Anthracostenosis or bronchial anthracofibrosis was not reported in Europe until 2008, and only 4 cases have been published in Spain, specifically in 2 articles in this journal, the latest in 2012.1,2 A PubMed review showed that in the past 5 years, fewer than 10 cases of patients with this respiratory condition have been reported worldwide.6–12
We describe a new clinical case associated with biomass exposure, with an initial suspected diagnosis of sarcoidosis versus tuberculosis.
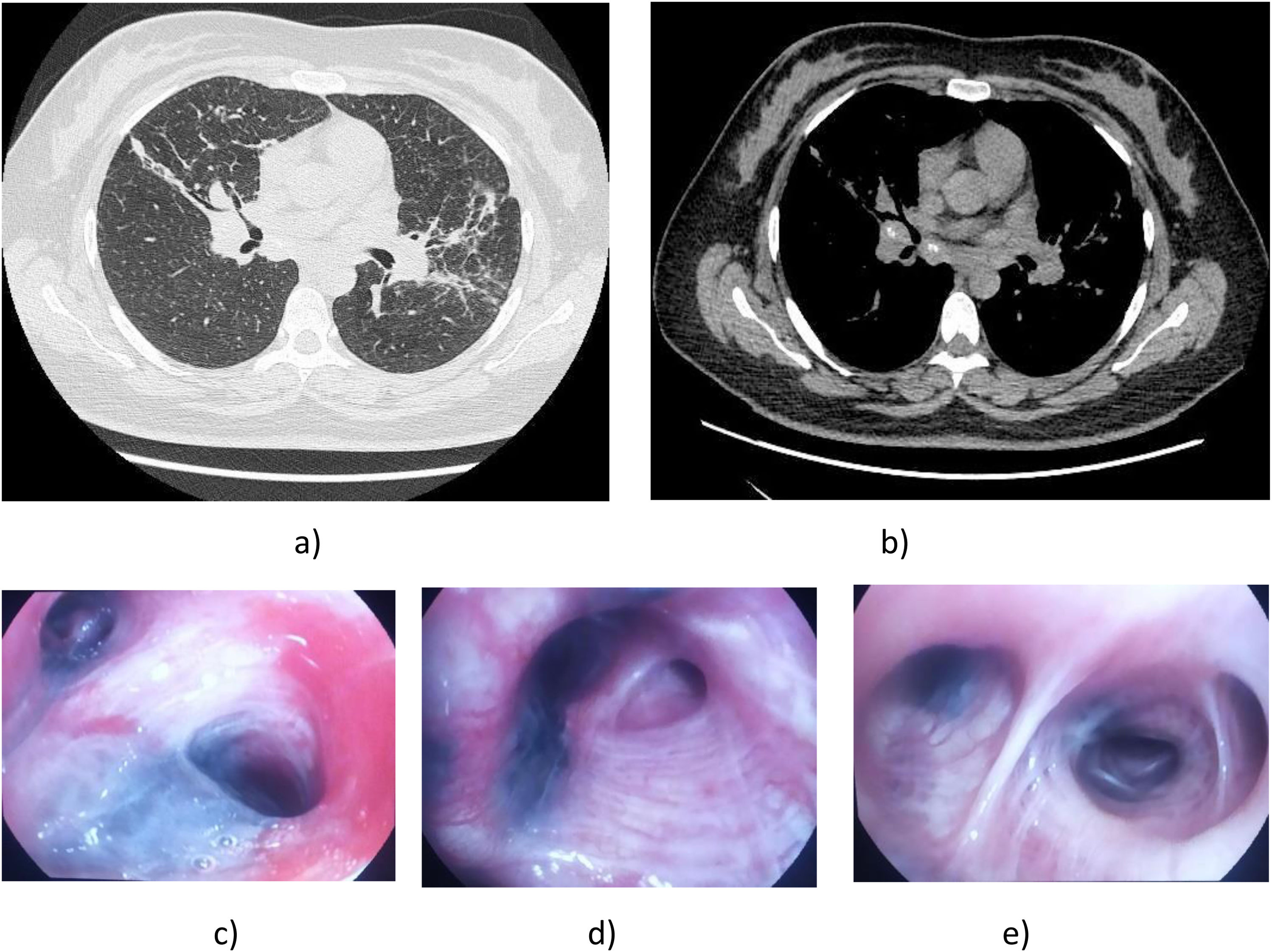
Our patient was a 36-year-old woman, with no history of toxic habits or past medical conditions, originally from Peru, who had been living in Spain for 7 years. She was not in contact with tuberculosis (TB) patients. However, until the age of 23, she was exposed to wood-based cooking and heating sources in her home country. She attended our respiratory medicine department with moderate exertional dyspnea. A chest X-ray revealed hilar thickening with areas of atelectasis/consolidation in the middle lobe and lingula, initially attributed to SARS-COV-2 infection, although all PCR tests and serology were negative. A subsequent chest computed tomography (CT) scan showed a very subtle micronodular pattern with perilymphatic distribution in both upper lobes, as well as atelectasis/consolidation areas, especially in the middle lobe and lingula, with mediastinal and hilar lymphadenopathies, raising suspicion of a granulomatous inflammatory/infectious condition as the primary diagnosis (TB or sarcoidosis)3 (Fig. 1a and b).
(a) CT slice in lung parenchymal window showing areas of consolidation/atelectasis predominantly in the mid-lung fields. A subtle bilateral micronodular pattern is also visualized. (b) CT slice in mediastinal window displaying significant bilateral hilar lymphadenopathies (>1cm in short axis diameter). (c) Endoscopic view showing thickened, anthracotic-appearing mucosa with almost complete stenosis of the entrance to the lingula. (d) Endoscopic view of the middle lobe, showing an extensive area of dark pigmentation with thickened mucosa causing marked narrowing of the medial segment of the middle lobe. (e) Endoscopic view from the intermediate bronchus, showing multiple areas of anthracotic pigmentation in the intermediate bronchus and in several segments of the right lower lobe.
Given the CT findings, fiberoptic bronchoscopy (FB) was performed, revealing extensive and numerous blackened areas of friable anthracotic-appearing mucosa throughout both bronchial trees (Fig. 1c–e). Near-complete stenosis of the lingula and the medial segment of the middle lobe was also observed, caused by mucosal thickening with prominent anthracotic pigmentation. This was biopsied several times, resulting in significant bleeding. Samples were collected by aspiration, and a bronchial lavage was performed, with negative microbiology results and normal cell counts. The biopsy histology showed fibrotic nodular lesions with anthracotic pigment and no evidence of malignancy. Endobronchial ultrasound (EBUS)-guided fine-needle aspiration (FNA) of the bilateral hilar, subcarinal, and right upper paratracheal lymph nodes retrieved anthracotic material, negative for malignancy and with no evidence of granulomas. Tpd date. no specific treatment has been initiated, as no Koch bacilli were isolated and lung function tests were normal.
Anthracofibrosis is a rare disease characterized by the deposition of anthracotic pigments in the bronchial wall that is associated with bronchostenosis and diagnosed by bronchoscopy.3,4
The primary causes of this condition include exposure to wood smoke (during cooking or domestic heating), as observed in our patient, and occupational histories, such as employment in the stone and coal industries. There is no significant association with smoking,2,4 but it has been associated with various diseases, including TB, chronic obstructive pulmonary disease, lung cancer, pneumonia, and interstitial lung disease (ILD).4,5,11
CT typically reveals bronchial stenosis with secondary atelectasis, mediastinal lymphadenopathies, and parenchymal changes caused by bronchial narrowing. Endobronchial tuberculosis, lung cancer and sarcoidosis are common causes of bronchial stenosis that mimic anthracofibrosis on imaging, requiring bronchoscopy and biopsy to confirm the diagnosis.9 Given the limited knowledge of anthracofibrosis in Spain, radiologists rarely report it as a primary diagnosis, and it may go unnoticed or undiagnosed.1,5
FB is the gold standard for diagnosing anthracofibrosis, and the presence of anthracotic pigmentation combined with multisegmental bronchostenosis may be sufficient for a definitive diagnosis.7,10 Endobronchial biopsy of affected segments often leads to profuse bleeding, as occurred in our patient, and the diagnostic yield for cytology may be inadequate. Melanoma must be considered in the differential diagnosis when extensive pigmented patches are observed during bronchoscopy, but EBUS-FNA can help narrow down the diagnosis.10,11
It is crucial to remember that anthracofibrosis is a distinct entity with generalized but nonspecific CT abnormalities that can often mimic tuberculosis, cancer and sarcoidosis and, rarely, even chronic thromboembolic pulmonary hypertension.3,9,12 Knowledge of this clinical condition is very limited among healthcare professionals, especially in urban areas where risk factors for its occurrence, such as ILD, are rare.
Declaration of generative AI and AI-assisted technologies in the writing processNo artificial intelligence was used for the writing or preparation of this manuscript.
Informed consentInformed consent was obtained from the patient for the publication of their clinical data and images.
FundingThis study received no funding.
Authors’ contributionsCNG prepared the initial draft of the manuscript. MJBB and MANB critically reviewed the content and approved the final version.
Conflicts of interestThe authors state that they have no conflict of interests.