Breast radiology: New horizons in times of pandemics
More infoThis study aimed to determine the ability of axillary ultrasonography to predict the number of lymph nodes with metastases found in sentinel node axillary surgery or axillary lymph node resection in patients recently diagnosed with breast cancer after percutaneous biopsy.
MethodsThis prospective study included patients diagnosed with invasive breast cancer by percutaneous biopsy. Axillary lymph nodes were classified at ultrasound examination as suspicious or not suspicious, and the number of suspicious nodes was compared with the number classified as suspicious in the surgical specimen.
ResultsWe included 142 patients, 4 of whom had bilateral cancer; 133 of the 146 tumors were clinically classified as T1-T2 N0. The median number of suspicious lymph nodes at ultrasound was 2 (1–6), and the median number of suspicious lymph nodes in the surgical biopsy specimen was 1 (1–16); the difference was not significant (p = 0.1). The correlation between the number of positive lymph nodes on axillary ultrasound and the number of metastatic lymph nodes in the surgical specimen was 72.7% p = 0.0002 and the concordance was 79% (95%CI 62.4%–95.6%) p = 0.0001. For diagnosing high axillary tumor load (≥3 metastatic lymph nodes) versus low axillary tumor load (<3 metastatic lymph nodes), axillary ultrasound had 86.6% sensitivity, 83.3% specificity, 92% PPV, and 71.4% NPV.
ConclusionOur results show that preoperative axillary ultrasound can differentiate between low and high tumor load and can be used as a tool to select the type of treatment. These results need to be confirmed in randomized multicenter studies.
El objetivo de este estudio fue determinar la capacidad del ultrasonido axilar para predecir el número de ganglios con metástasis encontrados en la cirugía axilar de ganglio centinela o linfadenectomía axilar, en pacientes con diagnóstico reciente de cáncer de mama efectuado por biopsia percutánea.
MétodosEstudio prospectivo que incluyó pacientes diagnosticadas con cáncer de mama infiltrante mediante biopsia percutánea, que fueron evaluadas con ultrasonido axilar, caracterizando los ganglios en no sospechosos o sospechosos, cuantificando estos últimos y comparando este número con el resultado patológico de la cirugía axilar.
Resultadosfueron incluidas 142 pacientes, cuatro de ellas con cáncer bilateral. Ciento treinta y tres de los 146 tumores se presentaron clínicamente como T1-T2 N0. La mediana de ganglios sospechosos en ultrasonido fue de 2 (1-6) y la mediana de ganglios sospechosos resultantes en la biopsia quirúrgica fue de 1 (1-16), sin diferencia significativa (p = 0,1). La correlación entre el número de ganglios positivos encontrados por ultrasonido axilar y el número de ganglios axilares metastásicos hallados en cirugía fue del 72,7% (p = 0,0002), y la concordancia del 79% (IC95% 62,4-95,6%; p = 0,0001). La sensibilidad del ultrasonido axilar para diagnosticar alta carga tumoral axilar, con tres o más ganglios metastásicos versus baja carga tumoral, con 0, 1 o 2 ganglios metastásicos, fue del 86,6%; la especificidad, del 83,3%; el valor predictivo positivo, del 92%, y valor predictivo negativo del 71,4%.
ConclusiónNuestros resultados muestran que el ultrasonido axilar dirigido antes de la cirugía es capaz de diferenciar entre una axila de baja carga tumoral y una de alta carga tumoral, y puede ser usado como una herramienta para seleccionar qué tipo de tratamiento elegir, lo que debe ser demostrado en estudios aleatorizados multiinstitucionales.
The objective of this study was to determine the ability of axillary ultrasound to predict the number of lymph nodes with metastases found in axillary sentinel node surgery or axillary lymphadenectomy, in patients with a recent diagnosis of breast cancer after percutaneous biopsy.
IntroductionEvaluation of the axillary nodes is an essential part of the management of breast cancer, considered to be a staging procedure, for determining the treatment of this disease. Surgical management of the axilla in breast cancer has become less aggressive, with sentinel node biopsy replacing axillary lymphadenectomy in many cases. This shift has primarily taken place over the last two decades, after the publication of the ACOSOG Z0011 study, which demonstrated that, in patients with T1 and T2 invasive breast cancers with axillary metastases and without palpable lymphadenopathy, there was no survival benefit from axillary dissection after positive sentinel node biopsy if only one or two nodes were involved (low axillary tumour burden).1 The Z0011 study made it necessary to redefine the role of axillary ultrasound, a non-invasive and low-cost method, which, together with fine needle aspiration (FNA) or core biopsy, was used to select positive cases that would go directly to axillary lymphadenectomy, skipping the previous step of the sentinel node biopsy.
Since then, the need for preoperative evaluation of the axilla with ultrasound and potentially FNA in patients with breast cancer before surgery has been discussed. Studies have shown that patients with suspicious lymph nodes in the axilla detected with preoperative ultrasound ultimately had a higher number of positive nodes in axillary lymphadenectomy compared to those with positive nodes identified in sentinel node surgery.2–6 Just as there are studies that have focused on the use of axillary ultrasound to identify patients with a high tumour burden in the axilla, that is, with three or more involved lymph nodes,2,3,7 there are also studies to identify the group of patients with low tumour burden (one, two or none).8–11
The objective of this study was to correlate the number of pathological nodes detected in the preoperative axillary ultrasound with the number of pathological nodes detected in the final axillary pathological study; that is, to determine the ability of axillary ultrasound to distinguish between axillae with low and high tumour burden.
MethodologyThis was a prospective study of diagnostic tests in patients with invasive breast cancer diagnosed at Clínica Las Condes de Santiago between April 2018 and April 2020. Informed consent was obtained from all the patients included in the study.
All consecutive patients with invasive breast cancer with an initial diagnosis at our clinic by percutaneous biopsy were included, as well as patients diagnosed by this procedure at external centres and who were re-evaluated with images and a review of histological material at our clinic before surgery. Patients who received neoadjuvant chemotherapy, patients in stage T4, patients with local recurrence after treatment, patients who had had previous axillary surgery for breast cancer treatment, patients who underwent surgery in another institution and patients who did not have axillary surgery were excluded.
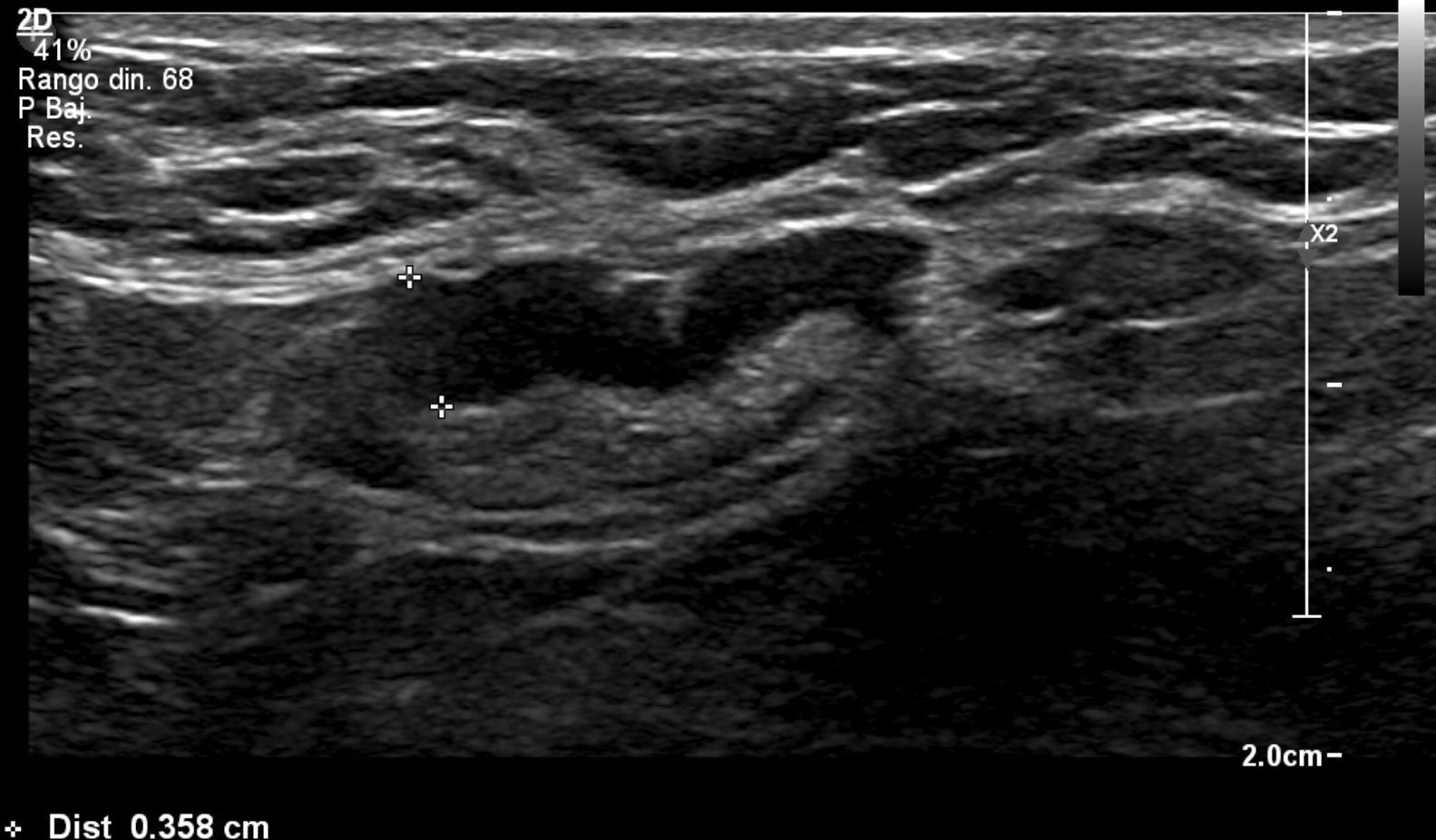
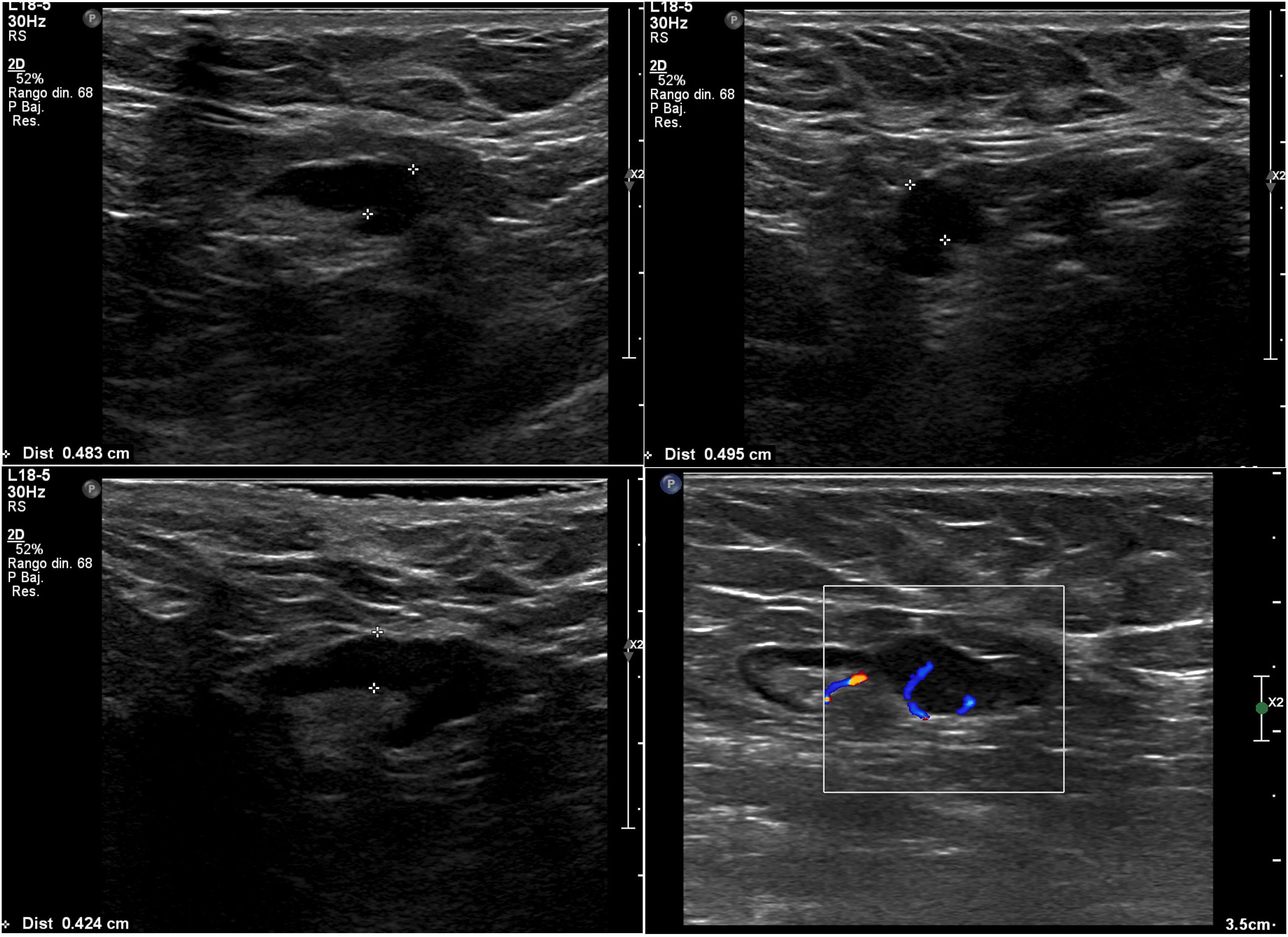
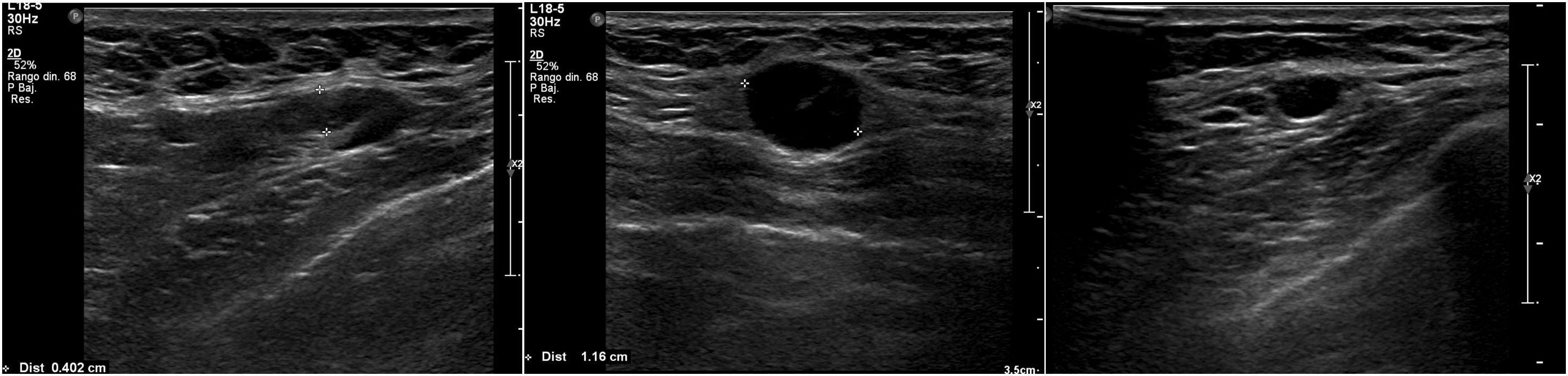
All patients included were evaluated by specialists in breast radiology using ultrasound directed at the axilla using a Siemens Epiq machine with an 18 MHz transducer. Evaluation of the axilla with ultrasound was performed during the diagnostic examination of a highly suspicious breast lesion or once the diagnosis of breast cancer was obtained prior to surgery. The nodes were categorised as not suspicious or suspicious, and the nodes classified as suspicious were counted. A node with eccentric or diffuse cortical thickening ≥3.5 mm (subdivided into ≥3.5 mm to <4 mm and ≥4 mm) or a node with loss of hilum was considered a suspicious sign. Axillae with 0, 1 or 2 metastatic nodes were classified as low tumour burden, and those with three or more metastatic nodes were classified as high tumour burden. Patients who underwent FNA for suspicious axillary lymphadenopathy at the request of their treating physician were recorded, which was left to the discretion of the treating physician.
The surgical study of the axilla included sentinel node biopsy, axillary lymphadenectomy or both, and was performed an average of 33 days (0-95) after the axillary ultrasound was performed. At our clinic, the choice to proceed directly to axillary lymphadenectomy can be due to clinically positive axilla with suspicious images and with confirmation by FNA. Sentinel node biopsy was carried out using radioisotope and isosulfan blue and was performed by specialist breast surgeons.
After surgery, the tumour characteristics were recorded, including tumour type, histological grade, tumour markers, size, multifocality and bilaterality, the type of axillary surgery performed, presence of metastatic nodes and number of nodes involved, and the characteristics of the tumour metastasis in the node.
The pathological result of the nodes from axillary surgery (whether sentinel node biopsy or axillary lymphadenectomy, if performed) was considered the gold standard for the analysis.
Statistical analysisFor the description of the categorical variables, absolute and percentage frequencies were used, and for the continuous variables, the median and minimum-maximum interval were used, because they were not distributed parametrically. The χ2 test was used to compare categorical variables and the Wilcoxon test for continuous variables.
In the diagnostic performance of ultrasound, a series of statistical analyses were performed: first, a Spearman correlation (Rho), and second, the association was determined with a logistic regression (OR). Then a ROC curve (AUC %) was drawn and the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated.
Statistical significance was set at p < 0.05. Stata 13 and Excel software were used.
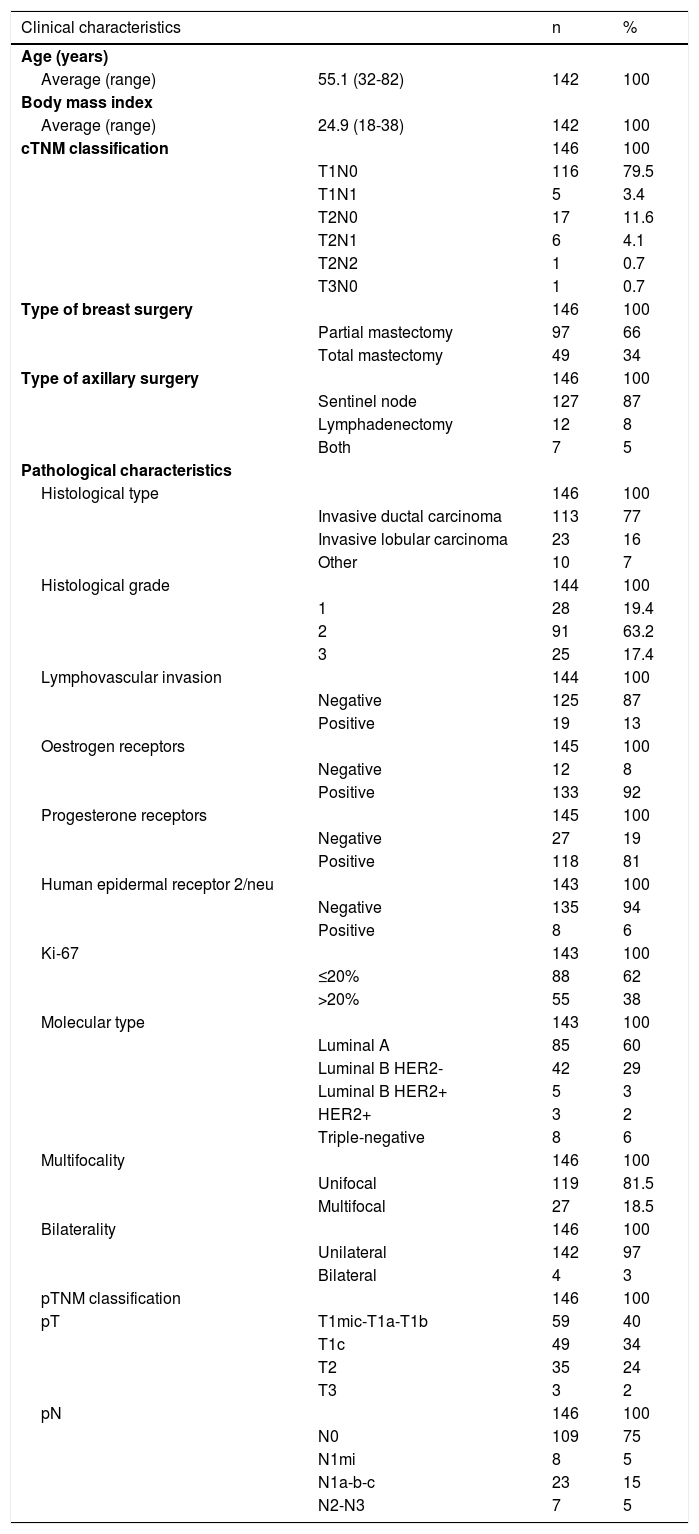
ResultsA total of 142 patients, all of them women, were included in this study. Four patients had bilateral cancer, so 146 tumours were studied. The clinical characteristics of the patients and the pathological characteristics of the tumours are summarised in Table 1. Of the 146 tumours studied, 133 presented clinically as T1-T2 N0.
Clinical and pathological characteristics of the study patients.
| Clinical characteristics | n | % | |
|---|---|---|---|
| Age (years) | |||
| Average (range) | 55.1 (32-82) | 142 | 100 |
| Body mass index | |||
| Average (range) | 24.9 (18-38) | 142 | 100 |
| cTNM classification | 146 | 100 | |
| T1N0 | 116 | 79.5 | |
| T1N1 | 5 | 3.4 | |
| T2N0 | 17 | 11.6 | |
| T2N1 | 6 | 4.1 | |
| T2N2 | 1 | 0.7 | |
| T3N0 | 1 | 0.7 | |
| Type of breast surgery | 146 | 100 | |
| Partial mastectomy | 97 | 66 | |
| Total mastectomy | 49 | 34 | |
| Type of axillary surgery | 146 | 100 | |
| Sentinel node | 127 | 87 | |
| Lymphadenectomy | 12 | 8 | |
| Both | 7 | 5 | |
| Pathological characteristics | |||
| Histological type | 146 | 100 | |
| Invasive ductal carcinoma | 113 | 77 | |
| Invasive lobular carcinoma | 23 | 16 | |
| Other | 10 | 7 | |
| Histological grade | 144 | 100 | |
| 1 | 28 | 19.4 | |
| 2 | 91 | 63.2 | |
| 3 | 25 | 17.4 | |
| Lymphovascular invasion | 144 | 100 | |
| Negative | 125 | 87 | |
| Positive | 19 | 13 | |
| Oestrogen receptors | 145 | 100 | |
| Negative | 12 | 8 | |
| Positive | 133 | 92 | |
| Progesterone receptors | 145 | 100 | |
| Negative | 27 | 19 | |
| Positive | 118 | 81 | |
| Human epidermal receptor 2/neu | 143 | 100 | |
| Negative | 135 | 94 | |
| Positive | 8 | 6 | |
| Ki-67 | 143 | 100 | |
| ≤20% | 88 | 62 | |
| >20% | 55 | 38 | |
| Molecular type | 143 | 100 | |
| Luminal A | 85 | 60 | |
| Luminal B HER2- | 42 | 29 | |
| Luminal B HER2+ | 5 | 3 | |
| HER2+ | 3 | 2 | |
| Triple-negative | 8 | 6 | |
| Multifocality | 146 | 100 | |
| Unifocal | 119 | 81.5 | |
| Multifocal | 27 | 18.5 | |
| Bilaterality | 146 | 100 | |
| Unilateral | 142 | 97 | |
| Bilateral | 4 | 3 | |
| pTNM classification | 146 | 100 | |
| pT | T1mic-T1a-T1b | 59 | 40 |
| T1c | 49 | 34 | |
| T2 | 35 | 24 | |
| T3 | 3 | 2 | |
| pN | 146 | 100 | |
| N0 | 109 | 75 | |
| N1mi | 8 | 5 | |
| N1a-b-c | 23 | 15 | |
| N2-N3 | 7 | 5 | |
The majority (122/146) of the axilla ultrasound evaluations were performed prior to percutaneous biopsy of breast cancer. In 11 of the patients, FNA was performed for axillary adenopathy, which was requested by their treating physician for palpable lymphadenopathy. In nine of these, the FNA was positive for malignancy.
There were two microinvasive cancers. In one of the bilateral cancer cases, a marker study was performed in only one of the cancers.
In the first instance, axillary surgery consisted of sentinel node biopsy in 91.7% (134/146), with seven patients also undergoing axillary lymphadenectomy during surgery after a positive rapid biopsy. The median number of sentinel nodes obtained was two (1-5). There were 12 patients in whom the surgeon decided to go directly to lymphadenectomy because of a clinically suspicious axillary node. The characteristics of the axilla on ultrasound and surgery are summarised in Table 2.
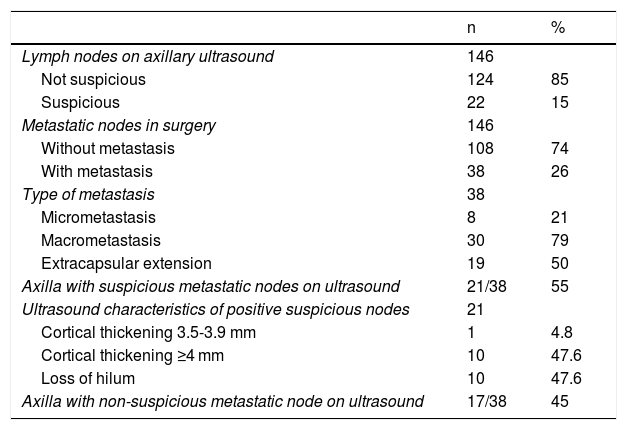
Characteristics of the axilla on ultrasound and in surgery.
| n | % | |
|---|---|---|
| Lymph nodes on axillary ultrasound | 146 | |
| Not suspicious | 124 | 85 |
| Suspicious | 22 | 15 |
| Metastatic nodes in surgery | 146 | |
| Without metastasis | 108 | 74 |
| With metastasis | 38 | 26 |
| Type of metastasis | 38 | |
| Micrometastasis | 8 | 21 |
| Macrometastasis | 30 | 79 |
| Extracapsular extension | 19 | 50 |
| Axilla with suspicious metastatic nodes on ultrasound | 21/38 | 55 |
| Ultrasound characteristics of positive suspicious nodes | 21 | |
| Cortical thickening 3.5-3.9 mm | 1 | 4.8 |
| Cortical thickening ≥4 mm | 10 | 47.6 |
| Loss of hilum | 10 | 47.6 |
| Axilla with non-suspicious metastatic node on ultrasound | 17/38 | 45 |
The median number of suspicious nodes on ultrasound was two (1-6) and the median number of suspicious nodes resulting from the surgical biopsy was one (1-16), with no significant difference between them (p = 0.1).
The area under the curve (ROC) of axillary ultrasound versus axillary node biopsy was 0.9087.
The correlation between the number of positive nodes found by axillary ultrasound and the number of metastatic axillary nodes found in surgery was 72.7% (p = 0.0002), and the agreement was 79% (95% confidence interval [CI], 62.4-95.6%; p = 0.0001).
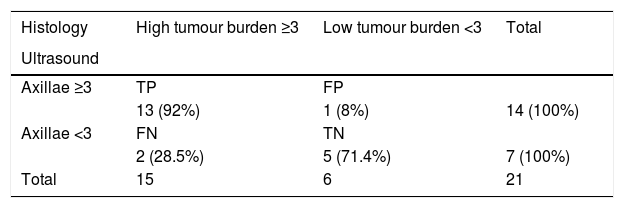
The sensitivity of axillary ultrasound to diagnose axillae with a high tumour burden versus those with a low tumour burden was 86.6%, the specificity was 83.3%, the PPV was 92% and the NPV was 71.4% (Table 3). There was no significant difference when comparing by type of surgical procedure.
Ability of axillary ultrasound to differentiate axillae with low tumour burden (0, 1 and 2 metastatic nodes) and axillae with high tumour burden (≥3 metastatic nodes).
| Histology | High tumour burden ≥3 | Low tumour burden <3 | Total |
|---|---|---|---|
| Ultrasound | |||
| Axillae ≥3 | TP | FP | |
| 13 (92%) | 1 (8%) | 14 (100%) | |
| Axillae <3 | FN | TN | |
| 2 (28.5%) | 5 (71.4%) | 7 (100%) | |
| Total | 15 | 6 | 21 |
Specificity: 83.3%; sensitivity: 86.6%; PPV: 92%; NPV: 71.4%.
FN: false negatives; FP: false positives; TN: true negatives; TP: true positives.
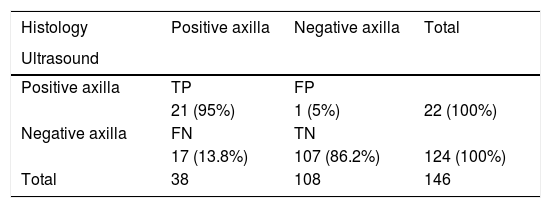
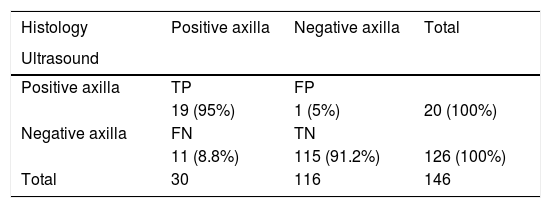
Regarding the overall diagnostic performance of axillary ultrasound to detect axillary metastases, of all the patients there was one false positive and 17 false negatives from a suspicious ultrasound; of the latter, six were axillary micrometastases. Consequently, in our series we obtained a sensitivity of 55%, a specificity of 99%, a PPV of 95% and a NPV of 86%. If we consider ultrasound to be a method that is based on morphological changes, then the diagnosis of micrometastasis is beyond its scope. Therefore, the values calculated without considering micrometastases were 63%, 99%, 95% and 91%, respectively (Tables 4 and 5).
Diagnostic performance of axillary ultrasound with micrometastases.
| Histology | Positive axilla | Negative axilla | Total |
|---|---|---|---|
| Ultrasound | |||
| Positive axilla | TP | FP | |
| 21 (95%) | 1 (5%) | 22 (100%) | |
| Negative axilla | FN | TN | |
| 17 (13.8%) | 107 (86.2%) | 124 (100%) | |
| Total | 38 | 108 | 146 |
Specificity: 99%; sensitivity: 55.2%; PPV: 95%; NPV: 86.2%.
FN: false negatives; FP: false positives; TN: true negatives; TP: true positives.
Diagnostic performance of axillary ultrasound without micrometastases.
| Histology | Positive axilla | Negative axilla | Total |
|---|---|---|---|
| Ultrasound | |||
| Positive axilla | TP | FP | |
| 19 (95%) | 1 (5%) | 20 (100%) | |
| Negative axilla | FN | TN | |
| 11 (8.8%) | 115 (91.2%) | 126 (100%) | |
| Total | 30 | 116 | 146 |
Specificity: 99%; sensitivity: 63%; PPV: 95%; NPV: 91.3%.
FN: false negatives; FP: false positives; TN: true negatives; TP: true positives.
Most of the axillary nodes that were metastatic and were suspicious on ultrasound had macrometastases and extracapsular involvement. There were 38 cases with metastatic nodes in the axilla and 21 of 38 had suspicious ultrasound signs. Of these 21 cases, only one had a cortical thickening of less than 4 mm (3.6 mm) as the only suspicious sign, which was micrometastasis. In 10 of the 21 patients, the suspicious ultrasound sign was cortical thickening of 4 mm or more. Of these 10 patients, one had micrometastases and nine had macrometastases, and of these nine, six had extracapsular involvement. The other 10 suspicious cases on ultrasound had nodes with loss of hilum, all of them with macrometastasis, and six of 10 with extracapsular involvement.
DiscussionEvaluation of the axilla by ultrasound is part of the preoperative study of patients with breast cancer and is widely used in clinical practice. With the current axilla management criteria established in ACOSOG Z0011,1 the quantification of the axillary nodes to differentiate low and high tumour burden in the axilla is of great importance in the management of patients diagnosed with breast cancer.
In this study, there was no significant difference between the number of suspicious nodes detected with axillary ultrasound and the number of metastatic nodes diagnosed at surgery, in a sample of patients predominantly in the early clinical stage (T1-T2 N0). The sensitivity of axillary ultrasound to diagnose axillae with high tumour burden (three or more metastatic nodes) versus those with low tumour burden (0, 1 or 2 metastatic nodes) was 86.6%, the specificity was 83.3%, the PPV was 92% and the NPV was 71.4%.
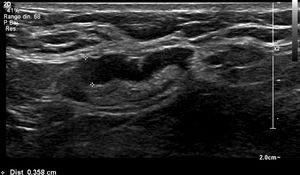
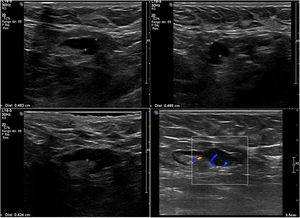
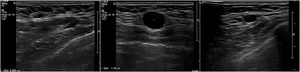
In the study by Caudle, two or fewer abnormal nodes on ultrasound had a 45% probability of having three or more nodes with positive histology in surgery;3 in the study by Farrell, a suspicious axillary node on ultrasound had 5.2 positive nodes in the final histology;4 and in the study by Pilewskie, more than one abnormal node in axillary images was significantly associated with three or more in the final histology.5 The best correlation and concordance between our study and those mentioned above may be related to the fact that our research is prospective, with a specialist who reviewed the axilla according to specific criteria and counted the number of abnormal axillary nodes while performing the examination, and not retrospectively as in the other studies. The ultrasound signs of suspicion of axillary metastasis that we used may also have had an influence, since, instead of choosing classic morphological criteria, such as those used by Bedi,12 which have a low positive predictive value, or those used by Amonkar,13 in which 47% of the lymph nodes defined as suspicious had a low tumour burden in axillary lymphadenectomy, we opted to conduct a literature search for suspicion criteria that could predict a high axillary tumour burden. These criteria were cortical thickening of more than 3.5 mm and loss of hilum, which were those that, in the study by Zhu,14 obtained the best sensitivity and specificity value for diagnosing three or more metastatic nodes.
We subdivided cortical thickening into two groups, the first from 3.5 mm to less than 4 mm, and the second 4 mm or more, and in the series there was only one patient who had metastatic nodes in surgery and in whom the only suspicious sign on ultrasound was a node with cortical thickening of 3.6 mm. In all the other patients, the metastatic nodes on ultrasound showed a cortical thickening of 4 mm or more and/or loss of hilum (Figs. 1–3).
In the retrospective study by Rooney,15 in which the author also looked for signs that better predict axillary involvement of three nodes or more, those with ultrasound signs of cortical thickening greater than 4 mm were also included as suspicious nodes, although in this study they also had to have a rounded shape and/or loss of hilum. Unfortunately, most of the studies aimed at evaluating axillary ultrasound to detect axillary metastases are retrospective and do not describe which ultrasound signs they used, leaving it to the discretion of the imaging specialist to assess whether they were classified as suspicious or not.3,5,10,16
The sensitivity, specificity, PPV and NPV of axillary ultrasound to diagnose axillary metastases in our study were 55.2%, 99%, 95% and 86.2%, respectively, similar to other studies such as that conducted by Barco, in which these values were 48%, 94%, 83% and 74%,10 and the study by Farshid, in which they were 39.8%, 94.6%, 79.8% and 78.1%.16 If we do not include micrometastases, which are beyond the scope of current images, and considering that there are studies that show that they would not have an impact on survival,17 in our study these values were 63%, 99%, 95% and 91.3%.
One of the limitations of our study is the small number of patients with metastatic nodes and the very low representation of HER2-positive and triple-negative cancers, since most of these patients underwent neoadjuvant chemotherapy. In our study, initial surgery consisted of sentinel node surgery in 91.7% of patients (134/146), with a median of two nodes obtained and only one sentinel node obtained in 35 of 134 cases, so it may be that some patients had more than two metastatic nodes undiagnosed in the axillary surgery. However, it should be noted that in the axillae with metastases that underwent sentinel node surgery, a single node was only obtained in three of them, two of these with lymphadenectomy as a second surgery. Moreover, in the patients with two or more metastatic nodes in sentinel node surgery, only three did not undergo subsequent lymphadenectomy. In one of these three, five sentinel nodes were obtained, three of them with micrometastases; in the second, two sentinel nodes were obtained with two nodes with micrometastases; and in the third, five sentinel nodes were obtained with two nodes with macrometastasis. It therefore seems unlikely that in our series there was a significant underrepresentation of the number of metastatic nodes in patients who only had sentinel node surgery. It can also be considered as a limitation that this study was conducted by an institution with specialist breast radiologists, which can influence the reproducibility of its results.
Despite these limitations, the findings of our study demonstrate that ultrasound has a good ability to differentiate between an axilla that is negative or with a low tumour burden and one with a high tumour burden, which can be very useful for determining the surgical course of action. In this context, it is worth mentioning the SOUND (Sentinel node vs Observation after axillary Ultra-souND) trial, which is an ongoing randomised study comparing sentinel node biopsy versus no axillary surgery in patients with breast cancer measuring less than 2 cm with clinically negative axilla and with negative preoperative axillary ultrasound.
As it is capable of detecting axillae with high tumour burden, axillary ultrasound would facilitate the selection of patients who may benefit more from the indication of neoadjuvant chemotherapy instead of primary surgery.
ConclusionAxillary ultrasound is able to differentiate between an axilla with low and high tumour burden and can be used as a tool to select which type of treatment to choose, which should be demonstrated in multi-institutional randomised studies.
FundingThis study received no specific grants from public agencies, the commercial sector or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Authorship- 1
Responsible for the integrity of the study: PNV.
- 2
Study conception: PNV.
- 3
Study design: PNV.
- 4
Data collection: PNV, BAD, PAH, MJC, CBA.
- 5
Data analysis and interpretation: PNV, BAD, PAH, MJC, CBA.
- 6
Statistical processing: MCC.
- 7
Literature search: PNV, BAD, PAH, MJC, CBA.
- 8
Drafting of the article: PNV, BAD, PAH, MJC, CBA.
- 9
Critical review of the manuscript with intellectually relevant contributions: STC, AIV.
- 10
Approval of the final version: PNV.
Please cite this article as: Neira Vallejos P, Aguirre Donoso B, Arancibia Hernández P, Behnke Arriagada C, Jacard Cangas M, Torres Castro S, et al. Ecografía axilar prequirúrgica en pacientes con cáncer de mama. Estudio prospectivo para valorar la capacidad de predecir la carga tumoral axilar. Radiología. 2022;64:28–36.