Congenitally corrected transposition of the great vessels implies double discordance: atrioventricular and ventriculoarterial. We present cardiac magnetic resonance images from a 9-year-old girl with congenitally corrected transposition of the great vessels, interventricular communication, and coarctation of the aorta who was treated with pulmonary artery banding, correction of coarctation, and posterior double switch. We also review the disease and the complications that should be evaluated after the surgical intervention.
La transposición congénitamente corregida de grandes arterias (TGAcc) implica una doble discordancia: atrio-ventricular y ventriculo-arterial. Presentamos las imágenes de resonancia magnética cardíaca de una paciente de 9 años con TGAcc, comunicación interventricular y coartación de aorta, a quien se le realizó cerclaje pulmonar, corrección de la coartación y posterior “doble switch”. Se realiza una revisión de la patología y de las complicaciones a evaluar tras la intervención quirúrgica.
Congenitally corrected transposition of the great arteries (ccTGA), ventricular inversion or L-transposition involves a dual discordance: atrioventricular (the right atrium connecting with the left ventricle through the mitral valve and the left atrium connecting with the right ventricle through the tricuspid valve) and ventriculoarterial (the outlet of the aorta anterior to and left of the right ventricle and the outlet of the pulmonary artery posterior to and right of the left ventricle).1
We present cardiac magnetic resonance imaging (MRI) of a 9-year-old patient with ccTGA, ventricular septal defect (VSD) and coarctation of the aorta who underwent pulmonary cerclage, coarctation repair and a subsequent double-switch operation (Figs. 1 and 2). The study was conducted in a Panorama 1.0 T system (Philips Medical Systems) and typical cine sequences were acquired: steady-state free precession (SSFP) white blood for functional assessment, black blood for anatomical evaluation, sequences for flow quantification in 2D phase-contrast (PC), angio-MRI with gadolinium (Dotarem® [gadoteric acid] 0.5mmol/ml, 0.2ml/kg) and double inversion recovery sequences in the assessment of late gadolinium enhancement. Post-processing was performed with Philips IntelliSpace Diagreso® auxiliary console software. For this study we conducted a review of the disease and the complications that must be evaluated following its correction.
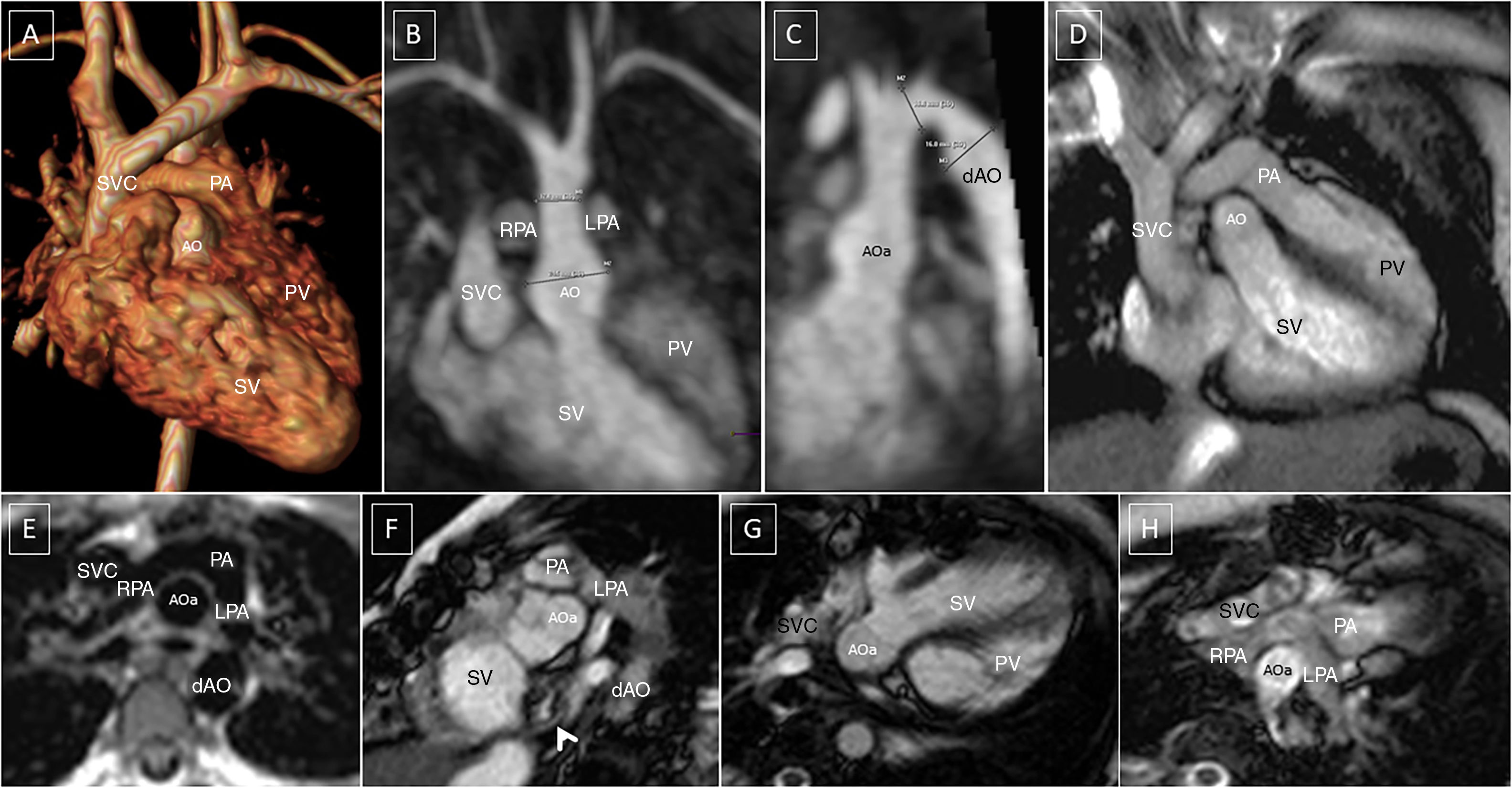
Post-operative changes in great vessels following arterial switch+LeCompte. Posterior ascending aorta. The pulmonary arteries wrap around the suprasinus ascending aorta. (A) Thoracic angio-MRI, 3D reconstruction, anterior view. (B) Oblique coronal maximum intensity projection (MIP). Ascending aorta sinus dilation. Pulmonary arteries on the sides. (C) Oblique sagittal MIP. Sinus dilation. Post-coarctation repair aneurysm in a fusiform pattern. (D) 3C long axis GE. Relationship of major vessels to outflow tracts. (E) Black blood axial SE. Typical image of pulmonary arteries and ascending aorta. (F) Short axis GE. Relationship between ascending aorta/systemic outflow tract and pulmonary artery. Stenosis in systemic atrial mesh (arrow). (G and H) 4-C GE, left ventricle (systemic) outflow tract and right ventricle (pulmonary) outflow tract.
AO: aorta; aAO: ascending aorta; dAO: descending aorta; LPA: left pulmonary artery; PA: pulmonary artery; PV: pulmonary ventricle; RPA: right pulmonary artery; SVC: superior vena cava; SV: systemic ventricle.
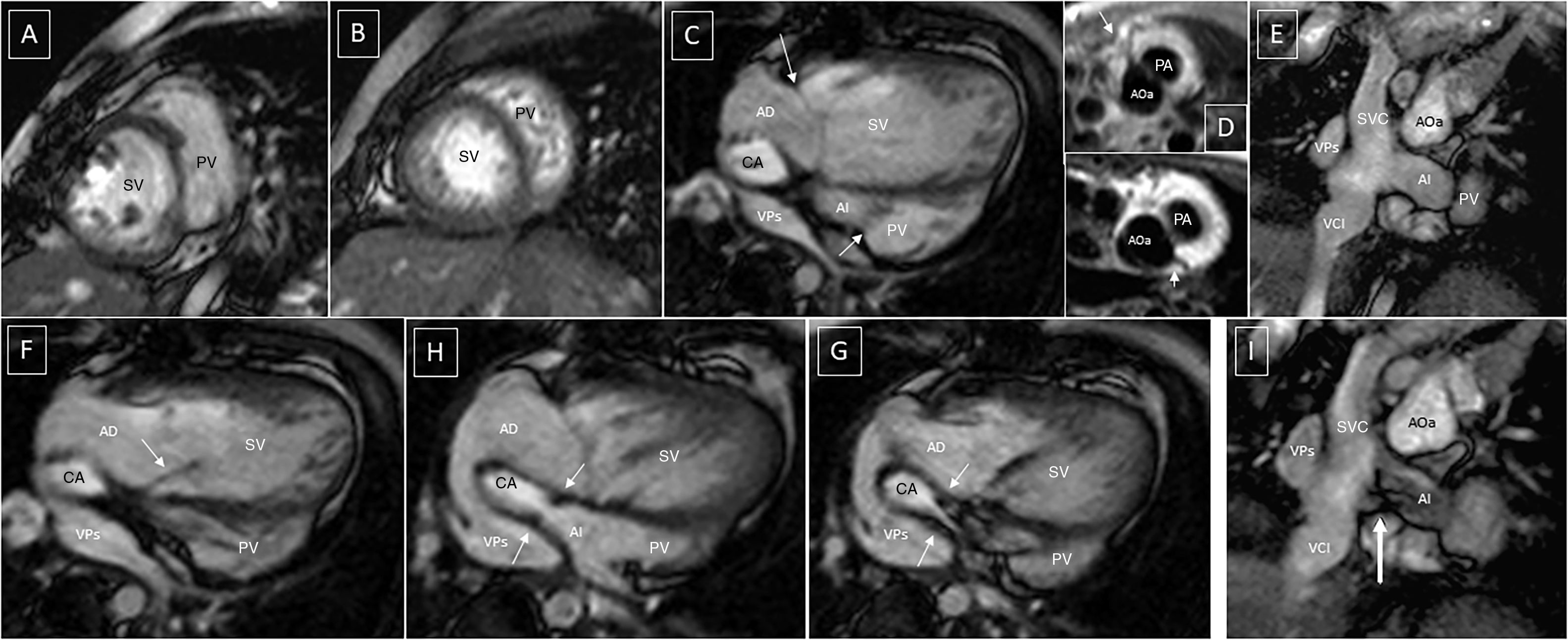
Post-operative changes in atria and vena cavae after atrial switch. (A and B) Short axis GE, compaction abnormality of left ventricle, medial and apical segment. (C, F, G and H) 4-C GE. (E and I) oblique coronal GE. Typical positioning of the atrial baffle. Apical displacement of tricuspid valve in C (arrows). Systemic AV valve insufficiency in F (arrow). Stenotic portion of the baffle with flow acceleration in G and H (arrows). Morphology of the vena cava “baffle” junction in E and I. Flow acceleration in the baffle anastomosis in I (arrow). (D) Axial black blood SE. Right coronary artery and ADA (top); circumflex artery (bottom). CA: caval axis; IVC: inferior vena cava; LA: left atrium; PVs: pulmonary veins; RA: right atrium.
ccTGA has an estimated prevalence of 0.04 cases per 1000 adults.1 It occurs when the heart rotates to the left (L-transposition) during embryogenesis, resulting in the morphologically left ventricle being positioned on the right.2 It may occur either in isolation or in combination with other heart defects: VSD (80% perimembranous),2 pulmonary stenosis and tricuspid disease.1 The aorta is supported by a muscular infundibulum and there is no continuity with the atrioventricular valve at this level. There is, however, mitral–pulmonary continuity as well as a subaortic cone which separates the tricuspid valve from the aorta. The outflow tracts show a parallel positioning with the pulmonary ring on the interventricular septum, which may lead to an obstruction of the outflow tract of the morphologically left ventricle (40%).2 There is often the presence of tricuspid valve dysplasia, with or without apical displacement of the septal veil, which causes valvular incompetence (30%).1,2
Other more complex abnormalities that may accompany it include: mitral/tricuspid atresia, abnormal pulmonary and/or systemic venous drainage, abnormal atrial/abdominal situs and the existence of a single ventricle.1,2
Although echocardiography represents the leading diagnostic test, cardiac MRI is the diagnostic test of choice when the ultrasound window is suboptimal and in post-operative assessment. ccTGA repair techniques are represented by:
- 1.
Anatomical correction or a double-switch operation (as in the case presented by our group). It is more complex and, therefore, seldom used in day-to-day clinical practice. This technique is intended to re-establish the morphologically left ventricle as systemic. To do this, intra-atrial flow is redirected using the Mustard–Senning technique and, in addition to this, an arterial switch is performed with swapping of major arteries, translocation of coronary arteries, positioning of pulmonary arteries in front of the aorta (Jatene procedure)2 and closure of the VSD with mesh. In order to be able to perform the anatomical correction, the morphologically left ventricle has to be able to withstand systemic pressure. If this is not possible, then the left ventricle must be previously prepared by maintaining high pressures following pulmonary banding.1,2
- 2.
Physiological repair, in which the right ventricle must withstand systemic pressure and only associated VSD disease is corrected.1
- 3.
Rastelli operation, when complex pulmonary stenosis and VSD which can tunnel to the aorta coexist. This consists of closure of the VSD with “fabric” mesh to redirect the flow from the left ventricle to the aorta, and implantation of a prosthetic duct between the right ventricle and the pulmonary artery.2
It is important to be familiar with the surgical techniques performed in order to know what type of complications should be taken into consideration during follow-up. Cardiac MRI enables examination of post-operative changes in a single study and anaesthetic procedure for the patient. In addition, it is capable of identifying both cardiac and extra-cardiac complications, which in most cases escape the diagnostic capacity of transthoracic ultrasound. As a result, it is an essential technique for suitable follow-up. Therefore, in a cardiac MRI study, in a double-switch anatomical correction, it is necessary to:
- •
On a Mustard–Senning level: rule out superior (most common) and inferior vena cava channel stenosis, primarily by means of SSFP cine sequences, with coronal slices at the level of the bicaval plane and 4-chamber plane, as well as angio-MRI in the late venous phase to achieve good filling of the inferior vena cava. Assess potential pulmonary venous channel stenosis and/or atrial baffle dehiscence, with axial/4-chamber SSFP cine sequences and angio-MRI. Finally, evaluate the presence of dilation of the azygos vein, which represents an indirect sign of probable stenosis or obstruction of the atrial baffle. All flow accelerations seen in SSFP cine sequences should cause possible stenosis or L–D shunts to be suspected and subsequently confirmed with specific planes and thinner slices in the direction of the initially visualised jet. Similarly, they may be analysed with 2D or 4D flow phase-contrast (PC) flow quantification sequences,4 which enable greater precision in their quantitative and qualitative evaluation.
- •
On a ventriculoarterial level (arterial switch): rule out obstruction in the outflow tract of the right ventricle, generally secondary to stenosis of the neopulmonary valve and/or supravalvular pulmonary stenosis at the surgical suture site (most common), as well as stenosis of the pulmonary branches associated with anatomical distortion following the LeCompte manoeuvre. Quantify the proportional flow in the right and left pulmonary artery by means of 2D or 4D flow PC sequences. In the outflow tract of the left ventricle, evaluate dilation of the neoaorta (primarily valvular ring and sinus region, corresponding to the site of translocation of the coronary arteries),2,3 neoaortic valve insufficiency and supravalvular aortic stenosis (at the surgical suture site). Regarding the ventricles, evaluate the function, volume and mass of the left ventricle1 and rule out silent infarction by means of evaluation of segmental abnormalities in SSFP cine sequences and/or evidence of late enhancement.3 Evaluate the function, volume and mass of the right ventricle (increased if there is obstruction in the outflow tract). Finally, rule out coronary artery disease, such as ostial (post-operative) stenosis/occlusion, proximal kinking and interarterial course.
Developments in advanced cardiac imaging techniques (cardiac MRI) in the last decade have enabled better pre- and post-operative evaluation and follow-up of patients with complex congenital heart diseases such as ccTGA. These combined with advances in surgical techniques in the same period result in better management of the course of the disease and an increase in the survival rates of patients with ccTGA.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Urmeneta Ulloa J, Aroca Peinado Á, Bret-Zurita M. Transposición congénitamente corregida de grandes arterias: valoración por resonancia magnética cardíaca tras corrección anatómica tipo “doble switch”. Radiología. 2019;61:262–265.