Mucormycosis is a severe but rare fungical infection. It commonly affects immunocompromised individuals and is associated with high rates of mortality despite the use of modern antifungal therapy and surgical debridement. Mucor fungal infections involving chest wall are extremely unsual. We present a case of sternal mucormycosis in a patient with HIV and lymphoma.
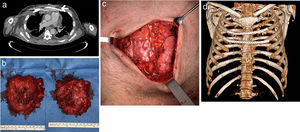
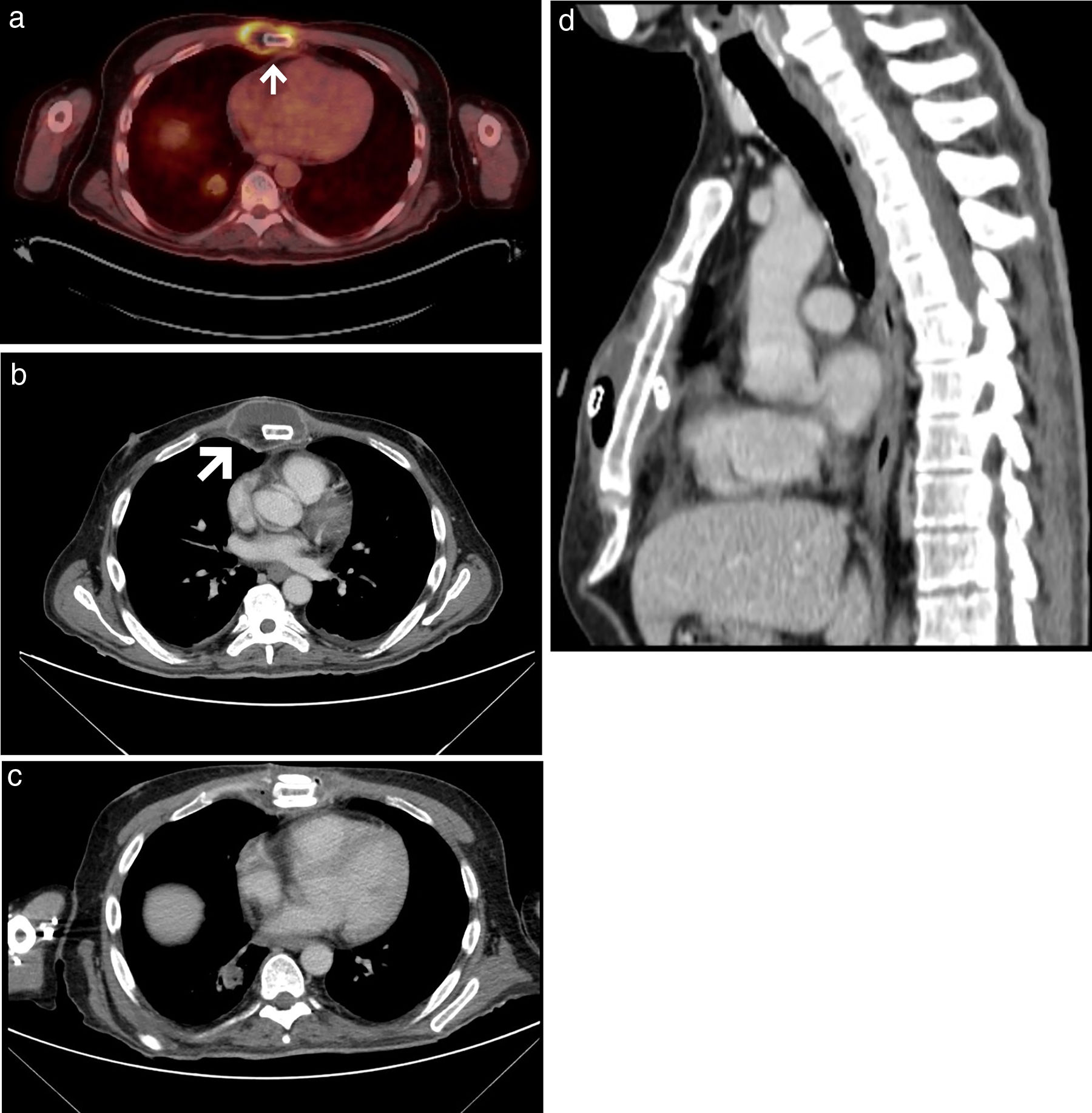
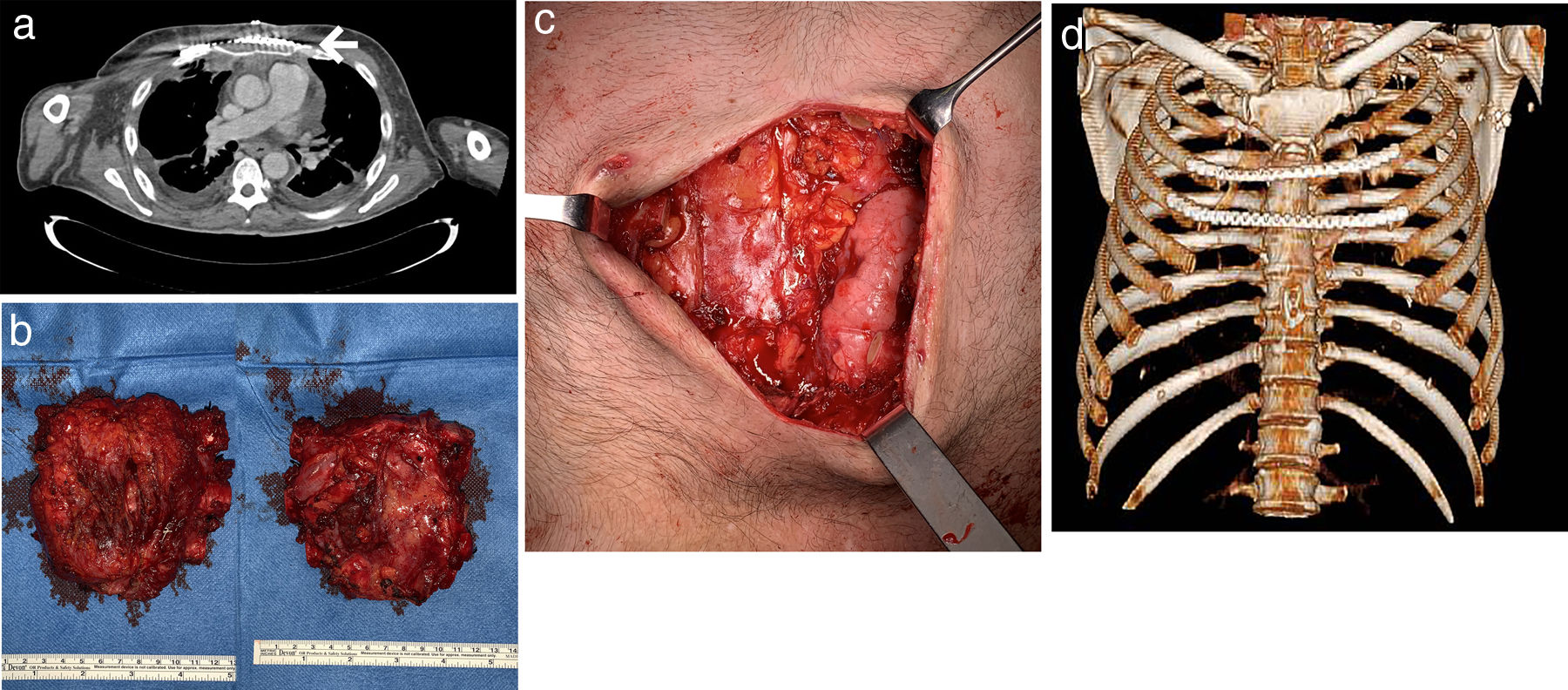
A 46-year-old man was referred to the thoracic surgery department of our hospital for management of sternal mucormycosis. Formerly, he was admitted to our hospital after an episode of upper gastrointestinal bleeding, and was finally diagnosed with B-cell non-Hodgkin's lymphoma (with bone marrow and central nervous system infiltration), previously unknown HIV and CMV infections, and a hemophagocytic lymphohistiocytosis. Despite aggressive medical therapy (chemotherapy, antibiotics, antiretroviral therapy, etc.), he continued to have persistent fever. A positron emission tomography/computed tomography (PET/CT) scan, performed for staging purposes of the patient's lymphoma, showed hypermetabolic soft-tissue lesions involving the left thigh, right leg, and sternal region consistent with abscesses (Fig. 1A, B). Due to progressive worsening of these lesions (both sternal and lower extremities) with increase in size, erythema and pain, surgical debridement was performed. Biopsy specimens were sent for microbiological and pathological analysis. Cunninghamella bertholletiae, resistant to azoles and echinocandins, was isolated in the samples, so treatment with amphotericin B lipid complex was started at high doses. Histology of specimens showed necrotic tissue with non-septate filamentous fungal hyphae with right angle branching suggestive of mucormycosis. A thoracic CT performed after surgical debridement (Fig. 1C, D) showed a worsening of the sternal collection, demonstrating an increase in the size of the collection and of the gas bubbles. Focal areas of disruption of the sternal cortical bone with intramedullary gas were also observed. Also, reappearance of soft tissue lesions was observed, for which a new debridement was necessary. After multidisciplinary discussion with haematology and infectious diseases teams, we decided to perform surgery in an attempt to control the sternal infection. A resection of both the sternal body and bilateral third to fifth costal cartilages was performed (Fig. 2B). A polypropylene prosthesis along with 2 titanium bars was used in order to reconstruct the anterior chest wall defect (Fig. 2A, C, D). The patient remained stable throughout surgery. He was extubated at the operating room before being admitted to the intensive care unit for observation. Histopathological examination showed an invasive fungal infection with extensive necrosis and invasion of the bone marrow of the sternum, as well as 90°-branched filamentous structures compatible with Mucor sp.
(A) Axial fused PET/CT image prior to surgery. White arrow showing an abscess. (B) Chest CT (axial image, mediastinal window) prior to surgery showing an abscess (white arrow) surrounding the sternum. (C, D) Chest CT (axial [C] and sagittal [D] images, mediastinal window) after surgical debridement.
(A) Chest CT (axial image, mediastinal window) after surgery showing the polypropylene prosthesis and one of the titanium bars (white arrow). (B) Photos chest wall resection piece (left front, right back) and (C) post-resection defect. (D) 3D volume-rendering CT image showing the chest wall reconstruction.
Postoperative recovery was uneventful and the chest drains were removed on postoperative day 5. The patient remained well and pain-free during serial follow-ups (5 months post-surgery) with no evidence of recurrence of sternal fungal infection.
Intensive intravenous antifungal treatment with liposomal amphotericin B and isavuconazole was maintained after surgery. Unfortunately, a new bone marrow biopsy performed 1 month after revealed extensive tumor involvement as well as hemophagocytosis, hemosiderophages, and fungal structures consistent with mucormycosis. Targeted treatment for the recurrent lymphoma was also initiated, but regrettably the patient did not respond to these therapies. Given that and the persistence of fungal disease with extensive bone marrow infiltration, antifungical and lymphoma treatment was stopped and it was decided to transfer the patient to a hospice care center. The patient died 7 months after surgery due to lymphoma progression and disseminated mucormycosis.
Mucormycosis is caused by fungi belonging to the order Mucorales. Mucormycosis in haematological patients is a severe infection with high mortality rate. Granulocytopenia, immunosuppression, diabetes, and penetrating trauma are the most prevalent predisposing diseases associated with mucormycosis.1 The most common sites of infection include lungs, sinuses, brain, and skin.2 The thoracic wall is an unusual location for mucormycosis, and very few cases of sternal mucormycosis have been described in the literature.3,4 Diagnosis is obtained from microscopic observation of clinical specimens. The combination of surgery and antifungal therapy is currently considered the treatment of choice for these patients.5 According to data from in vitro studies, animal models, and case reports, high dosages of liposomal amphotericin B or amphotericin B lipid complex are the current standard regimens of treatment.6 Surgery should be as radical as possible to significantly reduce the fungal burden, eradicate the disease, and prevent recurrence. Compared with that of other clinical manifestations, mortality was observed to be highest among patients with disseminated mucormycosis.7 Also, Cunninghamella infections associate higher mortality rate than that caused by other Mucorales.7
Only two cases of “spontaneous” (not post-surgical) mucormycosis with sternal or anterior chest wall involvement have been previously described in the literature.3,4 There are a few reports of sternal mucormycosis in cardiac patients who have undergone sternotomy. In both cases, resection surgery was performed and the thoracic wall was reconstructed with autologous rib grafts in one case and with an omental flap and skin grafts in the other, without local recurrence of the fungal infection. In our case, a reconstruction with a polypropylene prosthesis was performed and no recurrence occurred at the sternal level, so we believe that surgery was effective for locally controlling the fungal infection. Our patient's history of advanced (previously unknown) HIV infection, non-Hodgkin's lymphoma, and hemophagocytic syndrome may have negatively influenced his clinical evolution and explain the systemic mucormycosis.
In conclusion, resection and reconstruction with prosthetic material of chest wall mucormycosis in immunosuppressed patients may be a feasible option in well-selected cases, with low morbidity and mortality associated to the procedure and good local disease control.
Authors’ contributionThe authors have made substantial contributions regarding not only the conception and design of the manuscript, but also the drafting and critical revision of the article.
Source of fundingNo one has provided financial support for the preparation of this manuscript.
Conflict of interestThe authors declare no conflict of interest.





![(A) Axial fused PET/CT image prior to surgery. White arrow showing an abscess. (B) Chest CT (axial image, mediastinal window) prior to surgery showing an abscess (white arrow) surrounding the sternum. (C, D) Chest CT (axial [C] and sagittal [D] images, mediastinal window) after surgical debridement. (A) Axial fused PET/CT image prior to surgery. White arrow showing an abscess. (B) Chest CT (axial image, mediastinal window) prior to surgery showing an abscess (white arrow) surrounding the sternum. (C, D) Chest CT (axial [C] and sagittal [D] images, mediastinal window) after surgical debridement.](https://static.elsevier.es/multimedia/26596636/0000000300000004/v1_202112030727/S2659663621000576/v1_202112030727/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)