The currently available scientific evidence attests that mammographic screening and quality treatment have been able to reduce mortality attributable to breast cancer. Although screening is not without risks, population-based screening has clear advantages over opportunistic detection. Following the Council of the European Union's “Recommendations on cancer screening”, all the regional Autonomous Communities in Spain have screening programs that, in general, follow the same guidelines. The “European guidelines for quality assurance in breast cancer screening and diagnosis” serve as a reference that provides an overview of all aspects of screening. To achieve the foreseen objectives for the reduction of the morbidity and mortality attributable to breast cancer, screening programs must fulfill the established quality criteria and guarantee that patients have access to the best treatment options.
De acuerdo con el conocimiento científico actual, los programas de diagnóstico precoz mediante mamografía y la calidad de los tratamientos han logrado disminuir la mortalidad por cáncer de mama. Aunque no está exento de riesgos, el cribado poblacional tiene claras ventajas sobre la detección oportunista. Siguiendo las «Recomendaciones del Consejo Europeo sobre el cribado del cáncer», en España existen programas de detección precoz en todas las Comunidades Autónomas que, en líneas generales, siguen unas directrices comunes. «La Guía Europea para la Garantía de Calidad en el cribado y diagnóstico de cáncer de mama» es un documento de referencia y proporciona una visión general de todos los aspectos del cribado. Para conseguir los objetivos previstos de reducción de morbilidad y mortalidad por cáncer de mama es necesario que los programas de cribado cumplan con unos criterios de calidad establecidos y que se garanticen a las pacientes las mejores opciones terapéuticas.
Breast cancer is the most frequent tumor among Western women. It is estimated that the probability of developing it from 75 years on is 8%.1 In 2009, 6,130 deaths were recorded in Spain, with an adjusted mortality rate of 26, 35 per 100,000 women.2 In 2012, it is estimated that 27,000 cases will be diagnosed and 6,231 deaths will occur.3 It poses an important health problem in industrialized nations due to its high incidence, mortality and personal and social repercussion.4 In June 2003, the European Parliament urged the member countries to develop effective strategies aimed at improving screening, diagnosis and treatment of breast cancer.5,6
The main objective of population screening is to decrease mortality by detecting breast cancer early.7 Its natural history enables to detect it early, since in most tumors, there is a preclinical phase that is detectable between 1 and 3 or more years.8 Mammography continues to be the test of choice for screening9 and its effectiveness is widely proven.10 The results of the meta-analysis of 8 randomized clinical trials begun between 1963 and 1982, with 500,000 participants in Europe, The USA and Canada (HIP, Malmö, Two-Country, Edinburg, Stockholm, NBSS-1, NBSS-2 and Gothenburg), have shown that mammographic screening reduces mortality by breast cancer in 20–30%.10,11
The population screening programs began in several European countries at the end of the 1980s and in Spain, at the beginning of the 1990s.8 After a continued increase of breast cancer cases among Spanish women during the 1980s and 1990s, the decrease of the incidence rate from the year 2001 on (recorded for the 2001–2004 period) among women 45–65 years of age is possibly the consequence of generalizing mammographic screening, the treatment of high risk lesions and a less frequent use of substitutive hormonal therapies.12 Recent studies confirm that the mortality rate is also diminishing thanks to the early detection programs and the breakthroughs in the disease systemic treatment.4,13,14
Although breast cancer screening is widely accepted, it is not free from controversy4 and there often appear voices critical of the programs, their methodologies and results.15 30 years after the beginning of the first programs, there still remain some unresolved basic aspects (age of target population, periodicity of the screening, standardization of reading systems, etc.).
The objectives of this update are to show the current status of screening, to comment on the radiographic and radiologic guidelines collected in the «European guidelines for quality assurance in breast cancer screening and diagnosis»7 and to discuss the aspects that cause the most debate and controversy.
Principles of breast cancer screeningAt present, it is not possible to prevent the onset of a breast cancer, because the main known risk factors (sex, age, personal family antecedents, early menarche, late menopause, etc.) are not modifiable. Therefore, secondary prevention is the alternative to interrupt its natural history.16 Breast cancer complies with epidemiological criteria required to be susceptible of screening: high morbidity and mortality, high prevalence of the detectable preclinical status, possibility of effective treatment and the existence of a screening test of high sensitivity and specificity, low cost and few side effects.17 Screening targets an «asymptomatic » population; therefore, it must comply with requisites that are stricter than those demandable from diagnostic tests used on symptomatic patients.18,19
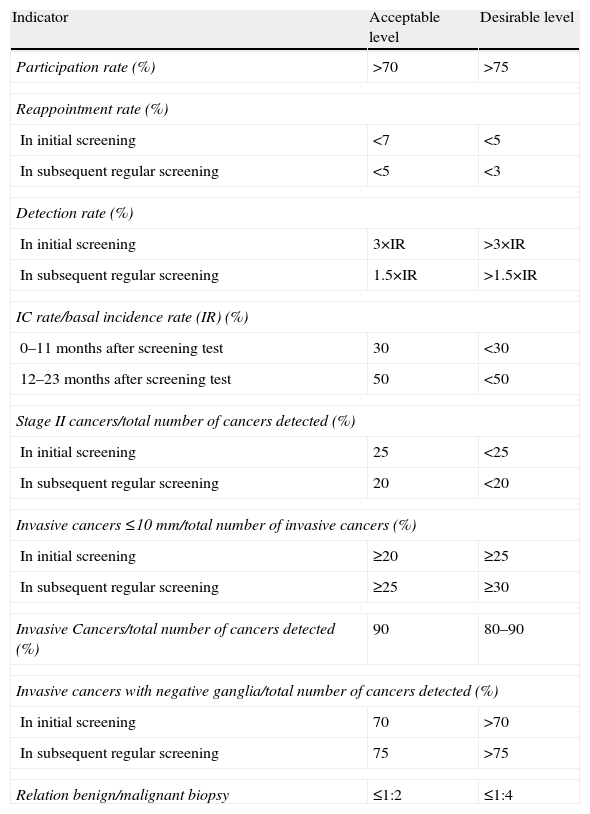
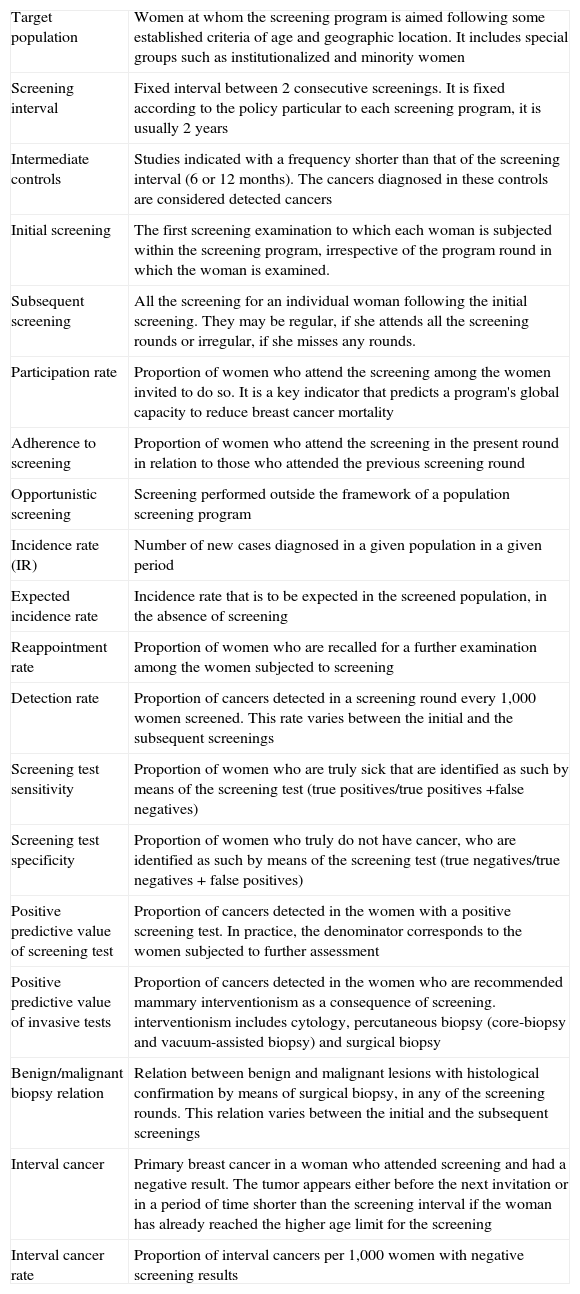
The European Guideline, in its fourth edition,7 collects the quality standards recommended and it is a reference document in all the aspects of screening.15 It contains documents on technical control, radiographic and radiologic, epidemiological, and quality control guidelines in pathology and surgery and it includes a summary with the indicators used to assess the screening process (Table 1) and a glossary with the terminology used (Table 2).
Performance indicators used to measure the impact a screening program has on the decrease of breast cancer mortality.
| Indicator | Acceptable level | Desirable level |
| Participation rate (%) | >70 | >75 |
| Reappointment rate (%) | ||
| In initial screening | <7 | <5 |
| In subsequent regular screening | <5 | <3 |
| Detection rate (%) | ||
| In initial screening | 3×IR | >3×IR |
| In subsequent regular screening | 1.5×IR | >1.5×IR |
| IC rate/basal incidence rate (IR) (%) | ||
| 0–11 months after screening test | 30 | <30 |
| 12–23 months after screening test | 50 | <50 |
| Stage II cancers/total number of cancers detected (%) | ||
| In initial screening | 25 | <25 |
| In subsequent regular screening | 20 | <20 |
| Invasive cancers ≤10mm/total number of invasive cancers (%) | ||
| In initial screening | ≥20 | ≥25 |
| In subsequent regular screening | ≥25 | ≥30 |
| Invasive Cancers/total number of cancers detected (%) | 90 | 80–90 |
| Invasive cancers with negative ganglia/total number of cancers detected (%) | ||
| In initial screening | 70 | >70 |
| In subsequent regular screening | 75 | >75 |
| Relation benign/malignant biopsy | ≤1:2 | ≤1:4 |
IC: interval carcinoma; IR: incidence of breast cancer in the absence of screening.
Glossary of terms used in breast cancer screening.
| Target population | Women at whom the screening program is aimed following some established criteria of age and geographic location. It includes special groups such as institutionalized and minority women |
| Screening interval | Fixed interval between 2 consecutive screenings. It is fixed according to the policy particular to each screening program, it is usually 2 years |
| Intermediate controls | Studies indicated with a frequency shorter than that of the screening interval (6 or 12 months). The cancers diagnosed in these controls are considered detected cancers |
| Initial screening | The first screening examination to which each woman is subjected within the screening program, irrespective of the program round in which the woman is examined. |
| Subsequent screening | All the screening for an individual woman following the initial screening. They may be regular, if she attends all the screening rounds or irregular, if she misses any rounds. |
| Participation rate | Proportion of women who attend the screening among the women invited to do so. It is a key indicator that predicts a program's global capacity to reduce breast cancer mortality |
| Adherence to screening | Proportion of women who attend the screening in the present round in relation to those who attended the previous screening round |
| Opportunistic screening | Screening performed outside the framework of a population screening program |
| Incidence rate (IR) | Number of new cases diagnosed in a given population in a given period |
| Expected incidence rate | Incidence rate that is to be expected in the screened population, in the absence of screening |
| Reappointment rate | Proportion of women who are recalled for a further examination among the women subjected to screening |
| Detection rate | Proportion of cancers detected in a screening round every 1,000 women screened. This rate varies between the initial and the subsequent screenings |
| Screening test sensitivity | Proportion of women who are truly sick that are identified as such by means of the screening test (true positives/true positives +false negatives) |
| Screening test specificity | Proportion of women who truly do not have cancer, who are identified as such by means of the screening test (true negatives/true negatives + false positives) |
| Positive predictive value of screening test | Proportion of cancers detected in the women with a positive screening test. In practice, the denominator corresponds to the women subjected to further assessment |
| Positive predictive value of invasive tests | Proportion of cancers detected in the women who are recommended mammary interventionism as a consequence of screening. interventionism includes cytology, percutaneous biopsy (core-biopsy and vacuum-assisted biopsy) and surgical biopsy |
| Benign/malignant biopsy relation | Relation between benign and malignant lesions with histological confirmation by means of surgical biopsy, in any of the screening rounds. This relation varies between the initial and the subsequent screenings |
| Interval cancer | Primary breast cancer in a woman who attended screening and had a negative result. The tumor appears either before the next invitation or in a period of time shorter than the screening interval if the woman has already reached the higher age limit for the screening |
| Interval cancer rate | Proportion of interval cancers per 1,000 women with negative screening results |
The Radiodiagnosis Specialist Technician (RST) is usually the only health professional who sees the woman.7 He or she must record information relevant for the radiologist (palpable anomalies, nipple retraction, spontaneous telorrhea, antecedents of previous surgery, etc.).7 In addition, he or she should have the skills to manage the woman's anxiety, explain the procedure and the importance of compression.7 The correct positioning is essential to obtain the maximum visualization of the mammary tissue and decrease recall due to an inadequate technique.7 Following the radiographic guidelines, the repetition rate should be below 3%.7
Radiologic guidelinesThe radiologist is the person responsible for the studies to be performed with the required quality.7 Interpretation of the mammography is a complex task since the radiologic presentation of breast cancer is variable and some lesions require a high level of training and specialization to be able to detect them.20 It is necessary to maintain optimal concentration and reading conditions, and pauses every 30–40minutes are recommended.7 If it is possible, it is recommended to perform a double reading because it increases the test's sensitivity 5–20%.7
At present digital mammography is replacing analogical mammography both in clinical practice and in Early Diagnosis Units.4,21 Transition from analogical to digital mammography has been slow, possibly due to multiple factors (high cost, learning curve, initial increase in the recall rate, etc.).21,22 Direct digital mammography offers several advantages such as reduction of the radiation dose,23 decrease of the repetition rate,22 the ease with which it is compared with previous studies and the use of tools that optimize detection (computer assisted manipulation of contrast, zoom and detection systems).24 From the point of view of health management, it enables to integrate the information into digital networks and eliminates costs associated to analogical mammography.4 The published studies that compare analogical and digital mammographies, among them the DMIST trial,25,26 have not demonstrated significant differences in diagnostic accuracy between both techniques.24,25 In addition, digital mammography presents potential benefits, above all in people younger than 50 years and in women with dense breasts.26 For Salas et al., the false positive rates and the indication of interventionist procedures were fewer with digital mammography than with the analogical one, although the detection rate was not significantly different.27 In the near future, it is very likely for digital tomosynthesis to presuppose an important breakthrough in early diagnosis when it detects tumors concealed in conventional mammography.28
In breast image diagnosis we must not forget the importance of ultrasound as the technique complementary to mammography. Performed by expert radiologists it makes it possible for us to characterize lesions and attribute to them the degree of suspicion with greater accuracy, selecting the areas that are more adequate for the percutaneous biopsy, to look for additional lesions and make the pre-surgical staging of the axilla.29
The reading of screening mammography is done later, that is why some women receive a second appointment. This reappointment or recall to perform a complementary examination is one of the main shortcomings of screening.30 There are several factors that influence the reappointment rate; some are related with the woman (age, glandular pattern, premenopausal or postmenopausal condition, body mass index, participation in prevalent or incident round, family antecedents of breast cancer, etc.)30,31 and others with the screening process (the radiologist's experience and training, quality of the mammography, the use of one or two projections, etc.).7 High reappointment rates increase the program's costs and, above all, they generate unnecessary anxiety in women.7,31 Contrariwise, low reappointment rates decrease the detection rate and increase interval cancers (IC).7 It is desirable to get acceptable reappointment rates (less than 7% in prevalent rounds and 5% subsequent ones) that ensure balance between sensitivity and specificity, in a way so that the largest possible number of tumors are diagnosed and that the damage caused by false positives is minimized.7,15,30 The previous studies must be available because they help reduce the reappointment rates and make it possible for some cancers to be detected, which would otherwise go undetected.7,15
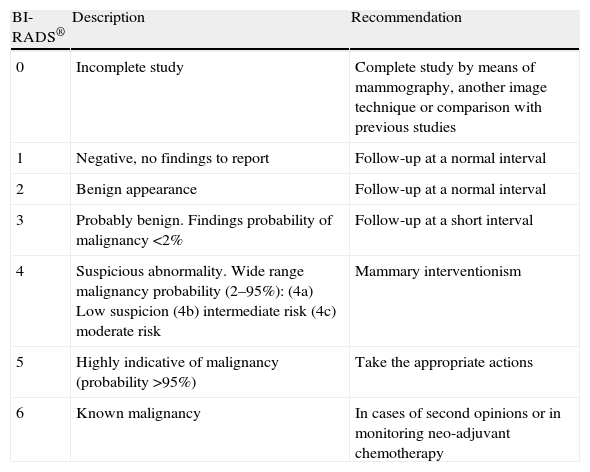
For the classification of the mammographic findings, the categories BI-RADS® from the American College of Radiology are used, and they have made it possible for the terminology to be standardized and for the lesions to be categorized establishing the degree of suspicion and recommending the action to be taken in each case32 (Table 3).
System of categorization and recommendations by the American College of Radiology (ACR), Breast Imaging Reporting and Data System (BI-RADS®).
| BI-RADS® | Description | Recommendation |
| 0 | Incomplete study | Complete study by means of mammography, another image technique or comparison with previous studies |
| 1 | Negative, no findings to report | Follow-up at a normal interval |
| 2 | Benign appearance | Follow-up at a normal interval |
| 3 | Probably benign. Findings probability of malignancy <2% | Follow-up at a short interval |
| 4 | Suspicious abnormality. Wide range malignancy probability (2–95%):(4a) Low suspicion(4b) intermediate risk(4c) moderate risk | Mammary interventionism |
| 5 | Highly indicative of malignancy (probability >95%) | Take the appropriate actions |
| 6 | Known malignancy | In cases of second opinions or in monitoring neo-adjuvant chemotherapy |
Viewing an anomaly in the screening mammography requires an additional study (complementary projections/or ultrasound) that confirms or rules out a lesion.7 If a suspicious lesion is confirmed, diagnostic confirmation and treatment interventionist tests will be indicated in case it is necessary. Multidisciplinary team work, the meetings held periodically by the different professionals involved, allows us to assess and follow up all the patients adequately.
The detection rate must be in keeping with the incidence rate expected for a given population (Table 1). A high detection rate at the expense of intraductal cancer (IDC) may indicate overdiagnosis.33,34 The proportion of IDC in relation to the infiltrating cancers should be greater than 10% (minimum standard) or even better greater than 15% (desirable standard).7 The proportion of invasive cancers ≤10mm with respect to the total number of cancers detected is an important indicator that reflects a program's quality.7 In the initial round, its minimum standard lies at 20%, but it is desirable for it to be above 25%. In successive screenings, the minimum and desirable standards should be above 25 and 30% respectively.7
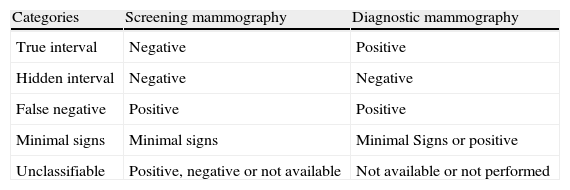
Screening mammography enables to detect most of the cancers suffered by the target population. Nevertheless, not all the women who attend screening benefit from it. It is estimated that 10–30% of the cancers may not be detected with mammography.35 A breast tumor diagnosed after a screening examination considered negative and before the appointment for the next scheduled checkup is referred to as IC.7,36 The IC rate is an indicator to assess a program's quality and future impact36 and it depends on breast cancer incidence and the periodicity and sensitivity of the screening test.36 IC is inevitable, but its number must be kept as small as possible because a high proportion reduces the program's effectiveness.7,36 False negatives should not surpass 20% of the total number of CI.7 Recording IC is an indispensable part of quality control and it requires follow-up of the target population to identify all the new cases of breast cancer that occur.7,36 ICs are classified into 5 categories according to what was proposed by the European Guideline (Table 4).7 The IC rate and the proportion of cases classified into the different categories vary depending on the method used to detect it and classify it radiologically.36 The protocol of the Breast Cancer Early Detection Programs36 proposes a methodology to assess IC and a systematic radiologic revision that ensure that data acquisition be thorough and the inclusion and exclusion criteria uniform.
Interval cancers classification into categoríes4
| Categories | Screening mammography | Diagnostic mammography |
| True interval | Negative | Positive |
| Hidden interval | Negative | Negative |
| False negative | Positive | Positive |
| Minimal signs | Minimal signs | Minimal Signs or positive |
| Unclassifiable | Positive, negative or not available | Not available or not performed |
Based on the UK Quality Assurance Guidelines for Radiologists, NHSBSP May, 1997, page 50.
At present all the Autonomous Communities (CC.AA.) have Breast Cancer Early Detection Programs offered to all the population considered to be at risk.8,17 The screening test is mammography with a two-year interval.8,17 Although operational criteria are common, there are some differentiating particularities among the Communities8,17,37 as to the age of the screening, the number of projections, the radiologist's minimum number of readings per year, the reading method, the intermediate revisions and the scope of the complementary studies.
The screening age covers from 50 to 64 years. Nevertheless, following the recommendations by the European Council6 as well as those by the National Health System,8,17 several CC. AAs have widened the upper limit to 69 years.8 Six Communities include, in addition, the 45-to-49 year group.8
The number of projections is 2 on each breast in the initial screening.8,37 In subsequent screenings, although some programs began with only one, double projection has also prevailed.37
The minimum number of readings a year is not demanded in all the Communities.8,37 Following the European guidelines, it is recommendable that every radiologist perform a minimum of 5,000 readings a year.7
The reading method is mostly double reading (with and without consensus).17 Nevertheless, the modalities vary among the Communities, and they may be simple (one reader), double with consensus (2 readers, who indicate the complementary test by agreement), double without consensus (2 readers with indication of a complementary test if one of them suggests it), and double with an arbiter (2 readers and, if they do not agree, a third radiologist to make the coinciding conduct prevail).37 Each reading model has advantages and disadvantages. The simple reading has lower economic cost, but it detects fewer cancers.38 The double independent reading increases detection in 9–20%, and in 13% for <1cm tumor. Its shortcoming is its higher cost and an increase in recall rate.38 The double reading with consensus manages to reduce recall to values similar to those of the simple reading.38 In order to optimize resources, it would be recommendable to perform a double independent reading and, in case of disagreement, consensus or arbitrage.15
For intermediate revisions (an interval of less than 2 years), the median percentage of indication is 3.21%, with a broad variability among the programs (0.78–6.43%), possibly because some programs include recommendations by family antecedents and not by radiologic findings.39
As to the complementary studies, some programs perform all the diagnostic confirmation tests (image and intervention examinations) in the hospital environment. Others, however, perform the complementary projections and ultrasounds at the Screening Units.
The screening processes are started by Management Units in charge of purging the databases, identifying and inviting the target population, issuing the result charts, following hospital referral and identifying CI.40 In Spain, variability with the programs work and the lack of uniformity in the data recording pose some problems when it comes to exploiting the results and comparing the indicators among the different Communities.30 In an effort to unify criteria, Breast Cancer Early Detection Programs on the Iberian Peninsula have been holding yearly meetings since 1998 where they present their results on a common basis and make proposals for consensus.37,39
The results of the main indicators corresponding to the year 200937 show a mean participation of 69, 10%, with considerable differences among the Communities (48–91.6%), and an adherence greater than 90%. The indication of complementary image tests is at 4, 67% (1.1–7.7%) and that of invasive tests at 0.65% (0.3–1.1%). The mean detection rate is at 3.62‰ (1.8–5.1‰). As to the type of tumors, for the most part, it reaches or approaches what is expected in a screening program8: invasive cancers 76.7%, IDC 14.1%, unknown tumoral type 9.2% and 67.6% of the tumors without ganglion affectation.37
Reflections on breast cancer screeningAccording to current scientific evidence, organized screening programs and quality treatment diminish breast cancer mortality. 4,13,41 The current programs that have been implemented for the longest time (Australia, Canada, Denmark, Finland, Iceland, Italy, Holland, Spain, Sweden and the United Kingdom) are publishing reduction of mortality of 16–36% among the women invited and of 24–48% among those who attend the screening.41 Nevertheless, the validity of the clinical trials that laid its foundations continues to be questioned, while the potential damage of population screening is stressed upon.30 Consequently, mammographic screening continues to be called into question periodically.15,42 The controversy started in the year 2000 with the article by Götzsche et al.43 that questioned the screening's justification. Later, Cochrane revisions concluded that their benefit was not proven.44 In 2009 several publications also had media repercussion because they argued that the benefits were fewer and the damages greater than had been believed so far45,46 that screening may start later and be less frequent to attain approximately the same benefit.45 Although there may still be controversy about the magnitude of the effects of screening, there is enough evidence at present to conclude that population screening diminishes breast cancer mortality4,14,41 and it enables the use of less aggressive surgical and oncological treatments.47,48
However, controversy on breast cancer screening conditions the lack of consensus in relevant issues:
- –
The age of the target population. There is consensus about the 50–69 year range8 in which the screening effectiveness has been proved widely.11,49 However, the expansion of screening onto younger women (40–49 years) generates controversy, possibly because the clinical trials that laid the foundations for population screening did not demonstrate a significant reduction of mortality in younger ages.8,11,30 Yet, recent studies suggest that it is also effective among 40–49 year-olds.50,51 In addition to the impact on mortality, the debate in this age group also focuses on the cost-risk-benefit relation52 due to the fact that the incidence of cancer is lower, tumors have a more rapid growth (less diagnostic advance and greater IC rate) and mammary density is greater (sensitivity and specificity of mammography decrease).10 The recommendation about the age to begin screening is different depending on regions and countries, and there is not unanimity among the different clinical guidelines.4 The lack of consensus generates contradictory messages among both population and professionals, and it causes an important volume of opportunistic screening before the age of inclusion in the programs.8,30
As to the women older than 70 years, in spite of the fact that breast cancer incidence is greater and the sensitivity of mammography is higher,35,53 they have generally been excluded from the population programs because a low participation rate was foreseen.54
- –
Periodicity of the screening. In Europe, biennial screening is classically recommended,7,8 while in the USA it was recommended annually.55 Recent publications indicate that between the 50 and 74 years of age the biennial interval attains almost all the benefit of the yearly screening, but with less damage.45
- –
Self-breast examination and physical examination. They present the disadvantage of increasing the false positives. The bibliography claims that «there is no evidence that self-examination of the breasts and clinical examination are recommendable» since their efficacy in the decrease of mortality is not proved.56,57 In our opinion, that is not reason enough not to recommend them, at least in dense breasts. It must be considered that the sensitivity of mammography is not as high as it was reported in the first recommendations of screening, where it was estimated at 85–95%.11 The actual sensitivity of mammography possibly ranges more widely from 68 to 95%, and it may even be lower in very dense breasts.49
- –
Number of mammographic projections. The European Guideline recommends the double projection in the initial screening, and leaves it up to the discretion of each program to make one or two projections in subsequent screenings.7 It has been demonstrated that double projection increases the test's sensitivity and specificity and reduces the reappointment rate7,49; therefore, in our opinion, the justification of single-projection screening in successive rounds should be only economic.
- –
Radiation-induced cancer risk. It is only demonstrated among the survivors of nuclear bombings in Japan and in patients subjected to radiation therapy.30,58 Digital mammography enables the use of a median glandular dose 22% lower than that of analogical mammography.23 The mean dose for a two-projection standard examination digital mammography is 3.7mGy as opposed to 4.7mGy with analogical mammography58 and it is considered insignificant to produce a radiation-induced cancer.59 Notwithstanding, the risk may increase as mammographies accumulate throughout life, especially when periodic mammography starts at younger ages.60
- –
Side effects of false positives. Psychological stress and physical morbility caused by reappointments and the indication of «unnecessary» interventionist procedures are considered among these.7,18,30,31 Most false positive findings only require complementary projections/or ultrasound to clarify the diagnosis of normalcy or benignancy.30 The percentage published about reappointment and indication of interventionism are variable according to the bibliography consulted, and the differences are clear between the US and European programs.30 Hubbard et al.61 publish recall rates of 16.3% in the first round and 9.6% in the subsequent ones, and interventionism rates of 2.5% in the initial round and of 1% in the subsequent ones. These authors estimate that after 10 years of participation in a yearly screening program, more than half the women will obtain a false positive result and between 7 and 9% an indication of biopsy. On their part, Castells et al. have estimated that one-third of the women who participate for 10 years in a biennial program may get a false positive result as recall.31 At present, it is considered that the participants should have adequate information about the probability of getting a reappointment and, ultimately, about the possibility of needing diagnostic confirmation invasive tests, so that every woman may decide whether she wants to participate or not in the screening, once the benefits and risks are known.31,61
- –
Overdiagnosis and overtreatment. These are two other negative effects of screening and they refer to the diagnosis and treatment of cancers which, in the absence of the screening, would never have caused any symptoms.10,17,62 Their exact magnitude is not clear, and they appear in the literature with very dissimilar figures33 Biesheuvel et al. estimated that the overdiagnosis range was between 4 and 54%.63 Jorgensen and Götzche have placed it between 52 and 54%, considering that one in every three 3 cancer diagnosed by screening corresponds to overdiagnosis.64 On the other hand, simulations conducted on theoretical models present estimations between 1 and 3%.65 A recent study that uses the incidence observed in the Dutch screening program and the theoretical incidence in the population without screening concludes that the overdiagnosis risk would be 2.8% in all the ages and 9.7% in the screening age, figures that are similar to what is estimated in the follow-up of the Swedish trial by Malmö (7–8%).66 Duffy et al. conclude that the benefit of screening is greater than the damage caused by overdiagnosis and that between 2 and 2.5 lives are saved for every case diagnosed in excess.67 Given that the current status of scientific knowledge does not permit to distinguish the breast cancers that will evolve from those that will not, at present, all the cancers detected are treated; thus a certain degree of overdiagnosis and overtreatment is inevitable.64
- –
High-risk women. The every-two-year follow-up strategies offered to women of intermediate risk are not sufficient for high-risk women.68 A high-risk woman is the one who are carriers of mutations in the BRCA1 or BRCA2 genes (an estimated risk of 50–80%) and those with important family antecedents, particularly in the case of bilateral breast cancer in early ages, and/or coexisting with ovarian cancers (estimated risk 30–50%).68,69 Most guidelines recommend beginning the examinations between 25 and 30 years of age.34 Mammographic screening in these ages presents inconveniences due to the lower sensitivity of mammography, prediction of elevated rates of IC and the possibility of provoking radiation-induced cancers.34 Other diagnostic modalities such as magnetic resonance and ultrasound are being proposed to compensate for the limitations of mammography among these women.70 Magnetic resonance is the technique with the greatest sensitivity (71–94% as opposed to 36–59% of mammography) although there are no randomized studies showing how it contributes to reducing mortality due to breast cancer.70 The results of the EVA trial concluded that the current magnetic resonance is superior to mammography, to diagnose both invasive and intraductal cancers.34 Studies conducted in high-risk women reflect a sensitivity of ultrasound between 33 and 65%.70,71 The results of the ACRIN 6666 trial in asymptomatic, high-risk women with dense breasts demonstrated that adding ultrasound to mammography in the screening increases cancer detection.71 Ultrasound is an economical technique and it does not use ionizing radiation, but its shortcoming is that it is operator-dependent and the number of false positives is greater than with mammography.71 Nowadays, magnetic resonance is beginning to look like the diagnostic modality of choice for high-risk women, which could even expand to those with a risk higher than 20%.34 Notwithstanding, both in the case of ultrasound and resonance, the actual usefulness of these techniques for screening will depend also on other factors such as the number of false positives and the availability of human and economic resources. In practice, there are still many issues to be solved regarding screening in high-risk women (beginning age, periodicity, techniques used, follow-up in population Screening Units or in multidisciplinary units, etc.).
- –
Breast density. High mammographic density is one of the factors predicting breast cancer risk.72 Women whose breasts are occupied more than 75% by dense areas have a risk 4–6 times higher than those whose breasts are made up of adipose tissue.73,74 In addition, in these cases the sensitivity of mammography is also lower.72 The options to improve detection rate may be shortening the screening interval (although it would possibly not increase either detection by lower mammography sensitivity in dense breasts) or else use other diagnostic combinations.72 The use of digital mammography, ultrasound and magnetic resonance may increase detection in very dense breasts in which, in addition, breast cancer risk is greater.72 Notwithstanding, and even though it seems clear that adding examinations would improve detection, in the context of a screening program, caution must be exercised and it must be assessed, by means of cost-benefit studies, whether it is profitable to indicate ultrasound and/or magnetic resonance in very dense breasts.
Early detection of breast cancer represents an important challenge for all the professionals involved. The good working order of a screening program is not limited just to the diagnosis of the cases, but rather it must ensure that they are managed adequately with specific health care circuits that guarantee homogeneous treatments based on the best scientific evidence. Work in multidisciplinary teams will guarantee that we may offer the patients the best therapeutic options to the end of actually making a positive impact on the disease's morbility and mortality, trying to minimize the negative effects of screening.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments have been conducted on human beings or animals for this research.
Data confidentialityThe authors declare that there are no patient data in this article.
Right to privacy and informed consentThe authors declare that there are no patient data in this article.
Authorship- 1
Person responsible for the study's integrity: MM.
- 2
Conception of the study: MM.
- 3
Design of the study: MM and AO.
- 4
Data acquisition: Not applicable.
- 5
Analysis and interpretation of data: Not applicable.
- 6
Statistical treatment: Not applicable.
- 7
Bibliographic search: MM.
- 8
Critical revision of the manuscript with intellectually relevant contributions: AO.
- 9
Writing of the paper: MM.
- 10
Approval of final version: MM and AO.
The authors declare that they have no conflict of interests.
Please cite this article as: Mellado Rodríguez M, Osa Labrador A. Cribado de cáncer de mama. Estado actual. Radiología. 2013;55:305–314.