Hyperpolarized (HP) gases are a new class of contrast agents that permit to obtain high temporal and spatial resolution magnetic resonance images (MRIs) of the lung airspaces. HP gas MRI has become an important research tool not only for morphological and functional evaluation of normal pulmonary physiology but also for regional quantification of pathologic changes occurring in several lung diseases. The purpose of this work is to provide an introduction to MRI using HP noble gases, describing both the basic principles of the technique and the new information about lung disease provided by clinical studies with this method. The applications of the technique in normal subjects, smoking related lung disease, asthma, and cystic fibrosis are reviewed.
Los gases hiperpolarizados (HP) son una nueva clase de agentes de contraste que permiten obtener imágenes por resonancia magnética (RM) de alta resolución temporal y espacial de los espacios aéreos pulmonares. La RM con gas HP se ha convertido en una importante herramienta de investigación no solo para la evaluación morfológica y funcional pulmonar normal sino también para cuantificar los cambios patológicos regionales que se dan en las enfermedades pulmonares. El propósito de este trabajo es introducir la RM con gases nobles HP, describiendo, para ello, tanto los principios técnicos básicos como la información sobre las enfermedades pulmonares que ofrecen los estudios clínicos realizados. También hacemos una revisión de sus aplicaciones en sujetos normales, en enfermedades pulmonares secundarias al tabaco, el asma y la fibrosis quística.
Conventional magnetic resonance (MR) is based on the signals emitted by the protons of the hydrogen nuclei in water molecules and at present, it plays a limited role in the lung, especially due to its low protonic density. The susceptibility effect induced by its many air–tissue interfaces and cardiac and respiratory movement are other additional limitations when it comes to obtaining images of the pulmonary parenchyma.
Hyperpolarized gases (HP) are some kind of new contrast agent that allows us to obtain MR images of the pulmonary air spaces. They are still considered drugs in the pipeline and they have not been approved for clinical use yet by the U.S. Food and Drug Administration (FDA). This is why the use of this modality is limited to some academic medical centers. Nevertheless, HP gas MRI has become an important research tool, not only for the morphological and functional assessment of the normal lung, but also for the quantification of regional pathological changes in pulmonary diseases.1–8 HP gas MRI contributes information on ventilation, gas exchange and alveolar structure and the distal airways. One of its many advantages over CT or gammagraphy is that it does not use ionizing radiation making it especially useful in children and repeated or serial studies.
The goal of this article is to introduce noble HP gas MRI, describing both the basic principles of this procedure and the new information it provides about lung diseases.
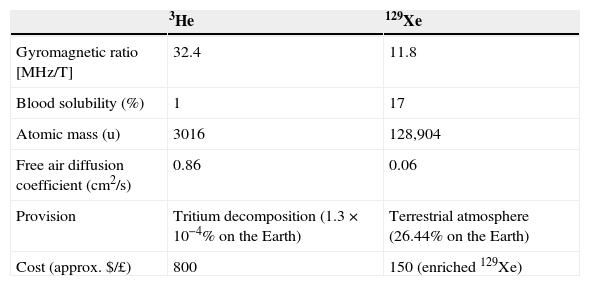
Magnetic resonance modality with hyperpolarized gasHyperpolarized gases: 3He and 129XeHP gases, when inhaled, allow us to obtain MRIs with high temporal and spatial resolution of the pulmonary air spaces. Two noble gas nonradioactive isotopes, 3He and 129Xe, can be hyperpolarized. Yet despite the fact that both gases can be hyperpolarized, HP gases 3He and 129Xe have different physiological properties (Table 1). 3He is not very soluble in blood or tissues making it a biologically inert gas. Once inhaled, it remains almost entirely within the air spaces. On the other hand, 129Xe's solubility in blood and tissues is relatively high—above all in lipids making it a biologically active gas, with anesthetic properties in high doses. It is precisely the solubility of xenon in biological tissues that makes it such a promising contrast agent for MRI. 129Xe allows us to assess together with MRIs the exchange of gases between the alveoli and the capillaries.9–13 The first HP gas MRI studies were obtained using HP 129Xe.14 However due to 3He's greater signal (a higher level of polarization and a higher gyromagnetic ratio) and due to the fact that it is safer, a large part of the initial work developed on humans with HP gas MRI has been done with 3He. But in the last few years there has been a growing demand for 3He for neutron detectors, and the demand has surpassed the supply index of the primary source – the decomposition of tritium. Now, 3He is expensive and worldwide reserves are limited. Instead 129Xe is very abundant on Earth. Until recently, polarizations were higher with 3He than with 129Xe yet a new technology has been developed to obtain larger quantities of highly polarized 129Xe.15 Due to the unlimited abundance of this gas in nature, to its lower and lower costs, to the breakthroughs in xenon polarizer technology and to the recent drop in the demand for 3He, 129Xe is starting to be used more commonly.
Hyperpolarized gases. Physical and physiological properties.
| 3He | 129Xe | |
|---|---|---|
| Gyromagnetic ratio [MHz/T] | 32.4 | 11.8 |
| Blood solubility (%) | 1 | 17 |
| Atomic mass (u) | 3016 | 128,904 |
| Free air diffusion coefficient (cm2/s) | 0.86 | 0.06 |
| Provision | Tritium decomposition (1.3×10−4% on the Earth) | Terrestrial atmosphere (26.44% on the Earth) |
| Cost (approx. $/£) | 800 | 150 (enriched 129Xe) |
He, helium; MHz, megahertz; T, tesla; Xe, xenon.
Using a polarizer, the nuclear spins of gas atoms are lined up in a small field outside the MR in such a way that polarizations of up to 50% can be achieved which is more than 100,000 times the polarization of hydrogen nuclei in a 1.5T magnetic field.16 The polarization process known as optical bombardment is performed by the method of spin-exchange with rubidium steam used as alkaline metal.15 Medical-grade nitrogen is used to attain a mixture of approximately 30% of HP gas and 70% of hydrogen with a total volume of approximately 1/3 of the forced vital capacity of the subject estimated through spirometry on the same day of the study. In order to minimize the possibility of having side effects from xenon in the central nervous system our group has come up with a xenon dose strategy to maintain the maximum alveolar concentration below 30%.
After polarization the gas is transferred to a plastic bag; it is transported to the MR and inhaled by the subject through a small plastic tube. The patient is asked to hold his breath for 20s or less. Vital signs, including O2 saturation in blood and heart rate, are monitored in all patients throughout the procedure.
The high levels of polarization obtained allow us to see the pulmonary air spaces through MRIs. We obtain images of the HP gases directly with the MRIs unlike the gadolinium-based contrast agents that are used to alter the relaxation properties of the nearby hydrogen nuclei.
Magnetic resonance imagesIn addition to the gas polarization several technical requirements are necessary. The MRIs are modified through a broadband amplifier that allows us to operate at the resonance frequencies of the HP gas. Specific radiofrequency coils are required.
When using different sequences, we can obtain both functional and morphological information of lungs. HP gas MRI can be used to study pulmonary function using to this end static and dynamic spin density images (ventilation image) or oxygen sensitive images. Also HP 129Xe allows us to obtain information on gas exchange. Additionally the pulmonary structure of alveoli and the distal airway can be assessed using weighted diffusion images.
- a.
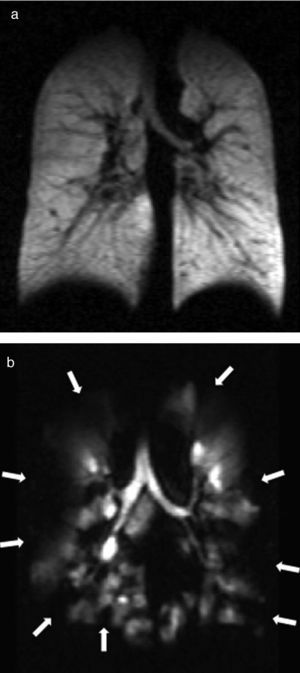
Ventilation image. It is obtained with spin density images while the subject is holding his breath after inhaling the HP gas. After breathing in the HP gas, the air spaces are filled and when obtaining the MRIs we get to see the changes in regional ventilation due to the air flow obstruction. Lots of gas reach the lung areas that are well ventilated and they look hyperintense in the MRI whereas the poorly ventilated areas appear hypointense (Fig. 1). This is conceptually similar to the pulmonary ventilation images of nuclear medicine. Dynamic ventilation MR is attained using ultrafast sequences and is used to show the distribution of gas in the lung parenchyma over time, through a subsecond-temporal resolution. Modern computer processing allows us to automate the data in an effort to quantify ventilation defects in HP gas MR.17
- b.
Measurement of the intrapulmonary oxygen pressure (O2P). The gas polarization loss index is highly influenced by the local environment. Oxygen due to its paramagnetic effect accelerates the longitudinal relaxation of the HP gas. By assessing the polarization loss index it is possible to calculate indirectly the local oxygen concentration18,19 and then find which ventilation/perfusion discordance areas really are.
- c.
Alveolar-capillary gas exchange. After inhaling HP 129Xe, the gas moves across the functional route of gas exchange in the lung spreading from the alveolar spaces to the alveolar septa (blood and tissue).20 Given that the spectral peaks associated with the 129Xe magnetization in the different pulmonary compartments have different chemical displacements it is possible to monitor the diffusion kinetics of 129Xe magnetization from one compartment to the other and differentiate the gaseous phase of 129Xe (xenon within the alveolar space) from that of the dissolution (129Xe within the alveolar epithelium, interstitium, capillary endothelium and blood).
Several data acquisition methods have been developed to obtain information on both gas exchange and gas alveolar-capillary uptake. In saturation recovery with chemical displacement a radiofrequency selective pulse is initially used to destroy the 129Xe magnetization in the dissolved phase. Then the recovery of 129Xe magnetization of the dissolved phase is seen when the HP 129Xe spreads from the alveolus to the alveolar interstitium.21
Compared to saturation recovery with chemical displacement that calculates directly xenon magnetization in the dissolved phase the method of contrast though xenon transference measures diffusion in the dissolved phase indirectly by analyzing the attenuation of the gaseous phase signal after multiple opportunities for interface diffusion.21 The pulmonary tissue-alveolar volume coefficient, the surface-volume coefficient and the thickness of the blood–gas barrier can then be determined.20
- d.
Diffusion images. In conventional MRIs contrast in the diffusion images depends on the molecular movement of water. This sequence is sensitive to the variations of this random molecule movement that can be altered in disease. The same principle is applied to diffusion-weighted HP gas MRI, this time to study the random movement of gas atoms within the alveoli. The studies are conducted using pulse sequences with bipolar diffusion gradients based on the Fast Low Angle SHot (FLASH) gradient echo.
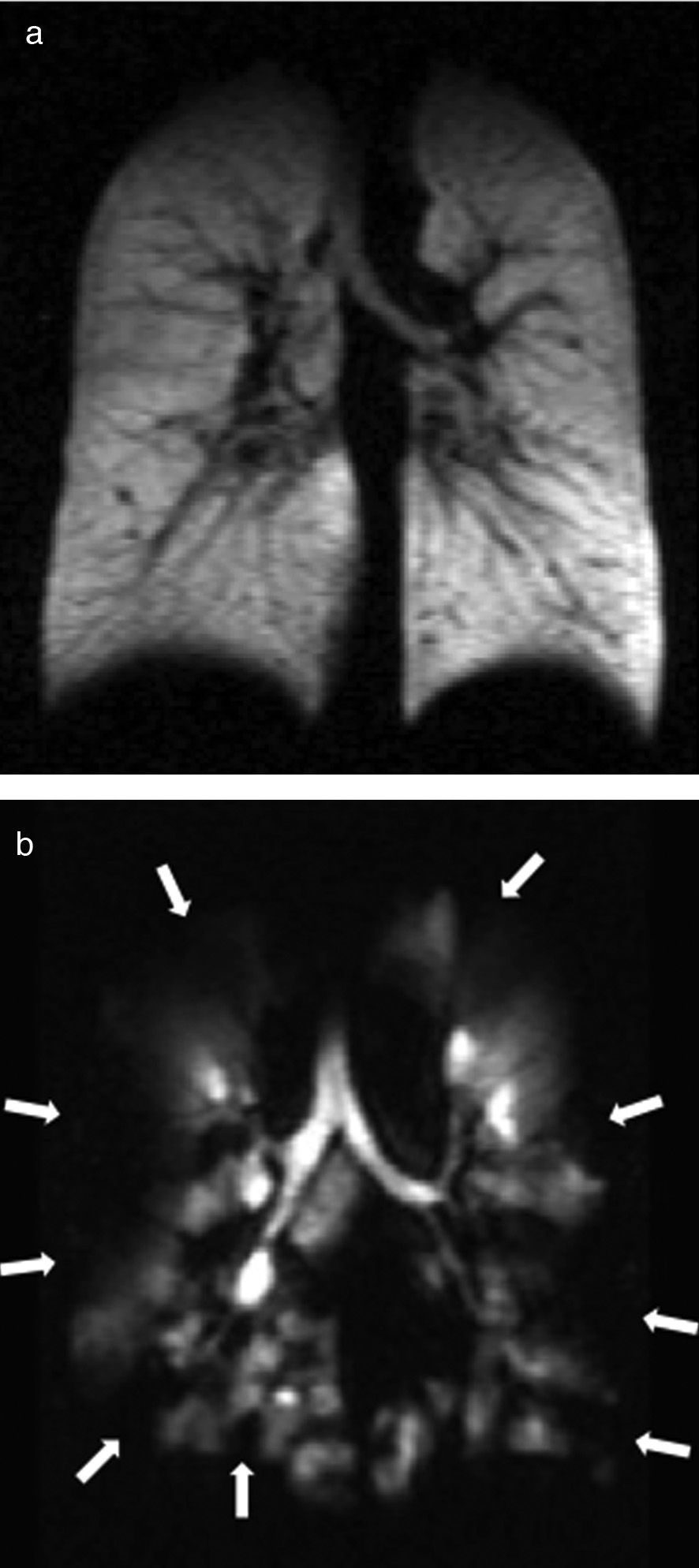
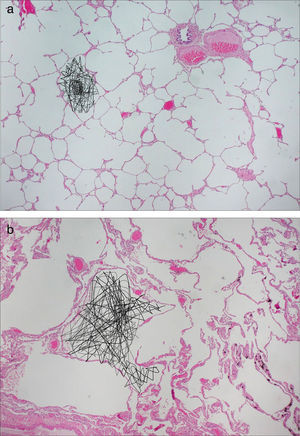
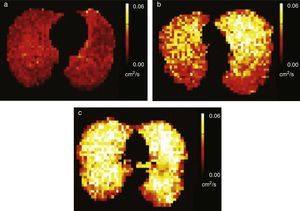
Diffusion images allow us to examine pulmonary microstructure. The diffuse movement of the HP gas molecules within the air space decreases the MR signal. From these data it is possible to determine an apparent diffusion coefficient (ADC) and a diffusion-associated median distance. Evidence suggests that the ADC provides a non-invasive quantitative measurement that correlates with the alveolar shape and size.22–27 In normal patients gas diffusion is restricted by the alveolar walls which results in low homogeneous ADC values. In patients with diseases that increase the alveolar size due to the expansion of the acinar airways or tissue destruction, such as emphysemas, diffusion restriction is reduced and the gas ADC increases significantly26–30 (Fig. 2). There have been publications of precise correlations between global and regional ADC estimated through HP gas MRI and alveolar size measured anatomopathologically.22,31–33
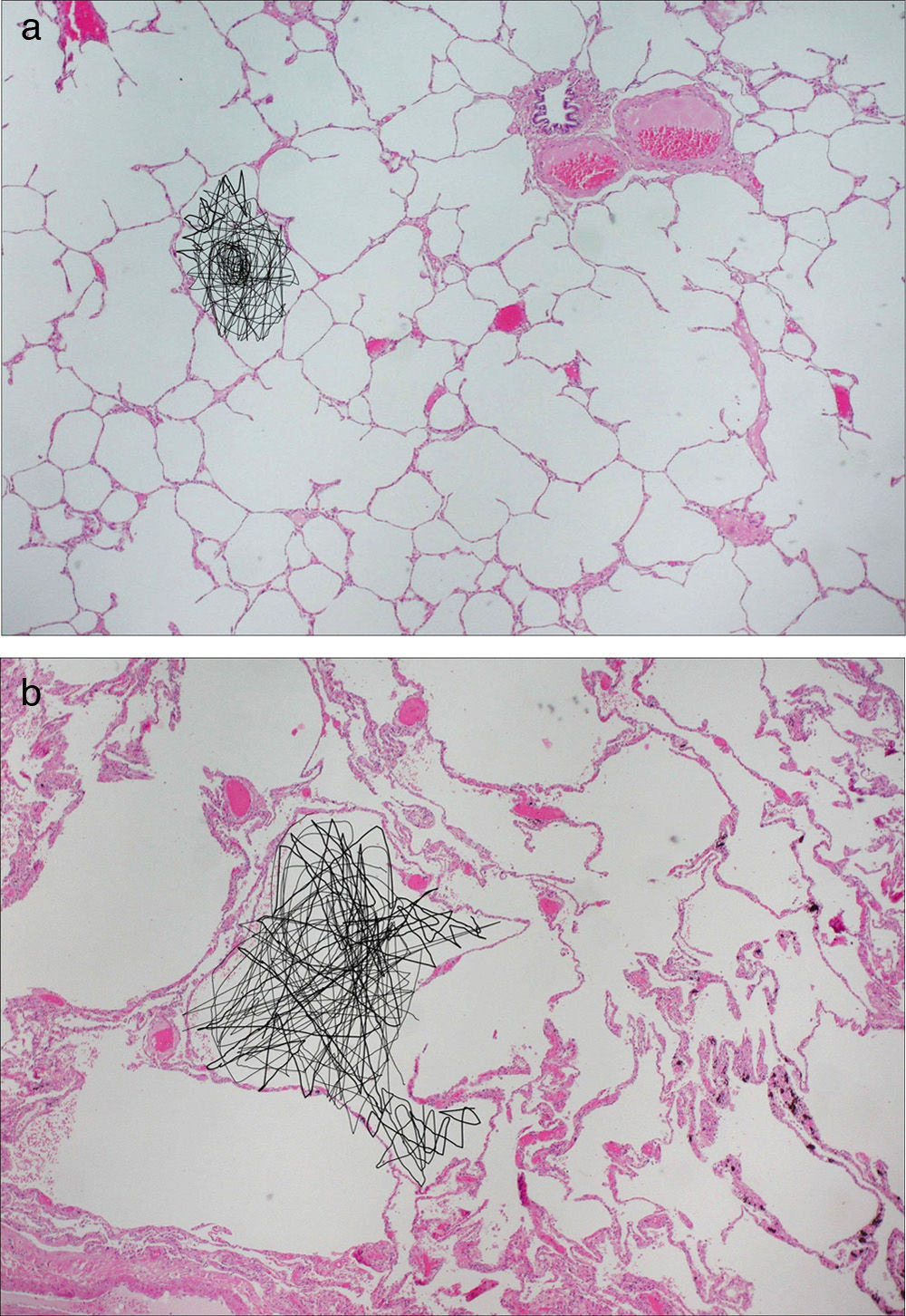
Figure 2.Diffusion-weighted HP gas MRI. Pulmonary anatomopathological image with schematics of the random movement of gas molecules in the alveoli. (a) Gas diffusion is limited by the alveolar walls in healthy subjects. (b) In emphysematous patients the limitations to diffusion are fewer due to the destruction of alveolar walls.
(0.33MB).He pulmonary morphometry based on diffusion-weighted HP He RM allows us to obtain quantitative measurements of both the acinar airways and the alveolar microgeometry. Based on the Weibel34 group's morphometric work pulmonary acini are cylindrical airway networks covered by alveoli. The 3He diffusion measurements are used determine acinar duct radii as well as the alveolar sleeve depth. It is possible to calculate standard morphometric parameters such as the median linear interception, the surface-volume coefficient and alveolar density.353He morphometry has proven to have an excellent correlation with the anatomopathological measurements.35
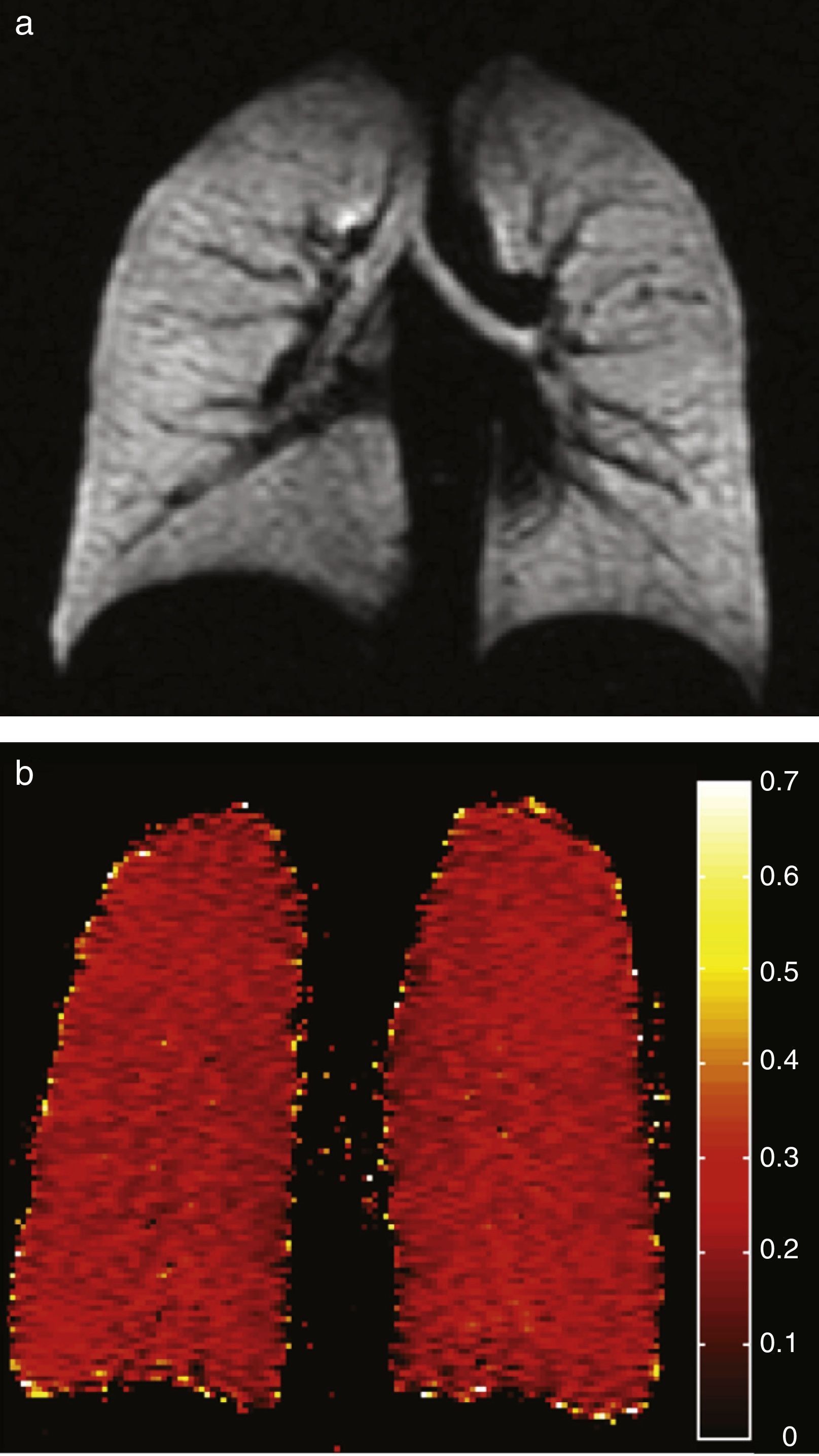
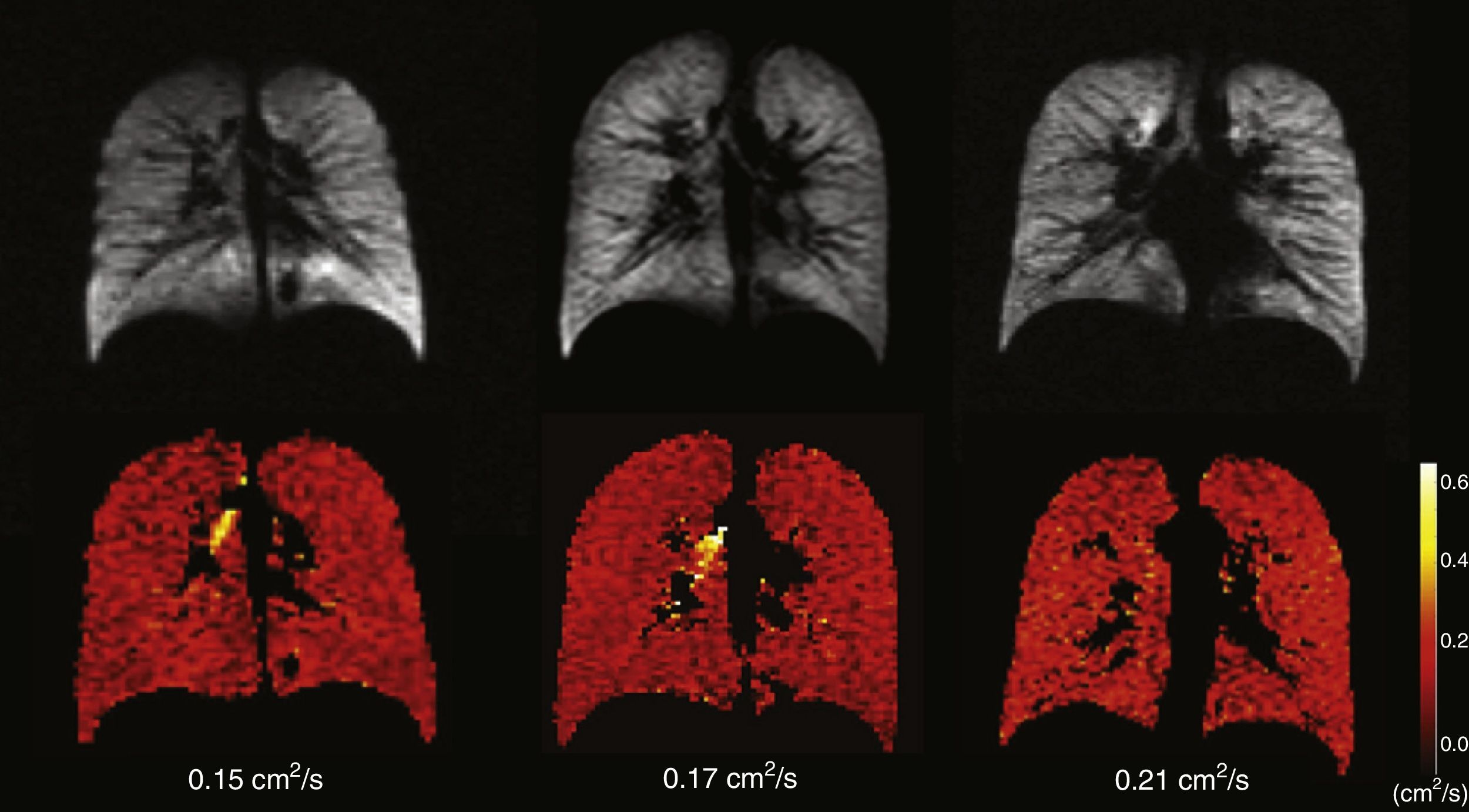
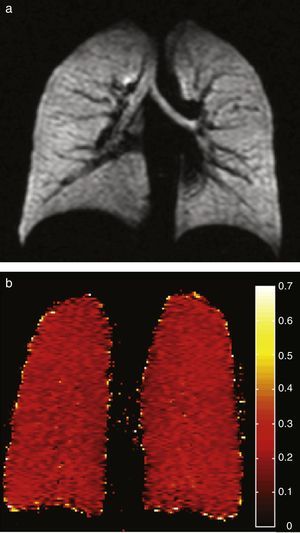
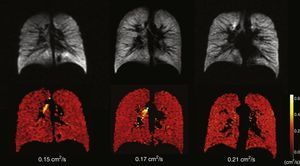
Healthy young adults usually have one homogeneous signal both in the HP gas ventilation and in diffusion MRI with uniformly low ADC values (Fig. 3). A study of 29 normal subjects whose ages ranged from 4 to 30 years (median 14 years, standard deviation 6.7 years) showed a strong trend toward a greater ADC in 3H MRI in older subjects likely due to a larger alveolar size as the lung grows indicative that the HP gas MRI is capable of examining the lung's normal growth and development bloodlessly (Fig. 4).36 The high sensitivity of the ADC for the detection of alveolar enlargement has also been described in older people who have never smokers.23
Age-associated ADC changes with hyperpolarized 3He. Ventilation images (below) and its corresponding ADC maps (above) in 3 healthy subjects; left to right: one child (10 years), one teenager (22 years) and one young adult (29 years). The 3 of them have normal homogeneous ventilation, without defects, and homogeneous low ADC values though progressive higher with age (0.15, 0.17 and 0.21cm2/s respectively).
Age-related changes in the ventilation pattern have also been described. It has been confirmed that healthy elderly people without known respiratory disease and with a normal spirometry have ventilation defects that are located in the periphery of lungs.37 With age, the percentage of the lung occupied by ventilation defects increases and the volume of these defects is no different from the volume of the ventilation defects of former smokers of the same age suffering from stage II COPD.38
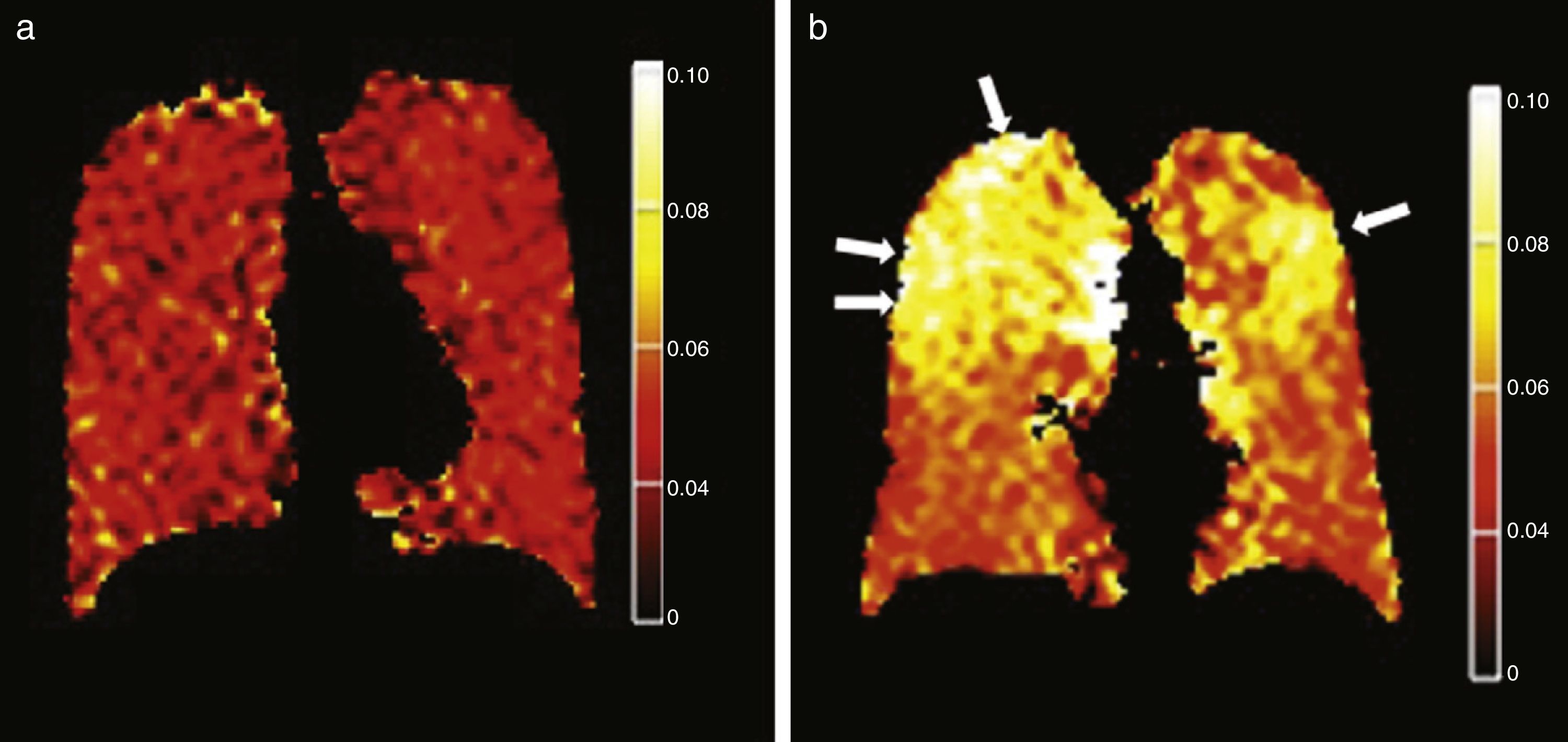
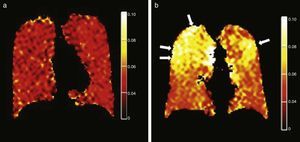
Tobacco related lung diseasesDiffusion-weighted HP gas MRI is one of the most developed HP gas modalities for the study of emphysemas.26,29,30,32,39 Diffusion-weighted HP 3He MRI has found enlarged air spaces in animal and human emphysematous models.26–323He ADC global and regional measurements have been compared in patients with emphysemas and normal subjects26,30,40 being a good correlation with spirometry confirmed.23,30,41 ADC maps obtained through diffusion-weighted HP 129Xe MRI can distinguish emphysematous patients from healthy subjects with results similar to those of 3He MRIs (Fig. 5).42 Diffusion-weighted HP 129Xe MRIs can be even more sensitive to the structural alterations of the lung than with 3He due to its lower diffusion coefficient.25,43
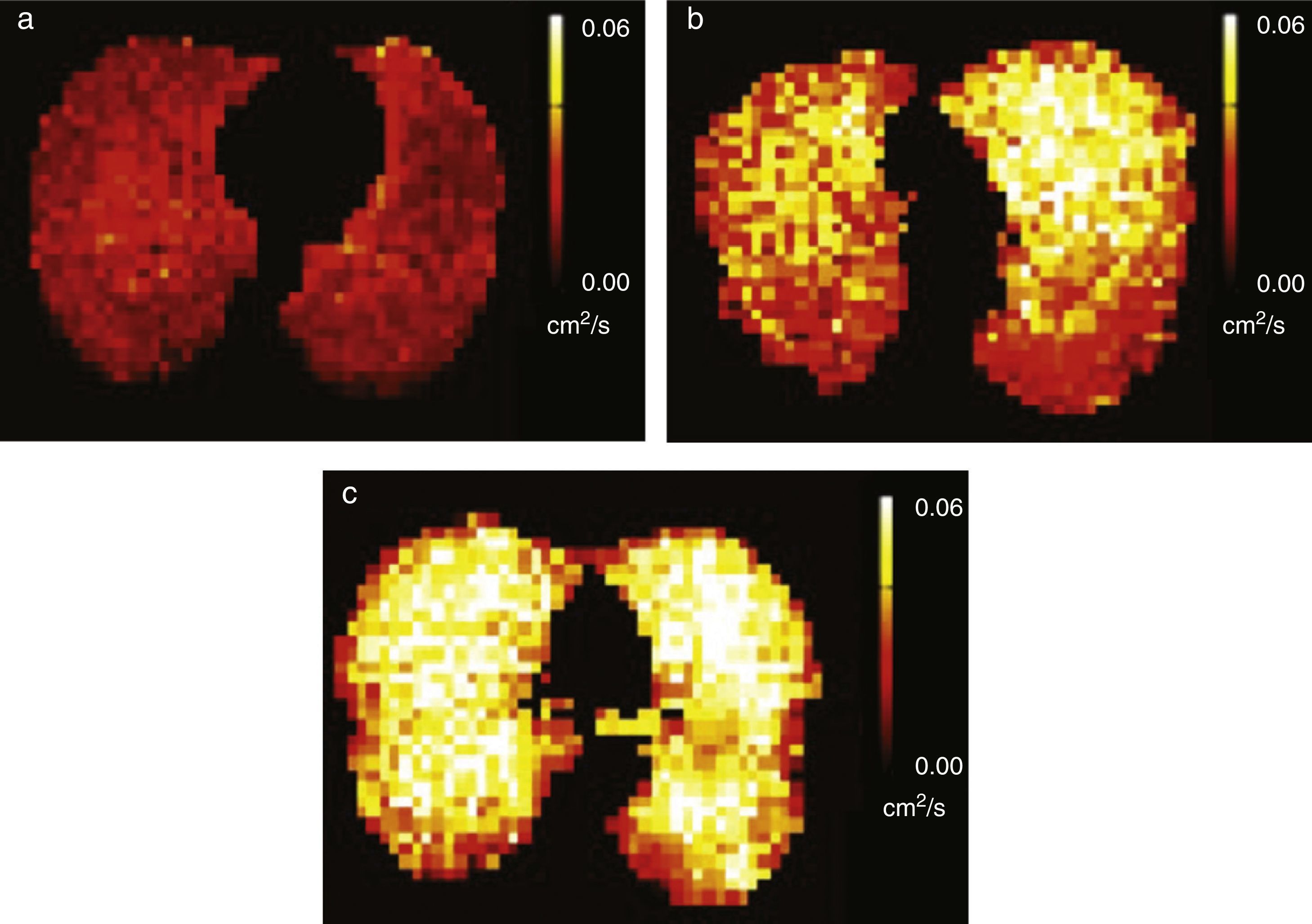
3He pulmonary morphometry can help detect alterations in the acinar structure of smokers with emphysematous changes with a greater sensitivity than that of conventional procedures (pulmonary function tests and CT).44 In a recent study diffusion-weighted HP 3He MRI detected alterations of the pulmonary function in smokers before symptom onset.29 The authors described a significant statistic correlation between the median ADC and the usual pulmonary function tests in asymptomatic smokers which strengthens the fact that this modality is more sensitive for the early detection of the disease. The ADC sensitivity to detect alveolar increase has also been described in passive smokers heavily exposed to tobacco smoke45 (Fig. 6).
Alveolar enlargement in passive smokers. ADC maps with hyperpolarized 129Xe gas of three different subjects, (a) one non-smoker with little exposure to tobacco smoke, (b) one passive smoker with heavy exposure to smoke and (c) one smoker show progressively higher ADC values and consequently larger alveolar size.
Asthma is the most common chronic disorder in children and it is a global problem affecting almost 300 million people of all ages, ethnic groups and countries.46 The economic burden of asthma in Spain is around ¢1480 million in the adult population.47
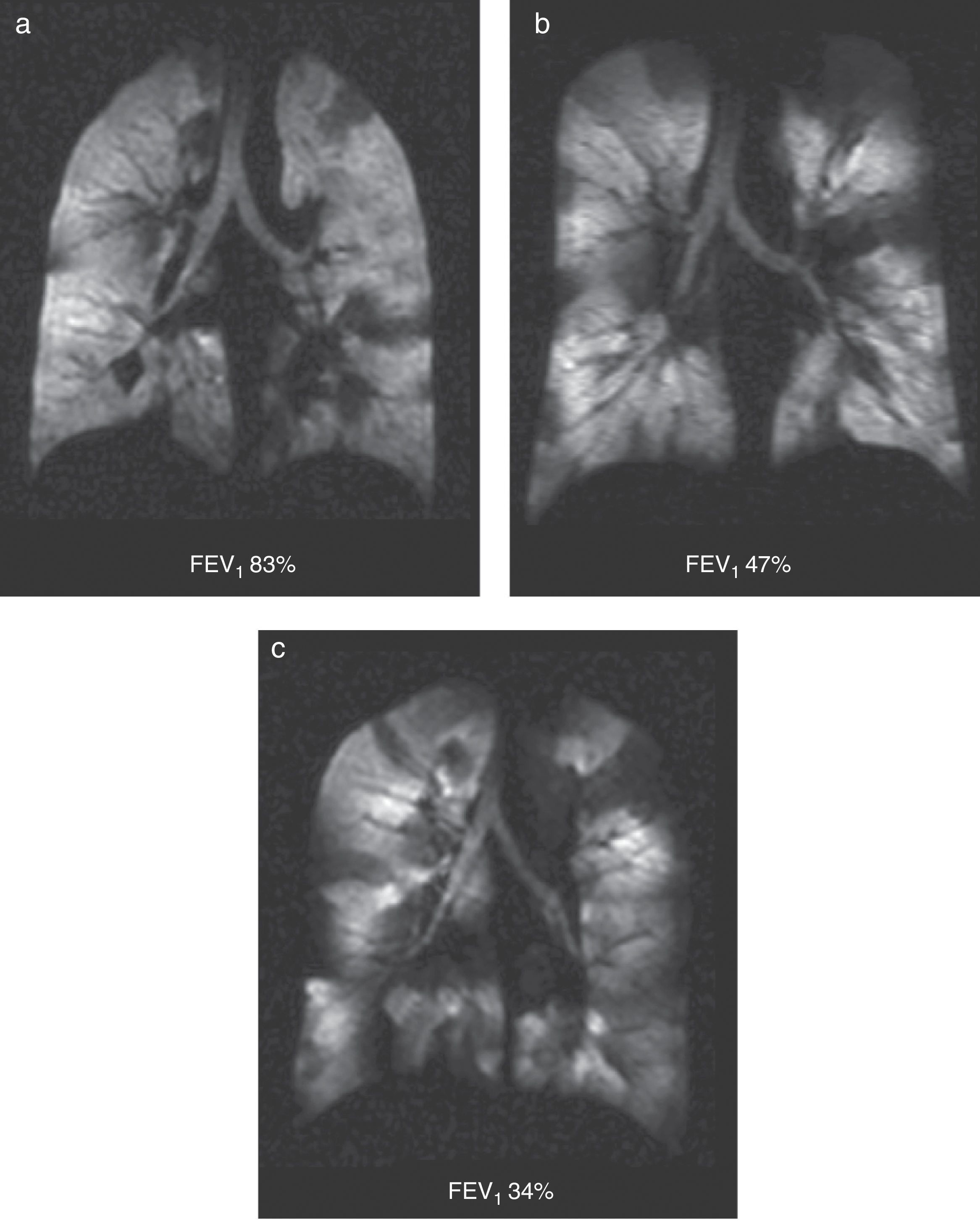
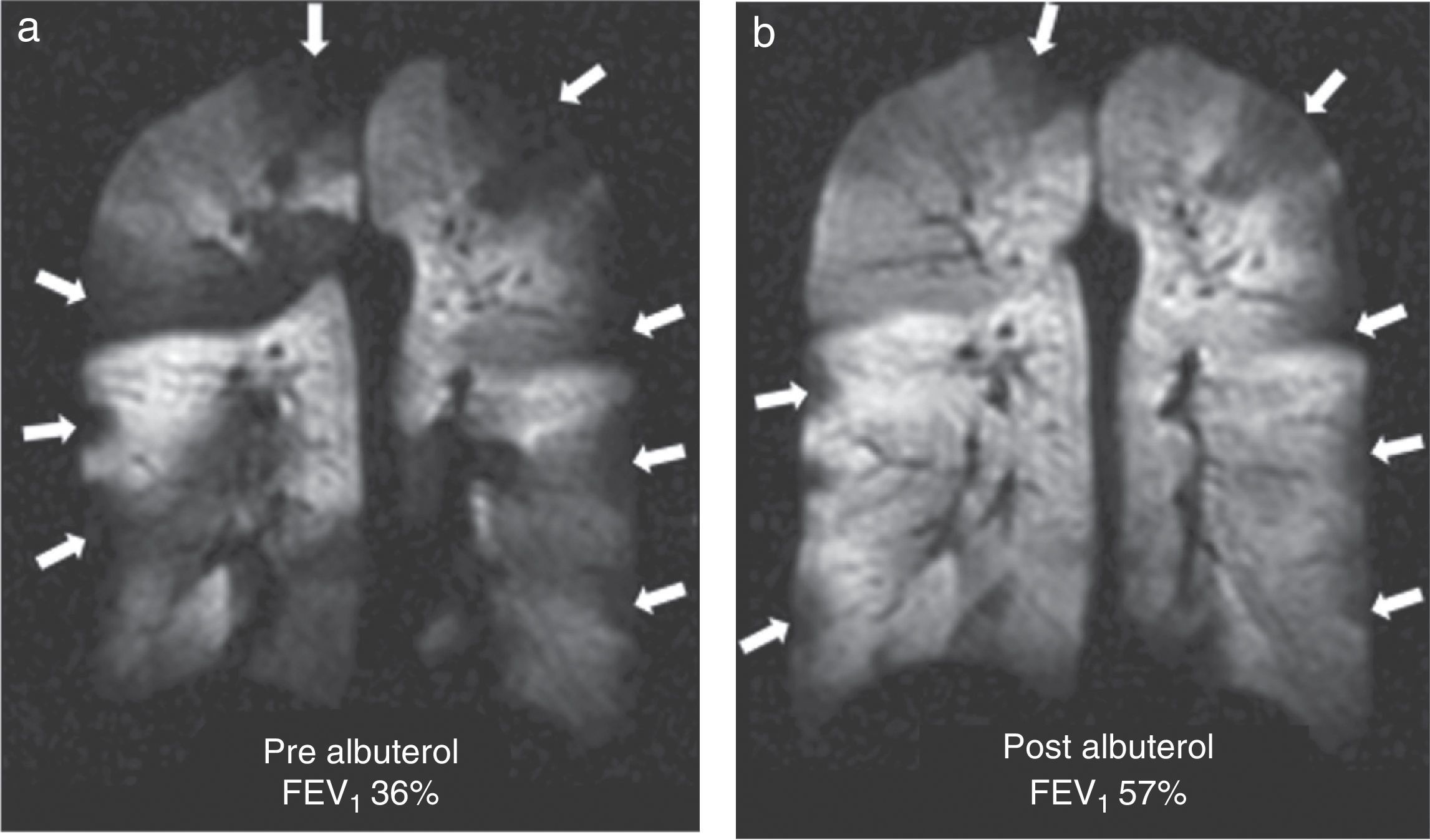
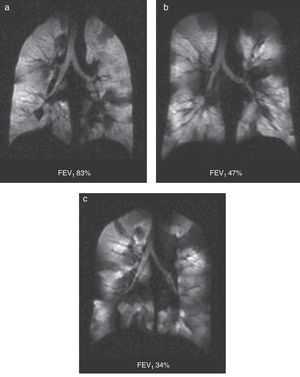
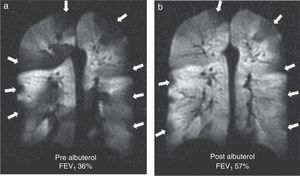
It is a chronic inflammatory lung disorder affecting mainly the small- and medium-sized airways. Symptoms are usually associated with variable obstructions of the airways and can be caused by bronchoconstriction, airway wall edema, mucous impact and airway remodeling. With the CT we can see in detail the bronchial walls and the air trapping. HP gas MRI can assess the functional alterations of the air flow in the lung secondary to structural changes in the airways. We have been able to confirm ventilation defects with HP gas MRI even in asymptomatic patients and those whose spirometries are normal48 (Fig. 7). The number of ventilation defects detected through HP 3He MRI has been associated with the seriousness of the disease established through spirometry or with the frequency of the symptoms.3 On the other hand the ventilation defects are medically induced through methacholine (an inhaled direct bronchoconstrictor)48 and reversed through an inhaled bronchodilator (albuterol) (Fig. 8).
Ventilation defects in asthma. Coronal ventilation images with 3He in 3 asthmatic patients with decreasing percent values of forced expiratory volume: (a) 83%, (b) 47% and (c) 34%. Multiple bilateral ventilation defects can be seen in the three images rising progressively as the forced expiratory volume diminishes even present with a normal spirometry (a).
HP gas MRI has given us a new perspective in the physiopathology of asthma. We have learned that the distribution of ventilation anomalies in the lung does not happen at random as we could expect if the whole lung was affected equally instead certain lung regions are more prone to be altered in the ventilation images indicative that the seriousness of the disease varies regionally.49,50 HP gas MRI findings can have important implications in regional therapies such as bronchial thermoplasty (RF ablation of the smooth muscle of the airway wall during bronchoscopy) since we can obtain better results with this therapy if we use images that help us guide this procedure.
Dynamic ventilation images allow us to analyze the kinetics of air trapping in asthmatic patients with a good concordance between the HP gas MRI and the CT trapping areas.51 The lack of radiation needed to perform MRIs makes MRIs an attractive alternative above all in longitudinal studies.
Cystic fibrosisCystic fibrosis (CF) is the most frequent fatal genetic disorder among the Caucasian population with an estimated incidence of one in 2500 alive newborn babies.52 This disease caused by the dysfunction of the CF transmembrane regulator (CFTR) proteine is characterized by more viscous exocrine secretions both in the lung and the gastrointestinal tract. In the lung it obstructs the bronchi leading to bacterial colonization, chronic pulmonary infection, bronchiectasis and fibrosis. Progressive pulmonary disease is the 1st cause of death in CF so it usually needs to be monitored through imaging techniques.
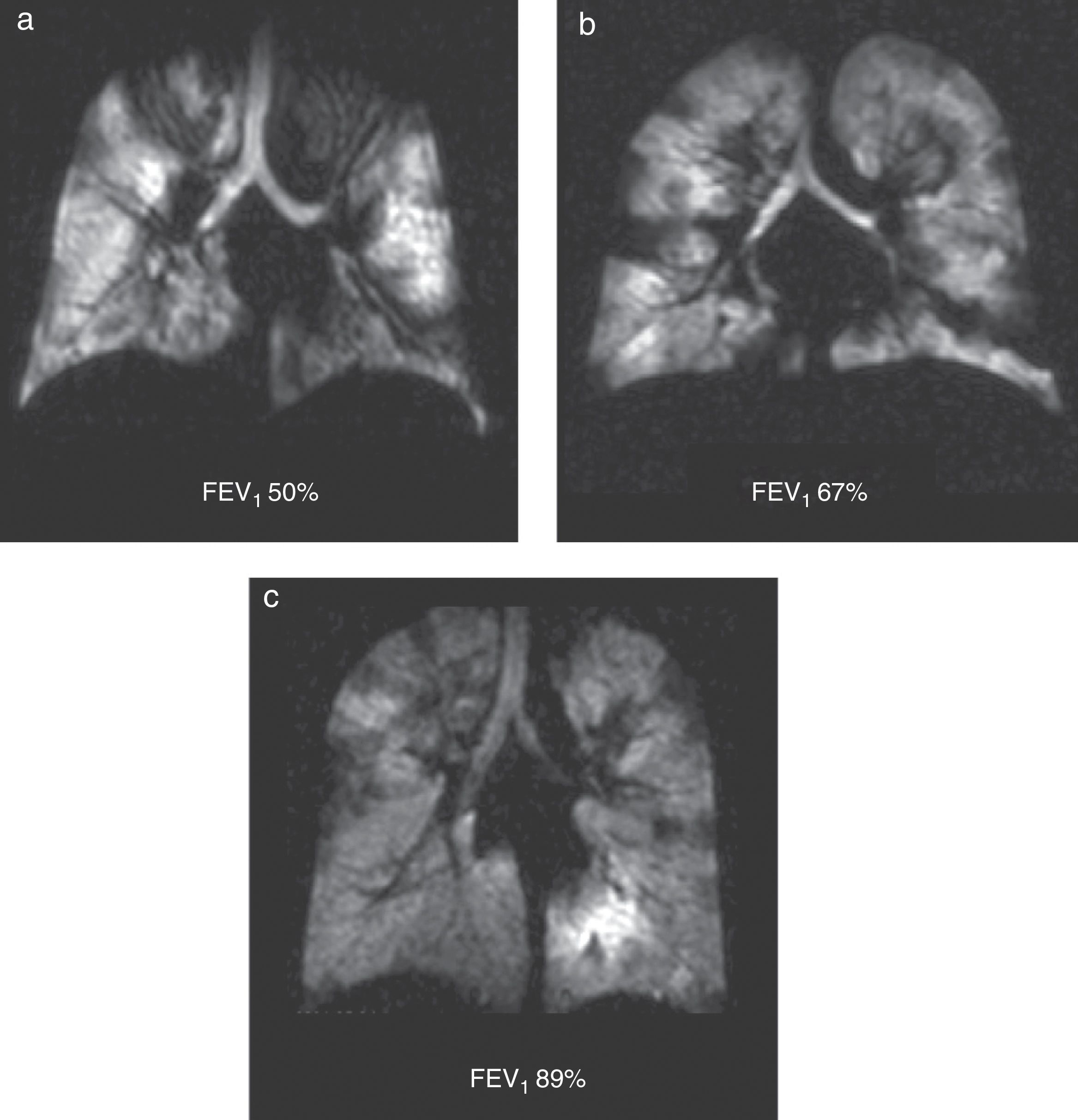
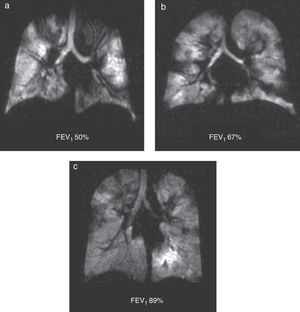
Through HP 3He MRIs we have been able to describe ventilation defects in CF patients—even in those whose pulmonary function tests are completely normal52 (Fig. 9). Ventilation defects change based on the bronchodilator therapy used and the mechanical clearance of the airways.52
HP gas MRI correlates well with structural anomalies of CT53 and spirometry52,53 yet its correlation with chest radiographies is bad.54 Correlation with spirometry is even more solid than that of CT.53 For all these reasons, HP gas MRI is regarded as a sensitive test to describe the early stages of this condition and it can become a biomarker of pulmonary CF given that it can be useful to monitor both its clinical state and the response to treatment.55
Future course of actionBecause it does not imply the use of ionizing radiation HP gas MRI is promising as a useful research tool for the investigation of the physiopathology of several pulmonary diseases and the assessment of the effectiveness of therapies. Yet this modality still needs further development before it can become a solid clinical tool. The important technical breakthroughs made in xenon polarizers and the recent scarcity of 3He indicate that 129Xe can eventually be used more commonly.
ConclusionHP gas MRI has become an important functional, morphological lung research tool from which we can obtain sensitive new information about the physiopathology of several pulmonary diseases.
Ethical responsibilitiesProtection of people and animalsAuthors declare that the proceedings followed abide by the ethical regulations of the corresponding human experimentation committee and the World Health Organization and the Helsinki Declaration.
Data confidentialityThe authors confirm that the protocols of their corresponding institutions and centers on publishing data from patients have been duly followed.
Right to privacy and informed consentThe authors have obtained the prior written consent from the patients and/or individuals aforementioned. This paper is in possession of the corresponding author.
Authors- 1.
Manager of the integrity of the study: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH.
- 2.
Study Idea: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH.
- 3.
Study Design: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH.
- 4.
Data Mining: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH.
- 5.
Data Analysis and Interpretation: N/A.
- 6.
Statistical Analysis: N/A.
- 7.
Reference Search: LF, TA, JPM, WFH.
- 8.
Drafting of work: LF, TA, JPM.
- 9.
Critical review of the manuscript with intellectually relevant remarks: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH.
- 10.
Approval of final version: LF, TA, JPM, EEDL, GWM, JFM, IR, WFH
Dra. Talissa A. Altes, M.D. received grants from NIH, University of Virginia Children's Hospital Medical Center, FAMRI, Siemens; consulting fees from Novartis, Guebert and lecture fees from Phillips. Dr. Grady Miller, M.D. received a grant from the NIH-NHLBI. Dr. Eduard de Lange, M.D. received a grant from NIH and royalties from Oxford University Press. Dr. F. William Hersman, M.D. received a grant from NHLBI. John Mugler received a grant from Siemens Medical Solutions. The remaining authors did not declare any conflicts of interest whatsoever.
Please cite this article as: Flors L, Altes TA, Mugler 3 JP, de Lange EE, Miller GW, Mata JF, et al. RM con gases hiperpolarizados: Nuevas perspectivas en el estudio de enfermedades pulmonares. Radiología. 2015;57:303–313.