haemoptysis and anaemia. Kidney involvement | Bilateral patchy alveolar pattern, normal heart size and no pleural effusion |
| Pulmonary infarction | Predisposing factors for pulmonary embolism, pleuritic pain | Thrombi in arteries leading to the injury, subpleural consolidation, inverted halo, basal, pleural effusion |
| Radiotherapy pneumonitis | History of radiotherapy weeks or months earlier | Ground glass, consolidation and traction bronchiectasis in radiation portal or its margin |
| Lipoid pneumonia | History of exposure, subacute/chronic course | Ground glass and basal consolidation, sometimes fat attenuation |
| Pneumonia from vaping | Use of e-cigarettes | More often diffuse pulmonary involvement, predominantly basal with subpleural sparing Variable patterns (DAD, DAH, HP, OP, lipoid pneumonia, AEP) |
| Acute damage from inhalation of toxins | Occupational, domestic or accidental exposure to toxins | Variable, laryngotracheal signs, pulmonary oedema, centrilobular nodules |
| Alveolar proteinosis | Subacute/chronic course. Few symptoms (clinical/radiological dissociation) | Ground glass and geographic cobblestone pattern |
AEP: acute eosinophilic pneumonia; AIDS: acquired immunodeficiency syndrome; DAD: diffuse alveolar damage; DAH: diffuse alveolar haemorrhage; HP: hypersensitivity pneumonitis; IDU: injection drug use; NSIP: non-specific interstitial pneumonia; OP: organising pneumonia; UIP: usual interstitial pneumonia.
The bibliographic references for this table appear in the Supplementary material.
One common cause of bilateral radiological involvement with dyspnoea is hydrostatic pulmonary oedema of cardiac origin.47 A diagnosis can be made from the central predominance of opacities, their cottony character, their association with Kerley B lines, cardiomegaly, pleural effusion and hilar enlargement and blurring; these findings are more evident on CT. Characteristic clinical data such as acute onset, orthopnoea and elevation of B-type natriuretic peptide will support the diagnosis.48 However, both diseases may be radiologically indistinguishable as pulmonary oedema is associated with passive atelectasis and consolidation in the posterior pulmonary regions adjacent to the accompanying pleural effusion. Meanwhile, pneumonia appears as a comorbidity in about 15% of patients admitted for heart failure in the non-epidemic phase.49 Co-existence of lung impairment due to COVID-19 and heart failure is common. This is a highly fatal combination, especially in elderly individuals with cardiovascular risk factors.50,51
Non-cardiogenic pulmonary oedema is a less common type of non-hydrostatic pulmonary oedema, with aetiopathogenic links to pneumonic diseases and with COVID-19, with which it can be associated.24,25 The most characteristic form of non-cardiogenic pulmonary oedema is adult respiratory distress syndrome (ARDS), which is, in fact, the most common form of progression in the most severe cases of COVID-19 pneumonia. It is characterised by diffuse acute inflammatory lung damage, with increased capillary permeability and loss of air space.52 However, ARDS is a heterogeneous syndrome with varied and complex mechanisms, the definition of which has varied over time.53 There is an underlying histological pattern of diffuse alveolar damage, common to many disease processes. Numerous pulmonary and extrapulmonary triggers (such as sepsis, pancreatitis, transfusion, drugs and trauma) can cause pulmonary oedema with variable fulfilment of the criteria for ARDS (Fig. 7). Their characteristic presentation on chest X-ray, with bilateral pulmonary opacities, constitutes a diagnostic criterion for ARDS,53 although interobserver agreement in its detection is only moderate.54 On CT, the acute phase manifests as opacities with ground-glass attenuation, consolidation or a cobblestone pattern with a bilateral, diffuse distribution.55 In ARDS of extrapulmonary origin, these signs tend to present an anteroposterior gradient, with preservation or lesser involvement of the anterior region of the lung and greater gradual attenuation, with consolidation in posterior regions.56 This distribution can be very similar to some cases of SARS-CoV2 pneumonia.
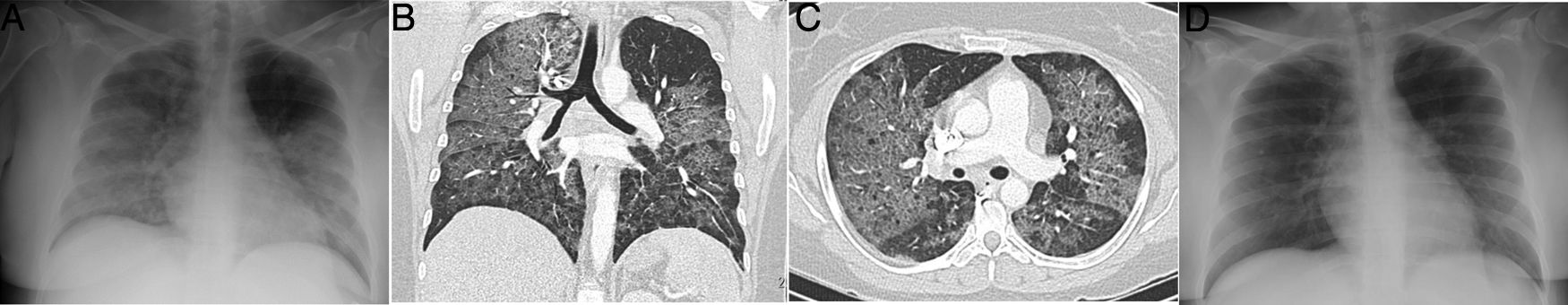
A 34-year-old woman, a multi-drug addict, who presented during the pandemic with fever, dyspnoea and hypoxaemia, as well as elevation of D-dimer. A portable chest X-ray (A) showed extensive bilateral consolidation. Pulmonary CT angiography was performed (coronal reconstruction in B; axial image in C) that showed a diffuse cobblestone pattern, with a central predominance and no pleural effusion. As non-cardiogenic pulmonary oedema due to drugs was suspected, the patient was treated with corticosteroids, with rapid complete resolution of clinical and radiological signs and symptoms at 24 hours (D), which is characteristic of the disease. The patient had presented a similar clinical picture one month earlier with an identical outcome.
Diffuse alveolar haemorrhage (DAH) is another cause of diffuse, bilateral lung involvement in patients with dyspnoea. The cardinal sign that would distinguish it from COVID-19 would be haemoptysis. However, it may be absent in a significant percentage of patients,6,57 and signs such as fever and serum markers of inflammation may be present, complicating differentiation. Anaemia of recent onset or other clinical evidence of diseases associated with DAH may help, since it is rarely idiopathic.44 From a radiological point of view, ground-glass opacities, consolidation and a cobblestone pattern are usually associated with a predominantly central or diffuse distribution, with only peripheral involvement being rare, unlike in COVID-19. Ill-defined centrilobular nodules may also be present in DAH.57
Pulmonary infarcts in venous thromboembolic disease manifest as peripheral subpleural lesions with ground-glass attenuation, consolidation or, characteristically, a central area of less attenuation or an inverted halo sign. They are often in the lower lobes and are associated with pleural effusion.58,59 These findings, along with fever, which is often associated, may raise suspicion of SARS-CoV-2 pneumonia (Fig. 2C). The presence of thrombi in the arteries leading to the lesion, frequent pleuritic pain and haemoptysis aid in diagnosis. Thrombotic phenomena commonly complicate other medical processes, including COVID-19.60
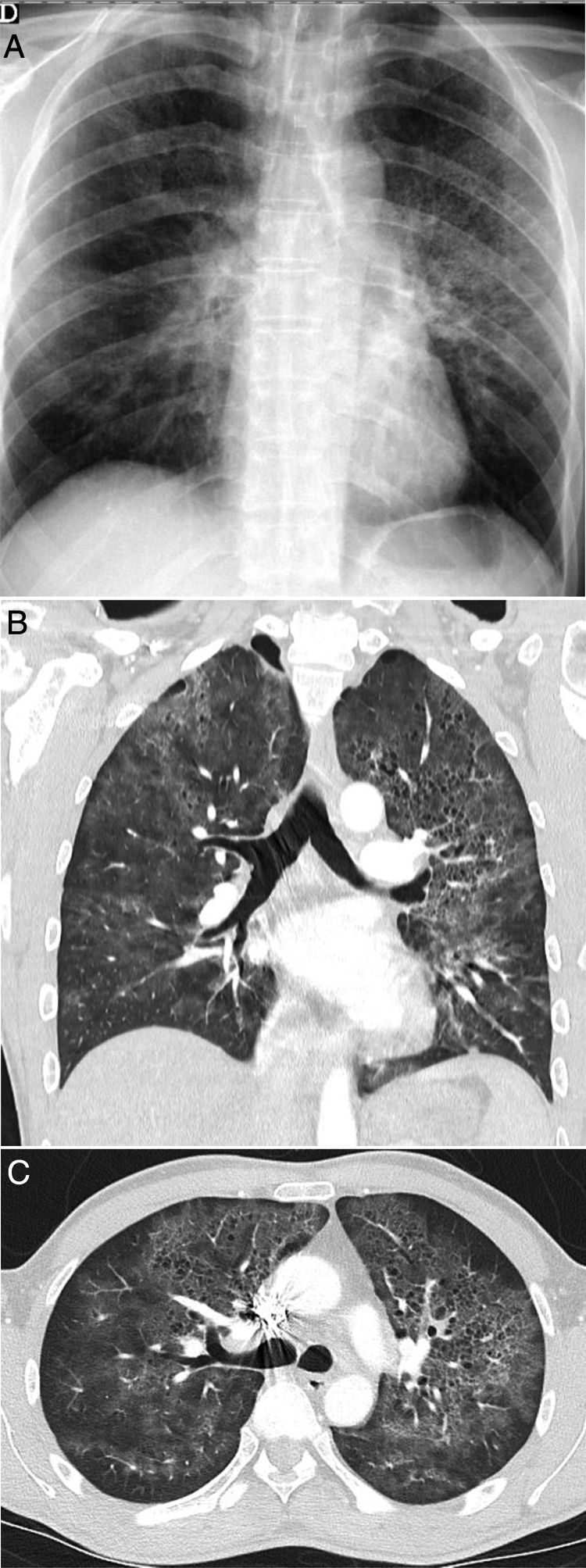
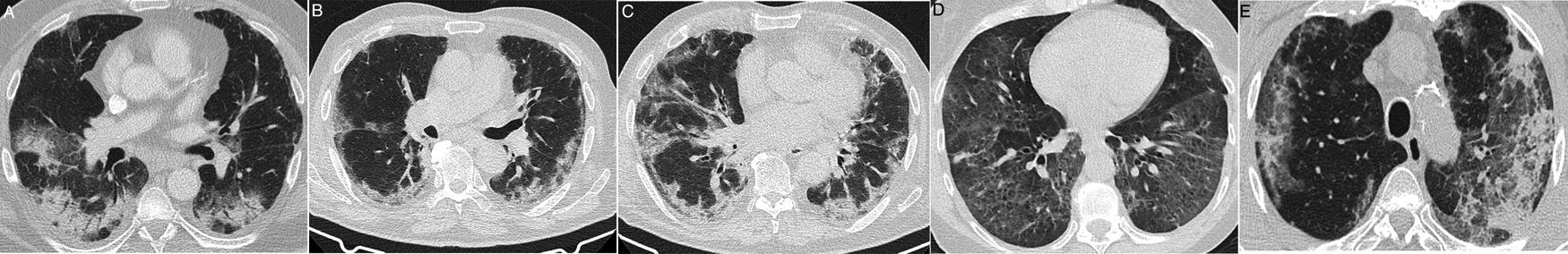
Some idiopathic or secondary diffuse interstitial diseases (collagen disease, drug toxicity, etc.) can have an acute or subacute clinical presentation or present exacerbations, which can lead to a differential diagnosis with COVID-19. A detailed description of such diseases is outside of the scope of this review. The diseases with the most clinical and radiological similarities to COVID-19 due to their appearance and distribution are organising pneumonia (OP), non-specific interstitial pneumonia (NSIP), desquamative interstitial pneumonia (DIP) and exacerbation of usual interstitial pneumonia (UIP).61 OP can occur idiopathically or be secondary to multiple processes, such as infections.61 Distinguishing the idiopathic form from that associated with viral pneumonia may be impossible in some cases,13,62 especially when a subpleural distribution predominates (Fig. 8A), as in around 40% of cases.63 It should not be surprising that some radiological signs described in OP, such as an inverted halo sign or a perilobular pattern, also appear in COVID-19 pneumonia.13 A predominance of consolidations with a peribronchovascular distribution could be a distinctive feature of OP. NSIP has many radiological similarities to SARS-CoV-2 lung involvement.64 However, its clinical presentation, generally associated with collagen diseases or toxicity, will allow its diagnosis. A subpleural-sparing band may appear in both diseases, although it is usually not as well delimited in NSIP (Fig. 8B and C). The loss of volume of the lower lobes and bronchiectasis within the ground-glass opacities are key radiological findings for diagnosing NSIP, although they may also appear in the course of COVID-19 pneumonia, such that it is very difficult to distinguish them. DIP is characterised by the presence of extensive, predominantly basal pure ground-glass opacities (Fig. 8D). In these cases, evidence of respiratory bronchiolitis and emphysema in the upper lobes in a patient who smokes aids in arousing suspicion. Acute exacerbation of UIP, but also of other interstitial diseases, can have a presentation similar to COVID-19. In these cases, knowledge of the patient's history or identification of reticulation, traction bronchiectasis and honeycombing associated with ground-glass attenuation opacities and central consolidations, characteristic of acute exacerbation, can aid in diagnosis.65 Although at the time of writing there are no data on the association of SARS-CoV-2 with a higher incidence of acute exacerbation, it is accepted that viral infections can trigger it, and therefore its association with COVID-1965 would be expected.
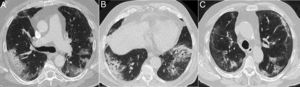
Interstitial diseases similar in appearance to COVID-19 that may manifest with dyspnoea and occasionally fever. A) Cryptogenic organising pneumonia with peripheral consolidation and air bronchogram. B and C) Non-specific interstitial pneumonia showing areas of ground-glass attenuation and peripheral consolidation with a subpleural-sparing band. D) Desquamative interstitial pneumonia in a patient who smokes, showing extensive areas of ground-glass attenuation and small air cysts. E) Chronic eosinophilic pneumonia showing peripheral consolidation partially sparing the subpleural region and characteristically affecting the upper lobes.
Hypersensitivity pneumonitis exhibits variable clinical and radiological signs.66 In its acute or subacute presentation, centrilobular nodules with ground-glass attenuation and a mosaic or hog's head cheese pattern are characteristic findings of the disease.67 Of these, the latter, with normal pulmonary lobules alternating with others of less attenuation due to air trapping as well as areas of greater ground-glass attenuation, is the one that could more often simulate SARS-CoV-2 pneumonia.68 However, in hypersensitivity pneumonitis and other diseases associated with this pattern, such as sarcoidosis and, in our experience, some collagen diseases and drug toxicity, the appearance is usually diffuse, without a predominance in the lung periphery as in COVID-19.
Eosinophilic lung diseases constitute a diverse group of diseases that can simulate COVID-19, especially acute eosinophilic pneumonia (AEP) from a clinical point of view and chronic eosinophilic pneumonia (CEP) from a radiological point of view.69 The peripheral eosinophilia present in all of them except AEP aids in suspecting them. AEP is a rare condition. Its characteristically acute presentation with fever and hypoxaemia, diffuse ground-glass opacities, and extensive thickening of septa as fundamental findings makes it difficult to differentiate it from many of the diseases discussed above. Peripheral consolidation of the chronic form may be similar to COVID-19, except for its upper predominance (Fig. 8E).
In cancer patients, the possibility of drug toxicity as the cause of a clinical and radiological picture with bilateral pulmonary opacities, fever and dyspnoea should always be borne in mind. Patterns of lung involvement such as OP, NSIP, a hog’s head cheese pattern or diffuse alveolar damage70 may call for consideration of COVID-19 in the differential diagnosis in epidemic phases (Fig. 9).
Pneumonitis secondary to drug toxicity. Its clinical presentation and parenchymal changes can simulate COVID-19 pneumonia. A) Patient with colon carcinoma being treated with the FOLFOX regimen (folinic acid, fluorouracil and oxaliplatin). B) Renal cell carcinoma treated with everolimus. C) Patient with lung neoplasm with ALK mutation treated with alectinib, who presented during the pandemic with dyspnoea; COVID-19 and other causes of the lesions were ruled out.
Finally, the pulmonary signs or complications of certain diseases (such as some diseases and haematological therapies) could cause confusion with COVID-19 in an epidemic phase, so it is essential to be aware of the patient’s medical history51 to include these rare diseases in the patient’s differential diagnosis (Fig. 2A), although a discussion thereof goes beyond the scope of this review.
ConclusionThis chapter has compiled the key distinguishing aspects of the diseases that can simulate SARS-CoV-2 pneumonia, either because their clinical presentation is similar or because their radiological characteristics are similar.
Authorship- 1
Responsible for study integrity: JJAJ.
- 2
Study concept: JJAJ, JMPM, EGG.
- 3
Study design: JJAJ, JMPM, EGG.
- 4
Data collection: JJAJ, JMPM, EGG.
- 5
Data analysis and interpretation: JJAJ, JMPM, EGG.
- 6
Statistical processing: N/A.
- 7
Literature search: JJAJ, JMPM, EGG.
- 8
Drafting of the paper: JJAJ, JMPM, EGG.
- 9
Critical review of the manuscript with intellectually significant contributions: JJAJ, JMPM, EGG.
- 10
Approval of the final version: JJAJ, JMPM, EGG.
The authors declare that they have no conflicts of interest.
Please cite this article as: Arenas-Jiménez JJ, Plasencia-Martínez JM, García-Garrigós E. Cuando la neumonía no es COVID-19. Radiología. 2021;63:180–192.