Diabetic retinopathy (DR) is the leading cause of irreversible blindness in people of a productive age in developed countries, macular edema being (DME) primarily responsible.1 DME occurs through the alteration of the blood–ocular barrier (BRB), with a multifactorial mechanism secondary to changes in cellular junctions, loss of pericytes and endothelial cells, dilation of retinal vessels, leukostasis and vitreoretinal traction.2,3 In general, it is due to the increase in inflammatory factors, such as prostaglandins and specific proinflammatory interleukins, and angiogenic substances which include vascular endothelial growth factor (VEGF). Decreases of the anti-angiogenic retinal pigment epithelium derived-factor (PEDF)4 also contribute to this mechanism.
DiagnosisDME diagnosis is clinically performed by fundoscopy; when the center of the macula (fovea centralis) is thickened or swollen, this is referred to as a clinically significant central-involved macular edema (CI-CSME) and when it is unaffected we refer to is as a clinically significant non-central-involved macular edema (NCI-CSME). Fluorescent angiography (FAG) is a contrast study which confirms the presence of DME and classifies it as focal and diffuse, which guides the pathophysiology. Focal DME is the result of internal BRB damage (microaneurisms), whereas diffuse DME is linked mainly to external BRB damage (pigment epithelium of the retina). Some patients can display DME with both angiographic patterns. FAG remains the “gold standard” when deciding DME treatment; nevertheless, in a publication about the value of FAG in the planning of DME treatment, we found that the capability of the specialist to decide the treatment with laser after photographic analysis without FAG matched with the analysis based on FAG in 85.7% of cases and showed slight variations between both analyses in 14.3%.5 Despite the fact that FAG is very useful clinically, it does not contributes that much to the evaluation of retinal morphology and its thickening pattern. In 1991, the optical coherence tomography (OCT) was patented, and nowadays is an essential complement to the ophthalmoscopy and FAG in patients with DME. OCT is currently the most precise technique for in vivo measurement of retinal thickness and the analysis of vitreomacular interface, being key in diagnosis and monitoring of treatment.
TreatmentTreatment is focused on reestablishing BRB, modulating inflammatory and angiogenic factors. The Early Treatment Diabetic Retinopathy Study (ETDRS) proved there was a reduction of 50% in the risk of moderate vision loss when treating the edema with a threshold laser (from 24% to 12% at 3 years), increasing the possibility of obtaining a slight visual improvement. However, in diffuse DME this improvement is limited. Recently, the use of vascular growth factor antagonists (anti-VEGFs) in intravitreal injections has proven to reduce neurovascularization and DME, with better results than laser monotherapy (REVEAL, RESTORE, VIVID, VISTA, DA VINCI, BOLT, etc.). The laser acts as a modulator of substances like PEDF and VEGF, in addition to the thermal destruction of the external layers of the retina, and reduces metabolic demand and oxygen expenditure, with the consequent reduction of VEGF. Anti VEGFs selectively block VEGF. Bevacizumab (BVZ) (Avastin™) is a recombinant humanized monoclonal (IgG1) antibody which unites all VEGF-A isoforms and has been used off-label via intravitreal injection since 2005 in different angioproliferative pathologies. Ranibizumab (RBZ) (Lucentis™) is a humanized antibody fragment directed against all VEGF-A isoforms, manufactured exclusively for its intravitreal use. Ranibizumab was approved by the FDA in 2012, and by the Federal Commission for the Protection of Sanitary Risks (COFEPRIS by its Spanish acronym) in 2014, for its intraocular use in DME treatment. Aflibercept (AFB) (Eylea™ in USA/Wetlia™ in Mexico) is a recombinant fusion protein, consisting of fractions of domains of the human receptors of VEGF type 1 and 2. It attaches to VEGF-A and the Placental Growth Factor (PGF) in a sort of “bait receptor”, avoiding its interaction with native VEGF receptors. It has also been approved by the FDA (2014), and is pending approval by COFEPRIS (by the end of 2015) for its intravitreal use in DME. Multiple studies have proven the safety and efficacy of these anti-VEGFs (ANCHOR, MARINA, PIER, PrONTO, SUSTAIN, SAILOR, HORIZON, READ, RISE, RIDE, RESOLVE, RESTORE, CATT, COPERNICO, GALILEUS, DA VINCI, VIVID, VISTA, BOLT, DRCRnet, etc.). In 2011, the CATT study proved the safety and efficacy of RBZ as well as BVZ in the treatment of wet-age related macular degeneration (angioproliferative disease). Neither were statistically superior (safety and efficacy) to the other. This study supported the bases to continue the off-label use of intravitreal BVZ. In March 2015, the DRCnet published the results of their study, “Protocol T”. The study compared the safety and efficacy of these anti-VEGFs in DME. It reported an improvement in visual acuity in patients with central-involved macular edema, but this effect depended on the initial visual acuity. When initial visual acuity loss was minor (20/30 to 20/40), on average, there were no apparent differences between the 3 anti-VEGFs. Nevertheless, in patients with low initial visual acuity (20/50 or worse), AFB proved to be more effective in visual acuity improvement. Intravitreal steroids have shown encouraging results in the treatment of DME; they inhibit over-regulation of inflammatory molecules and VEGFs. There are the dexamethasone intravitreal implant (Ozurdex™) and the fluocinolone intravitreal implant (lluvien™), the first one approved by the FDA and pending approval by COFEPRIS (by the end of 2015) for its use in DME. The latter has been approved for its use only in Europe. Preservative-free triamcinolone (ATLC™) is another steroid also approved by the COFEPRIS for intraocular use. Nowadays, steroids are recommended for resistant cases; however, some ophthalmologists consider them as a first line treatment or as an adjuvant therapy to laser and anti-VEGFs. Because the vitreomacular interface (posterior hyaloid and inner limiting membrane) plays an important role in the development of DME, in tractive or non-respondent cases its withdrawal is indicated via vitrectomy.
Different schemes in DME treatment have been described. Threshold laser is recommended in a selective fashion in a single use and, if necessary, repeated in intervals of no less than 12 weeks. Pharmacological therapy with anti-VEGFs has been proposed, from having one injection and repeating only when the ophthalmologist considers it necessary (Pro Re Nata PRN), to uninterruptedly every 4 weeks for 24 months (monthly dose). Currently, there are two schemes, “treat and observe” (T&O) and “treat and extend” (T&E), the first one consists of applying a “loading phase” of three anti-VEGF doses or until accomplishing the maximum visual effect and resolution of the edema, with a 4-week interval between each injection, and after that in a PRN mode. In the latter (T&E), after the “loading phase”, the treatment is extended in rows of 2 weeks until achieving intervals no longer than 12 weeks. Re-treatment or treatment extension criteria will depend on visual acuity and tomography findings, loss (T&O) or non-loss (T&E) of more than five letters, or an increase (T&O) or decrease (T&E) of macular thickness of over 100 microns. In case of presenting edema reactivation in the scheduled appointment (T&E), a shortened treatment is indicated. Based on the possible synergy between laser, corticosteroids and anti-VEGFs, the combination of these three at the same time has been proposed, with two intentions; accomplishing a greater visual and anatomical effect, and accomplishing the least amount of treatment repetition in the long term. Nowadays, there is no exact algorithm for DME treatment, thus it remains a debate topic in retina conferences.
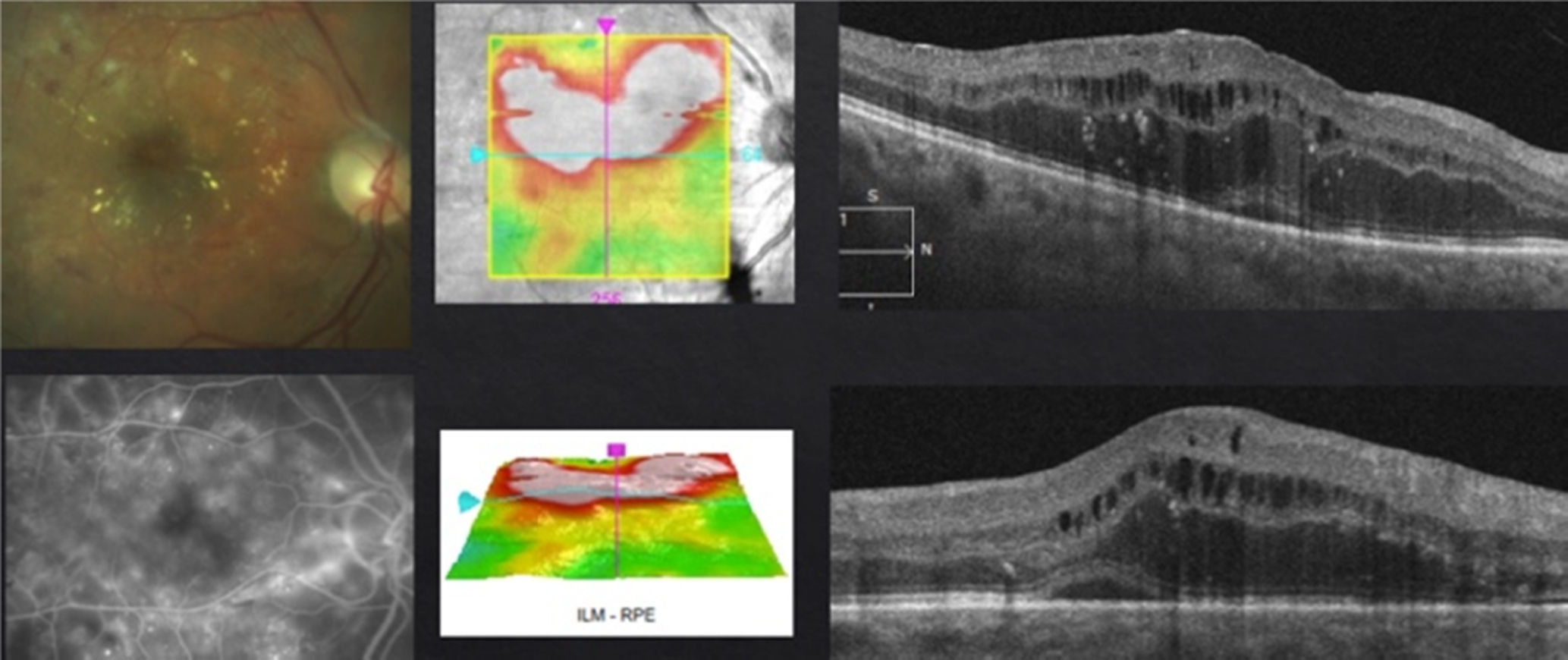
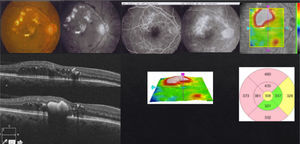
My personal approachStrict glycemic and lipid controls, systemic blood pressure and renal stability are fundamental. I decide on the treatment depending on clinical, angiographic and tomographic presentations. Based on reports from medical literature, as well as my personal experience,6–8 I consider as a first line treatment laser or intravitreal anti-VEGFs and intravitreal steroids or vitrectomy as a second line treatment. The anti-VEGFs I use will depend on the patient's economic capabilities; I choose BVZ in patients with limited economic resources. Despite the fact that the DRCRnet suggests a superiority of AFB in relation to RBZ in initial low vision cases, I usually begin with RBZ, and just in case I do not obtain a good response, I switch to AFB or BVZ. Monotherapy with anti-VEGFs, or in combination with laser, in my experience, has proven to be superior to combined therapy with steroids (TLC) during the “loading phase”.7 Therefore unless it is a case of macular ischemia (MI) or vitreo-proliferative diabetic retinopathy (VPDR), where the laser (MI) and the anti-VEGFs (MI and VPDR) may be questionable, I utilize them as first option of treatment. In case of chronic DME with little to no response to pharmacological (anti-VEGFs or steroids) and laser treatment, either with or without VMI thickening and/or VMTS, I indicate vitrectomy (Fig. 1). Vitrectomy is also the first option treatment in fibrovascular tractive retinal detachment with macular involvement secondary to vitreoproliferative diabetic retinopathy. It is important not to confuse VMI thickening or VMTS with a macular involvement tractive retinal detachment. As I mentioned before, in this case vitrectomy is my first option of treatment, contraindicating even the use of anti-VEGFs due to risk of fibrosis with detachment exacerbation. I apply pan-retinal photocoagulation if necessary.
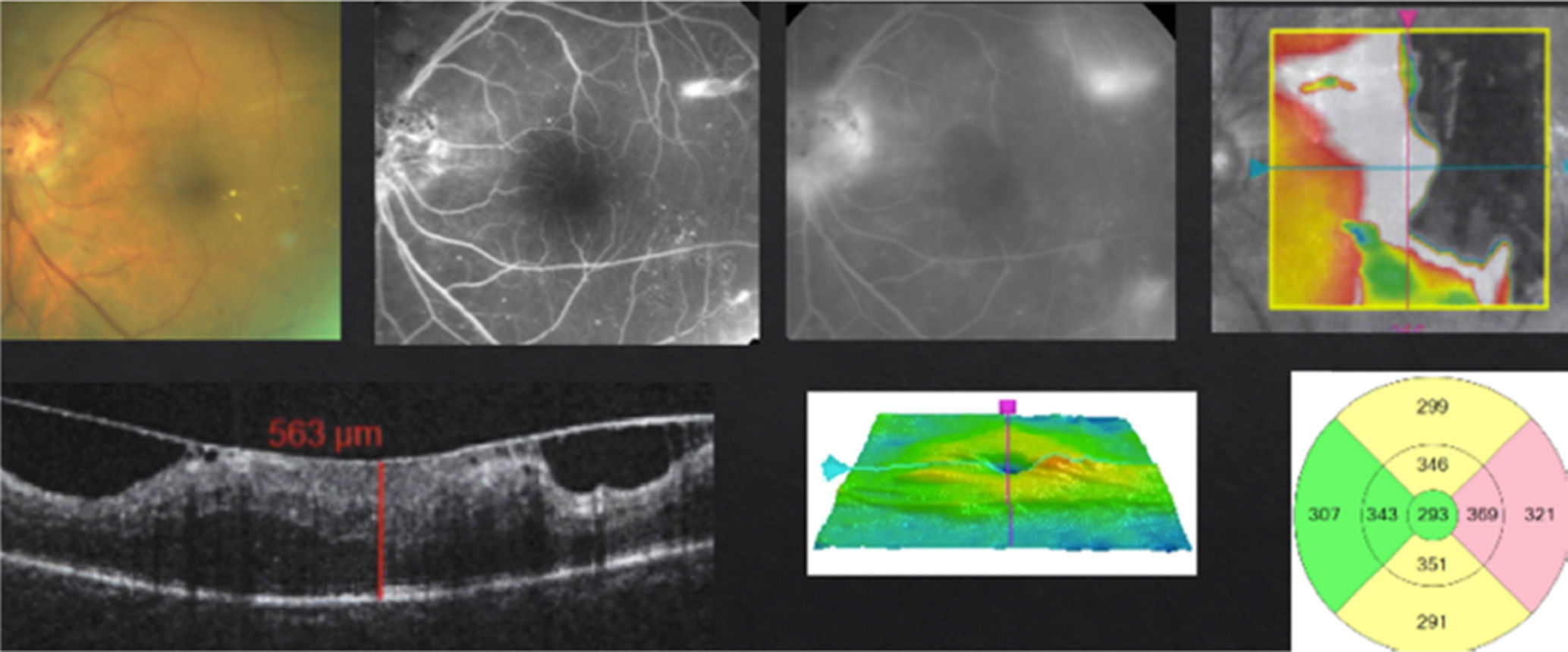
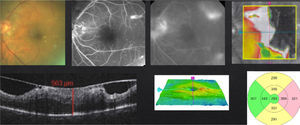
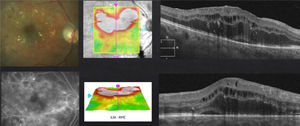
In case of presenting a NCI-CSME with a good visual acuity (better than 20/25), verified through FAG as a focal DME and an OCT showing a not involved fovea (Fig. 2), despite showing an anti-VEGF superiority over laser treatment in my experience,8 the decision to use monotherapy with laser as a first line treatment is my choice (risk of complication with intravitreal injection). Different from that suggested by the ETDRS, I use the invisible or subthreshold modified laser modality previously published,9 with the objective of lowering the complications of the threshold laser. It is possible to repeat this therapy in intervals of no less than 12 weeks. I apply the laser as a modified “scatter” in the edema zone (respecting the foveolar center) and, differing form multiple authors and the ETDRS, never direct to microaneurisms.9 I use anti-VEGFs, steroids or vitrectomy, in that order, as rescue therapies (when there is a poor or negative response with laser).
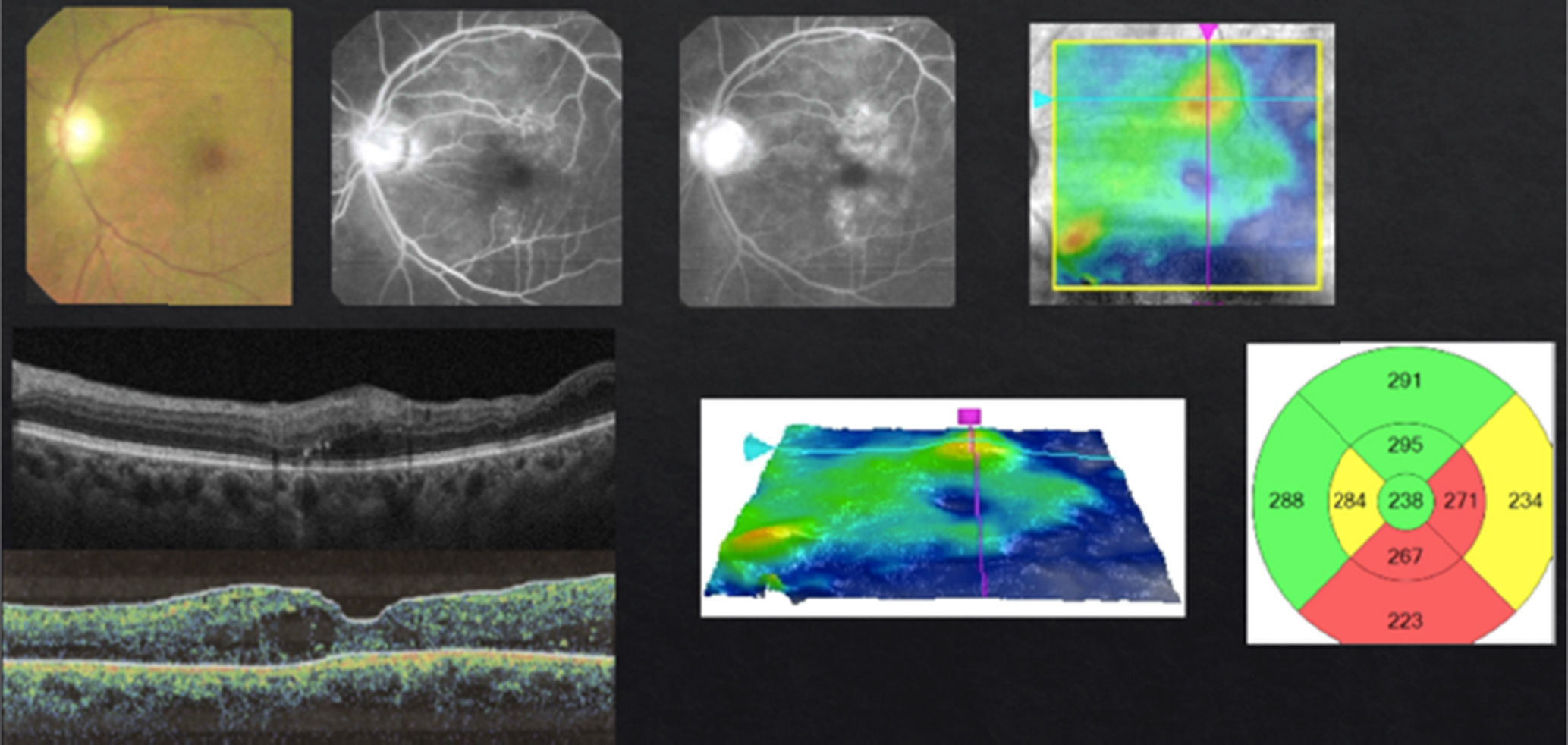
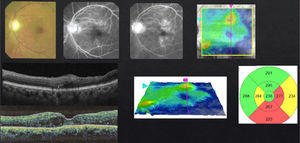
In case of presenting a CI-CSME with a slight decline in visual acuity (20/30 to 20/40), verified through FAG as a focal DME, with leakage that extends to the central zone and an OCT showing foveal involvement (Fig. 3), I suggest a combined therapy with anti-VEGFs and subthreshold lasers in the microaneurism zone, respecting the foveolar center. I apply the laser 1–3 weeks after the first injection, with the objective of a possible reduction in the frequency of future re-treatments. The injection scheme that I conduct is “Treat & Extend”. If necessary, I repeat the laser treatment with intervals of no shorter than 12 weeks between each session. Steroids and/or vitrectomy are just in case of a poor or null response.
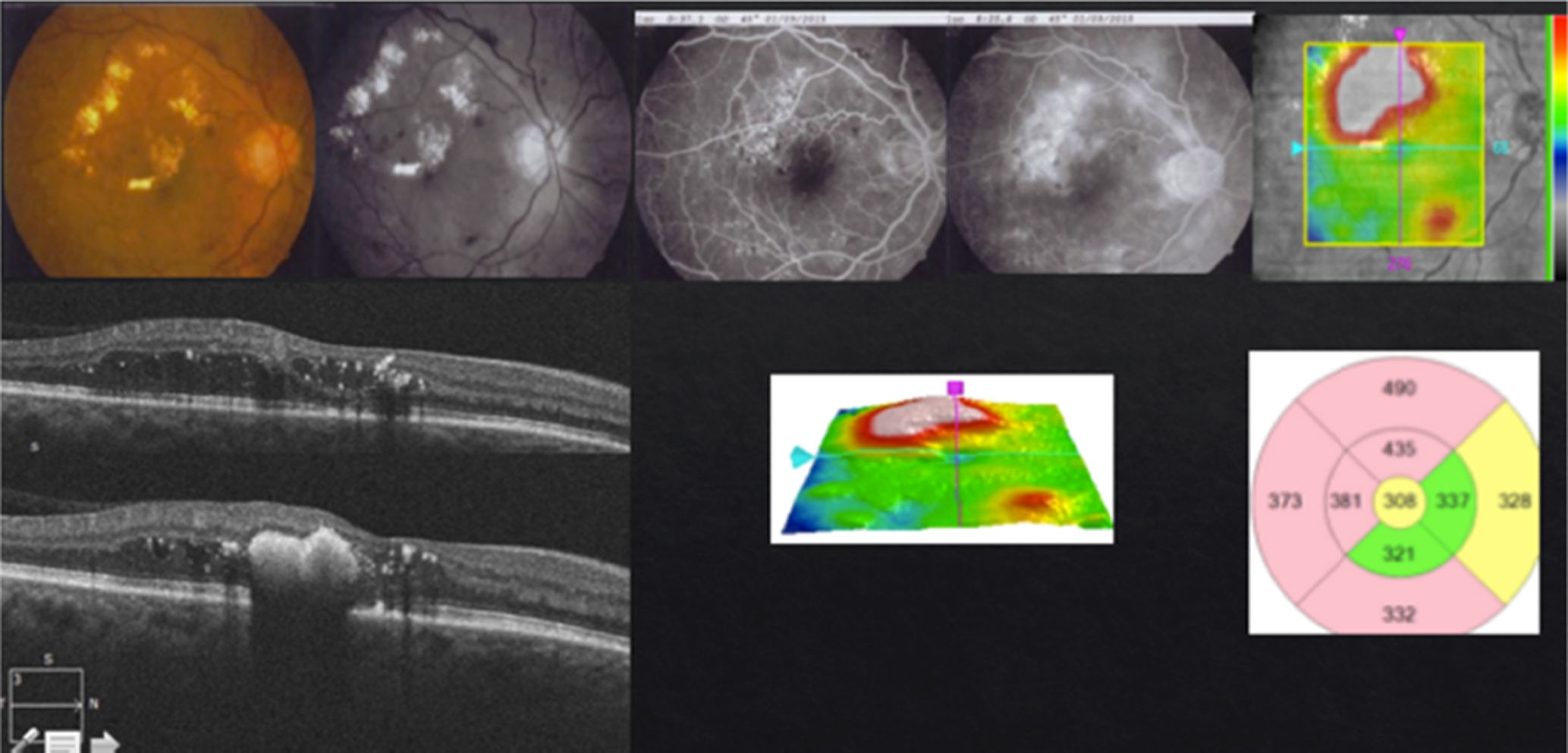
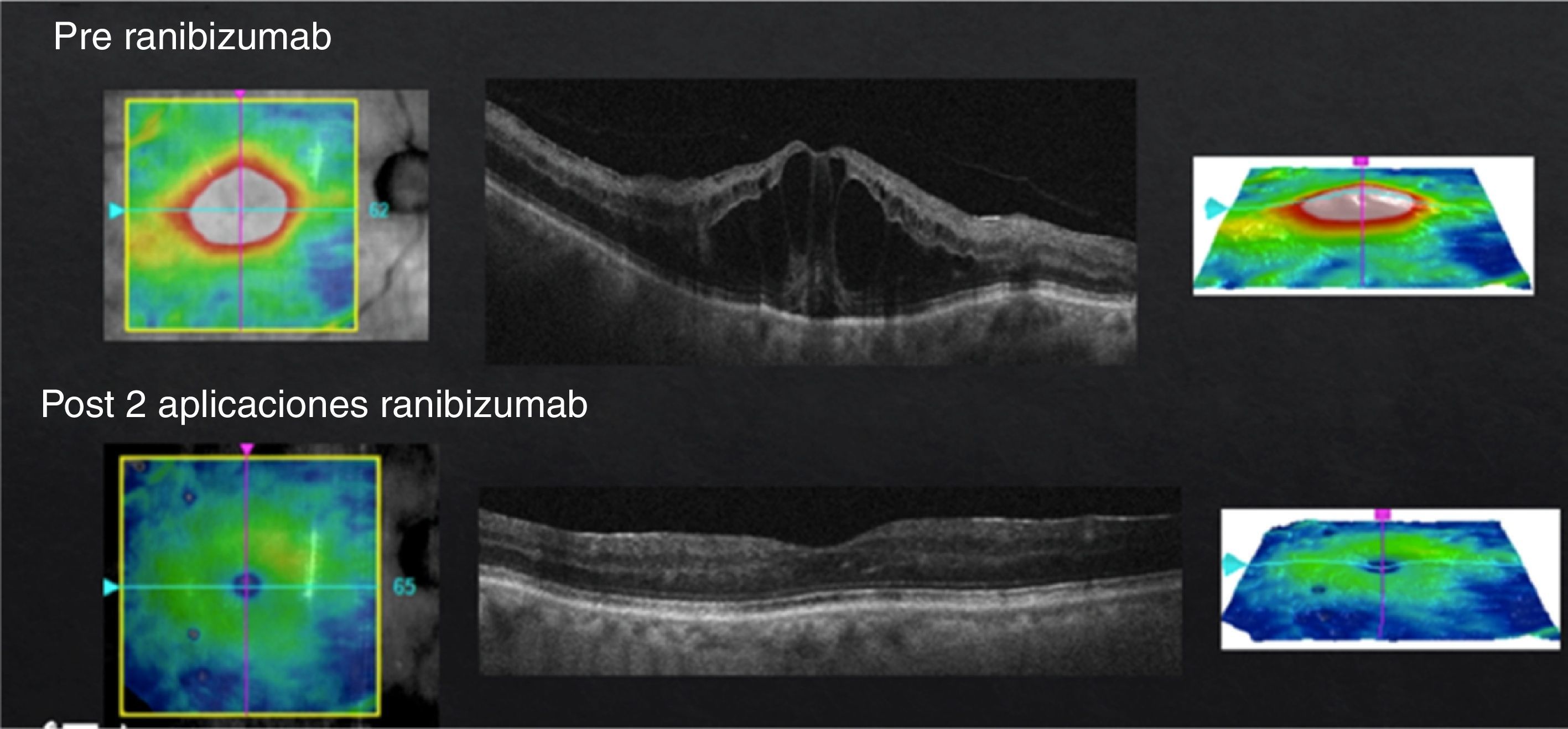
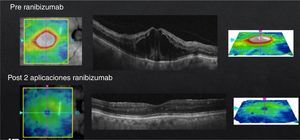
In case of presenting a CI-CSME with a bad visual acuity (less than 20/40), verified through FAG as a diffuse or mixed DME and an OCT with foveal data either in a beehive or petaloid shape, or with an added neurosensorial detachment (Fig. 4), monotherapy with anti-VEGFs under the “Treat & Extend” scheme is what I do. It is important to stress that, despite having a good response from the first or second injection during the “loading phase”, they ought to be repeated on at least three occasions (Fig. 5). In case of a partial response, I add subthreshold lasers as a rescue treatment, with the purpose of stimulating the production of PEDF and obtaining (by stimulation of the epithelium) a better response to anti-VEGFs. In case of a null response with anti-VEGFs, I do not apply lasers and cut straight to steroids. Given the case, if the patient shows positive results to the steroid therapy, the best is to try to continue this stability returning to anti-VEGFs. If this is not possible, I repeat steroid treatment at the time of recurrence. Similar to the other cases, if there is no response to medical therapy I perform a vitrectomy. Something that may also occur, despite satisfactory results with an anti-VEGFs, is that the patient may develop chronical tachyphylaxis or resistance to the anti-VEGF, suddenly manifesting a negative response. In this case, switching to a different anti-VEGF may reestablish a positive response.
In summary, anti-VEGFs are currently the “gold standard” in diffuse DME treatment. Laser remains as the “gold standard” in the treatment of focal DME without foveal involvement. Likewise, laser is used as an adjuvant therapy to pharmacological treatment (anti-VEGFs and steroids). Steroids are used in little or non-respondent cases (with or without VMI thickening and/or VMTS) resistant to first line treatment (laser and anti-VEGF) and as an adjuvant therapy to this same therapy. On the other hand, steroids may apply as a first choice of treatment of ischemic DME. A vitrectomy is indicated in case of chronic DME with little or no response to pharmacological and laser treatment, either with or without VMI alteration and/or VMTS, and as a first option of treatment in fibrovascular tractive retinal detachment.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.