Inflammatory mastitis of unknown etiology includes the entities periductal mastitis and idiopathic granulomatous mastitis. These relatively uncommon processes usually affect women of childbearing age. They usually present as a palpable mass that is painful on palpation. These lesions have an insidious clinical course and are difficult to diagnose. In some cases, they can resemble malignant disease, especially in some very developed cases where they can resemble inflammatory carcinoma. When considered all together in the appropriate clinical context, the magnetic resonance imaging signs enable us to approach a specific diagnosis. These entities share clinical and radiological characteristics with malignant processes, so biopsies are fundamental to rule out malignancy.
Las mastitis inflamatorias de etiología desconocida incluyen a la mastitis periductal y la mastitis granulomatosa idopática. Son procesos relativamente infrecuentes que suelen afectar a mujeres en edad fértil. Suelen presentarse como una masa palpable dolorosa a la palpación, de curso clínico tórpido y difícil diagnóstico. En algunos casos pueden semejarse a patología maligna y especialmente algunos casos muy floridos al carcinoma inflamatorio. La RM en estas mastitis proporciona signos que nos permiten en su conjunto y en un contexto clínico adecuado, aproximarnos a un diagnóstico específico. Estas entidades comparten características clínico y radiológicas con procesos malignos, siendo fundamental descartarlo mediante biopsia.
Mastitis is the inflammation of mammary parenchyma independently of its cause, representing reactive inflammatory changes which in some cases are the result of infection and in others when the aetiology is not entirely clear.
Non-infectious mastitis is relatively infrequent and usually affects women of child-bearing age. It usually presents on examination as a painful palpable mass, with an insidious clinical course and is difficult to diagnose. In some cases it may resemble a malignant pathology,1,2 especially in some very advanced cases where they can resemble inflammatory carcinoma. For these reasons, biopsy is usually required for diagnosis.3
The aim of this study is to describe the characteristics of periductal mastitis (PDM) and idiopathic granulomatous mastitis (IGM) on magnetic resonance imaging (MRI).
Material and methodsA retrospective study was conducted on ultrasound-guided core needle biopsies (CNBs) performed by our department during the period between September 2017 and September 2022. Of the 1605 biopsies performed, we identified 17 cases of non-infectious mastitis (PDM and IGM). Mammography and ultrasound were performed in all cases, and MRI was performed in eight cases.
The study was approved by the hospital’s Ethics Committee and no informed consent was required from the participants.
Ultrasounds were performed using an Acuson Antares system (Siemens® AG, Munich, Germany) with a 10–13 MHz linear transducer. The ultrasound study assessed the presence of duct ectasia, abscess, fistula, hypervascularisation in colour Doppler and axillary lymph nodes were assessed according to the Bedi criteria.4 Axillary lymph nodes were also assessed on the basis of the fine needle aspiration (PNA) biopsy. The CNBs were performed using a 14 G × 10 cm (Marquee; Bard®, Murray Hill, NJ) needle and the PNAs were conducted using a 10 cm3 syringe and 20 G needle.
The MRI scans were performed with a Signa HDxt 1.5 T (GE® Healthcare) and a MAGNETOM Aera 1.5 T (Siemens® Healthineers). Sequences obtained included: T2-weighted FSE, bilateral axillary coronal T1-weighted, dynamic axial acquisition (T1 FLASH 3D, SPAIR—one baseline and five post-contrast) with images obtained before and after a bolus injection of 0.1 mmol/kg weight of gadolinium and automatic subtraction of the pre- and post-contrast images. The b values used were: 0 and 700 with the Signa equipment and 50 and 700 with the Aera equipment. The variables assessed by MRI were T2 signal intensity, enhancement type, enhancement diameter, enhancement distribution and pattern, curve type and apparent diffusion coefficient (ADC) value, taking three ADC values and obtaining the average value.
Radiologists specialised in breast imaging at the hospital interpreted the mammograms, ultrasound scans, MRI and the performance of percutaneous procedures (FNA and CNB). The radiologists had 7, 17, 20 and 28 years of experience, respectively. The MRI scans were performed after the pathological diagnosis of the biopsy.
Microsoft® Excel was used for data management.
ResultsThe findings are summarised in Table 1. The mean age of the patients was 35.5 years (range 26–42 years). The mean diameter of the lesions was 58 mm (range 28–94 mm). On T2-weighted sequences, the lesions showed signal hyperintensity in seven cases. Duct ectasia was observed in five cases. Duct ectasia predominates in PDM (Fig. 1). However, in our series we found two cases of IGM showing very clear duct ectasia (Fig. 2). The mean ADC value was similar, 1.159 in PDM and 1.209 in IGM. All cases showed non-mass-like enhancement, with regional distribution in five cases, segmental in two and one in multiple regions. Regarding the internal enhancement pattern, clustered rings were found in all cases (Fig. 3). Five cases displayed type II enhancement curves and three displayed type III. Two IGM showed very diffuse involvement (Fig. 4) in contrast with PDM.
Inflammatory mastitis of unknown aetiology.
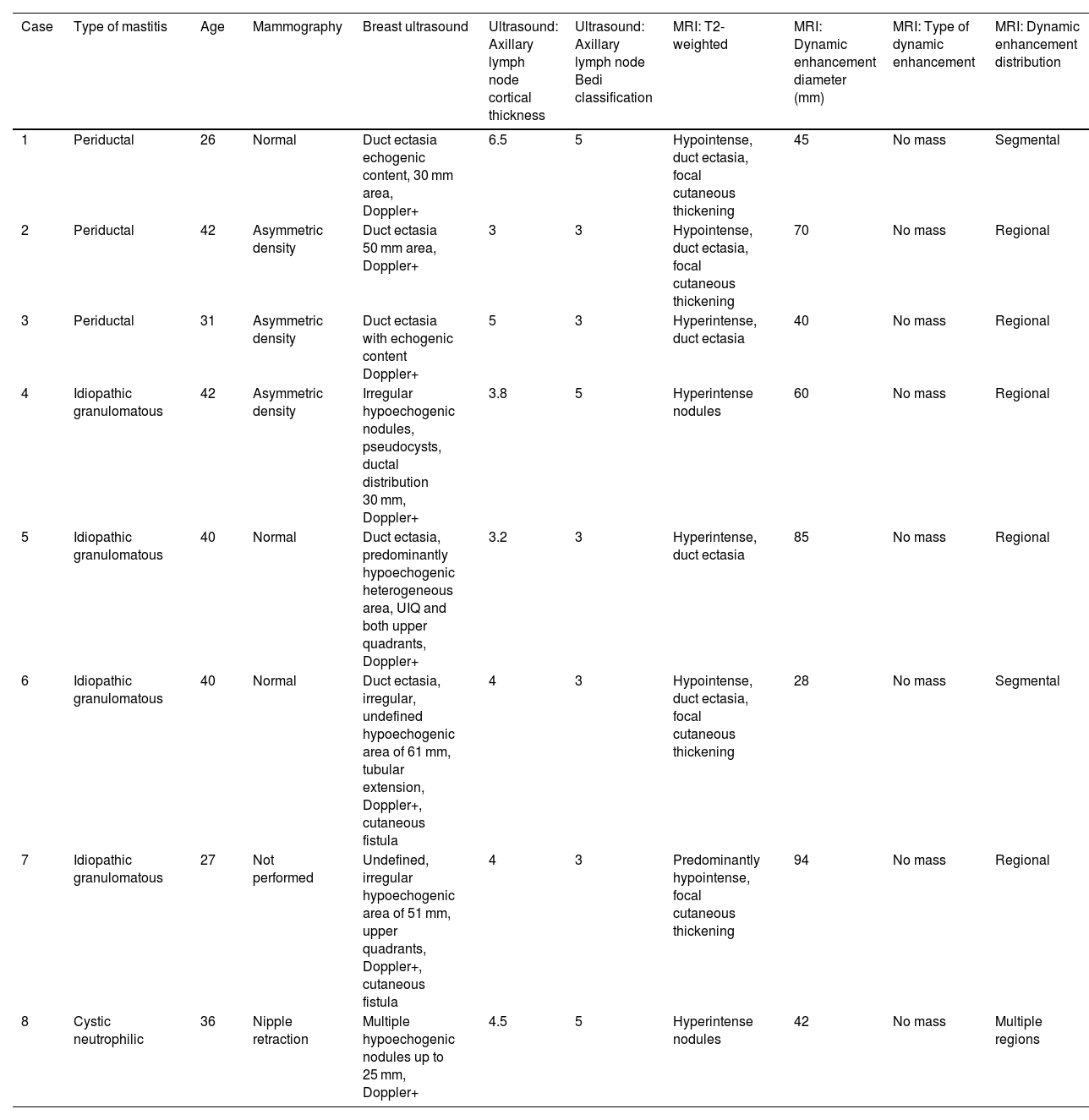
| Case | Type of mastitis | Age | Mammography | Breast ultrasound | Ultrasound: Axillary lymph node cortical thickness | Ultrasound: Axillary lymph node Bedi classification | MRI: T2-weighted | MRI: Dynamic enhancement diameter (mm) | MRI: Type of dynamic enhancement | MRI: Dynamic enhancement distribution |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Periductal | 26 | Normal | Duct ectasia echogenic content, 30 mm area, Doppler+ | 6.5 | 5 | Hypointense, duct ectasia, focal cutaneous thickening | 45 | No mass | Segmental |
| 2 | Periductal | 42 | Asymmetric density | Duct ectasia 50 mm area, Doppler+ | 3 | 3 | Hypointense, duct ectasia, focal cutaneous thickening | 70 | No mass | Regional |
| 3 | Periductal | 31 | Asymmetric density | Duct ectasia with echogenic content Doppler+ | 5 | 3 | Hyperintense, duct ectasia | 40 | No mass | Regional |
| 4 | Idiopathic granulomatous | 42 | Asymmetric density | Irregular hypoechogenic nodules, pseudocysts, ductal distribution 30 mm, Doppler+ | 3.8 | 5 | Hyperintense nodules | 60 | No mass | Regional |
| 5 | Idiopathic granulomatous | 40 | Normal | Duct ectasia, predominantly hypoechogenic heterogeneous area, UIQ and both upper quadrants, Doppler+ | 3.2 | 3 | Hyperintense, duct ectasia | 85 | No mass | Regional |
| 6 | Idiopathic granulomatous | 40 | Normal | Duct ectasia, irregular, undefined hypoechogenic area of 61 mm, tubular extension, Doppler+, cutaneous fistula | 4 | 3 | Hypointense, duct ectasia, focal cutaneous thickening | 28 | No mass | Segmental |
| 7 | Idiopathic granulomatous | 27 | Not performed | Undefined, irregular hypoechogenic area of 51 mm, upper quadrants, Doppler+, cutaneous fistula | 4 | 3 | Predominantly hypointense, focal cutaneous thickening | 94 | No mass | Regional |
| 8 | Cystic neutrophilic | 36 | Nipple retraction | Multiple hypoechogenic nodules up to 25 mm, Doppler+ | 4.5 | 5 | Hyperintense nodules | 42 | No mass | Multiple regions |
| MRI: Dynamic enhancement pattern | Mean value CDA (×10−3 mm2/s) | Curve type | |
|---|---|---|---|
| 1 | Clustered rings | 1.133 | 2 |
| 2 | Clustered rings | 1.051 | 3 |
| 3 | Clustered rings | 1.292 | 2 |
| 4 | Clustered rings | 1.352 | 3 |
| 5 | Clustered rings | 1.500 | 2 |
| 6 | Clustered rings | 1.008 | 3 |
| 7 | Clustered rings | 0.956 | 2 |
| 8 | Clustered rings | 1.221 | 2 |
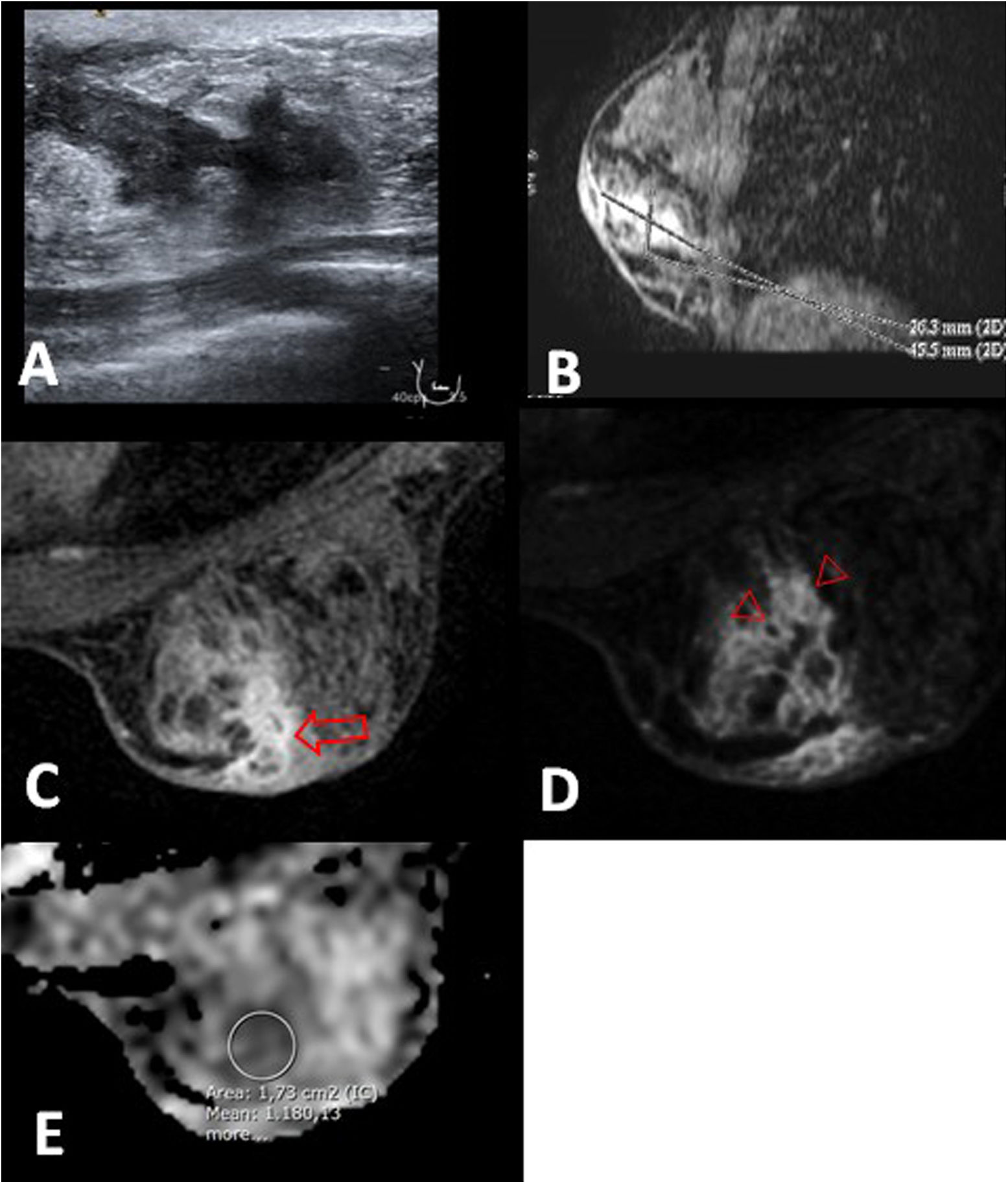
Periductal mastitis in a 26-year-old patient. A) Ultrasound shows duct ectasia with echogenic content in the right breast. B) Sagittal reconstruction that shows non-mass-like enhancement with a segmental distribution that occupies an area of 45 mm. C–E) Post-contrast MRI image, first phase, T1-weighted fat-saturation (C) and subtraction (D) show the duct ectasia with mural enhancement (arrow) and clustered ring pattern (arrowheads). E) The ADC map shows one of the three studied values, whose mean was 1.133 × 10−3 mm2/s. See the focal hyperintense cutaneous thickening near to the inflamed area (C and D).
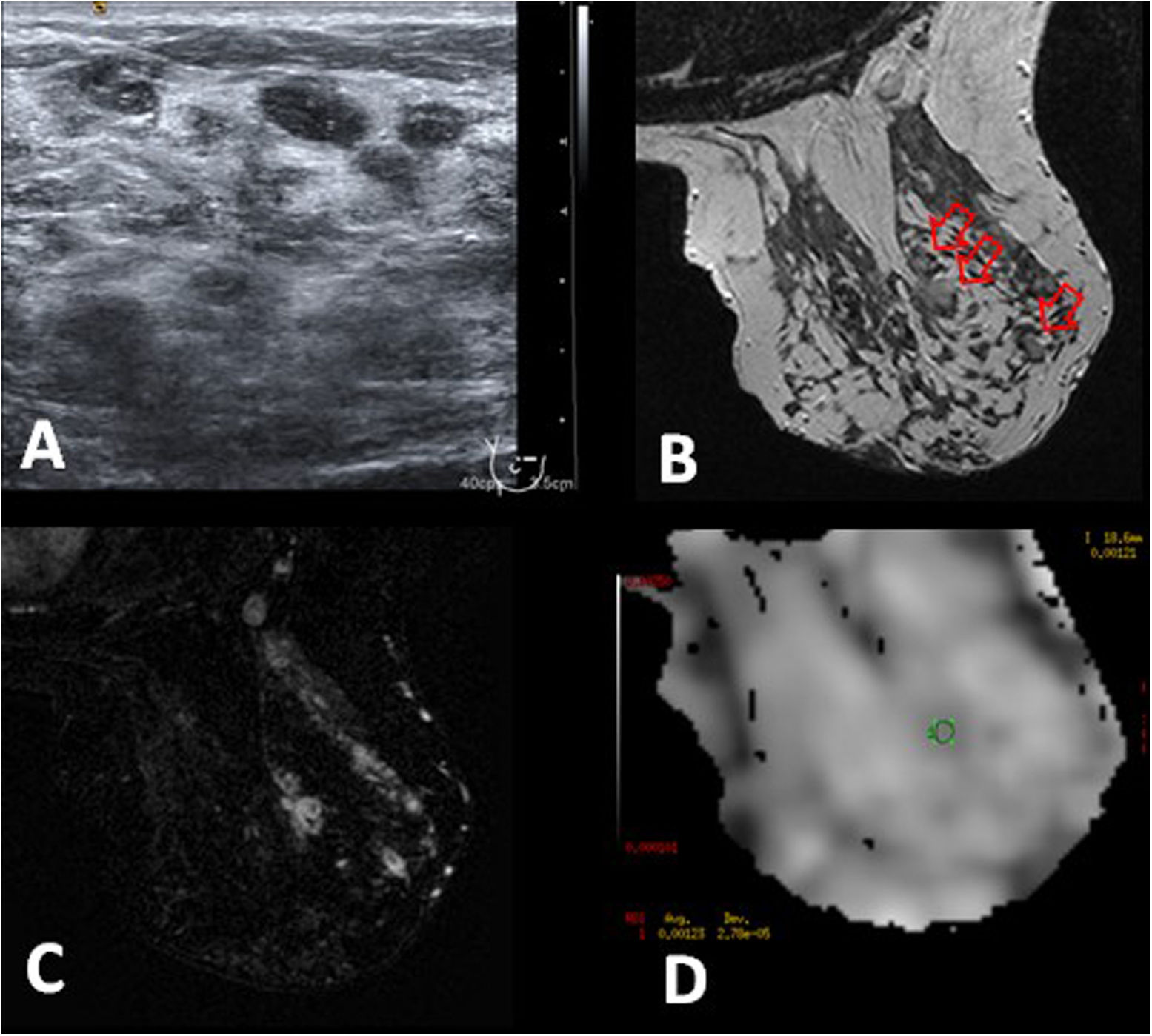
Idiopathic granulomatous mastitis in a 40-year-old patient. A and B) Ultrasound shows duct ectasia with echogenic content in the right breast, with a cutaneous fistula (arrow). C) T2-weighted MRI that shows thickened duct walls with hyperintense content (arrow). D and E) Post-contrast, T1-weighted MRI with fat-saturation and subtraction, show duct ectasia with mural enhancement (arrow in D) and the fistular tract to the skin (arrow in E) with focal cutaneous thickening and enhancement. F) Inflamed area consists of non-mass-like enhancement with a segmental distribution in a clustered ring pattern (arrowhead) that occupies an area of 45 mm. The ADC map showed a mean of 1.008 × 10−3 mm2/s (not shown).
Idiopathic granulomatous mastitis in a 42-year-old patient. A) Ultrasound showed pseudocysts smaller than 1 cm in the right breast. B) T2-weighted MRI shows multiple hyperintense nodules smaller than 1 cm (arrows). C) Post-contrast, T1-weighted MRI with fat-saturation and subtraction, shows non-mass-like-enhancement with regional distribution and clustered ring pattern related to the hyperintense nodules identified in T2 and which correspond to microabscesses. D) The ADC map shows one of the three studied values, whose mean was 1.352 × 10−3 mm2/s.
Idiopathic granulomatous mastitis in a 27-year-old patient. Ultrasound showed irregular hypoechogenic areas in the right breast that were associated with the Doppler signal. A) T2-weighted MRI that shows extensive discrete hyperintense areas and focal skin thickening with associated fistula tract (arrow). B) Post-contrast, T1-weighted MRI with fat-saturation and subtraction shows a 94 mm non-mass-like-enhancement with regional distribution and clustered ring pattern (arrows). C) Maximum intensity contrast projection image showing extensive inflammatory involvement occupying the upper quadrants of the right breast. Note the thickness of normal skin in most of the breast, except the area of the fistula tract (arrow in A). D) Three ADC values were studied, whose mean was 0.965 × 10−3 mm2/s.
All cases showed lymph nodes with thickened cortex, with a reactive appearance (Bedi 3) in five cases, and a metastatic appearance (Bedi 5) in three cases. FNA did not reveal atypical or malignant cells in any case.
DiscussionThere are multiple terms associated with mastitis and its complications in the form of abscesses. Dixon1 classifies them as associated or not with lactation (puerperal and non-puerperal) and secondary to skin infection. The forms of puerperal mastitis are related to infection. He divides non-puerperal mastitis into two types: periareolar (PDM), which can develop secondary infections, and abscesses and fistulas, and peripheral, which are usually associated with diabetes, rheumatoid arthritis, steroid treatment, idiopathic granulomatous mastitis (IGM) and trauma.
Hughes2 describes the difficulty in understanding the duct ectasia/PDM complex as a result of the multiple names associated with non-puerperal mastitis, with the possible connection between duct ectasia, PDM, subareolar abscess and periareolar fistula, considering IGM a different process.
There are several proposed classifications of non-infectious mastitis.1–3,5 De la Orden et al.5 offer a very systematic classification that differentiates between different degrees/entities: duct ectasia, recurrent periareolar mastitis (PDM) and IGM. Some authors suggest that rather than being different processes, they could be phases of the same entity since clinical and radiological findings often overlap.6 IGM starts with exposure of epithelial cells to a toxic substance, causing dilatation and erosion with lipid leakage that unleashes a granulomatous inflammatory response in the stromal connective tissue.6
The MRI findings of the mastitis in our series show predominantly non-mass-like enhancement with regional or segmental distribution and a clustered ring pattern, with a type II time-intensity curve and a mean ADC value of 1.190 × 10-3 mm2/s, associated with abnormal axillary lymph nodes. Enhancement has been described most frequently as non-mass-like, and the regional or multi-regional distribution is consistent with reports.7–9
Mammary duct ectasia as an MRI sign has received little attention in previous series7,8 probably because it is more clearly seen in early stages and is more readily evident on ultrasound. The presence of very clear duct ectasia in two of the reported IGM is demonstrative of the overlap between the signs that occur in both entities. In these two cases the radiologist initially suspected PDM and the biopsy reported IGM. In our opinion, this fact suggests that we are dealing with different phases of the same entity, as suggested by other authors,6 and probably indicates that some PDM may progress to IGM.
Fig. 5 shows a case of cystic neutrophilic granulomatous mastitis with no differential findings on MRI. This type of mastitis may show a distinct histological pattern characterised by suppurative lipogranulomas that are composed of central lipid vacuoles rimmed by neutrophils and an outer cuff of epithelioid histiocytes, often associated with Corynebacterium species (mainly Corynebacterium kroppenstedtii).10 Due to the difficulty in isolating the bacillus or the need to use appropriate media to reveal it, it is likely that these cases have been underdiagnosed and may account for a subgroup of IGM. The importance of recognising this entity lies in offering treatment with lipophilic antibiotics not always used in empirical treatments directed at more conventional pathogens, which may lead to the inflammatory process becoming chronic.
Cystic neutrophilic granulomatous mastitis in a 36-year-old patient. Ultrasound showed multiple hypoechogenic nodules which were associated with the Doppler signal (not shown). A) T2-weighted MRI image showing hypointense nodules (arrows). B and C) Post-contrast, T1-weighted MRI with fat-saturation (B) and sagittal reconstruction in subtraction (C) which show a non-mass-like-enhancement with multiregional distribution and clustered ring pattern (arrows). D) Three ADC values were studied, whose mean was 1.221 × 10−3 mm2/s. E) The pathology reveals the presence of epithelioid histiocytes forming a non-necrotising granuloma with a central cyst-like space surrounded by neutrophils with predominantly lymphoplasmacytic inflammation (20x). PCR came back positive for Corynebacterium kroppenstedtii.
A number of MRI findings help us in the differential diagnosis with malignancy. The subareolar location of the inflammatory involvement is more frequent in benign mastitis9,11 and whereas in inflammatory carcinoma it is usually more central or posterior. Focal skin thickening is more frequent in benign mastitis compared to diffuse skin thickening related to malignancy7,12; a diagnostic criterion for inflammatory carcinoma is skin involvement in the form of erythema affecting at least one third of the breast. The presence of duct ectasia suggests inflammatory disease, and is therefore useful to differentiate non-infectious mastitis (PDM and IGM) from malignant processes.3 The clustered ring enhancement pattern, hyperintense on T2-weighted imaging, has been reported more frequently in benign mastitis, explained by the presence of abscesses and small infected cysts.7,9,11,13 In malignant processes we can see hypointensity in T2-weighted images that would be secondary to fibrotic tissue due to desmoplastic reaction.9,13 In addition, the presence of a cutaneous fistula suggests a non-puerperal inflammatory mastitis rather than malignant pathology.6 Collections or abcesses can be observed in puerperal (infectious) mastitis and in all cases of advanced mastitis (Table 2).3
MRI findings for puerperal mastitis, mastitis of unknown aetiology, and invasive breast carcinoma.
| MRI findings | Puerperal mastitis | PDM | IGM | Invasive carcinoma |
|---|---|---|---|---|
| Skin thickening | Focal | Focal | Focal | Diffuse in inflammatory carcinoma |
| Duct ectasia | Infrequent | Frequent | Presence possible | Infrequent |
| Lesion in T2 | Hyperintense collection or abscess | Hyperintense | Hyperintense | Mainly hypointense |
| Type of enhancement | Secondary to inflammation | Predominantly non-mass-like | Predominantly non-mass-like | Variable |
| Enhancement distribution | Variable | Frequent: regional or segmental | Frequent: regional or segmental | Variable |
| Enhancement pattern | Variable | Frequent: clustered rings | Frequent: clustered rings | Variable |
| Curve type | Type I or II | Frequent: type II | Frequent: type II | Type II or III |
| ADC | Variable | Low | Low | Low |
In the dynamic study type III curves are more often present in malignant neoformative processes13,14 which is due to arteriovenous anastomoses and a large number of high flow capillaries, which means the contrast passes rapidly from the intravascular space to the interstitial compartment.9
Benign pathology usually shows hyperintensity in T2-weighted images and in diffusion-weighted imaging (DWI). Malignant pathology shows this hyperintensity in DWI and the use of ADC maps is useful.15 In general, malignant lesions show diffusion restriction, seen as diffusion hyperintensity and low ADC values, which is due to high cell density and limited extracellular density.16 The low frequency of cases with diffusion hypointensities in our series is consistent with benign pathology showing mostly diffusion and T2 hyperintensities.9,17,18 ADC values are lower than reported in other studies. Zhang et al. obtained values of 1.558 ± 0.34 for PDM and 1.244 ± 0.32 for IGM, which might be because the PDMs in Zhang’s study are more advanced and have formed chronic abscessed areas that increase the ADC value.18 Although the mean ADC value of all our cases (1.190) is superimposable to that of malignant tumours, the rest of the MRI findings and the clinical findings help to differentiate them.
Most of our cases showed suspicious axillary lymph nodes with thickened cortex (Bedi 3 and 5), which made it advisable to perform FNA to rule out malignancy. This finding is in line with reports,9 with FNA or CNB being the recommended tools for pathological study.
MRI has been described as the most sensitive imaging technique for diagnosing inflammatory carcinoma, as it can identify multiple small, confluent, enhancing masses with global skin thickening.19 One case in our series showed these features on MRI, with extensive parenchymal involvement, a less common finding in benign mastitis, with very low ADC. The cutaneous involvement was focal and not diffuse, which helped to guide the diagnosis as this differs from the clinical criteria for inflammatory carcinoma, and was subsequently confirmed by biopsy (Fig. 4). The differential diagnosis with inflammatory carcinoma, a rare and aggressive form of breast cancer with a clinical presentation that may mimic benign mastitis and therefore delay treatment, should always be kept in mind.20
One limitation of this study is the small number of cases due to the fact that it is an infrequent pathology and, in most cases, MRI is not performed.
ConclusionsMRI findings of PDM and IGM in our series were similar. They showed predominantly non-mass-like enhancement with regional or segmental distribution, a clustered ring enhancement pattern, a type II time-intensity curve and a mean ADC value of 1,.190 × 10−3mm2/s, with abnormal axillary lymph nodes. Duct ectasia in these forms of mastitis suggests that we are dealing with different phases of the same entity. Given their similarities, the clinical and imaging characteristics can provide guidance in the differential diagnosis with malignant processes, which must be ruled out using biopsy.
FundingThis investigation received no specific funding from public, commercial or not-for-profit sector agencies.
Author Contributions- 1
Research coordinators: MM, XB
- 2
Development of study concept: MM, XB
- 3
Study design: MM, XB
- 4
Data collection: MM, XB, BU, SG, BG, EM
- 5
Data analysis and interpretation: MM, XB, SG
- 6
Statistical analysis: MM, XB
- 7
Literature search: MM, XB
- 8
Writing of article: MM, XB
- 9
Critical review of the manuscript with intellectually relevant contributions: XB, BU, SG, CS
- 10
Approval of the final version: MM, XB, BU, SG, BG, EM, CS
None.