The spectrum of hemifacial microsomia, or facio-auriculo-vertebral spectrum, is a complex of craniofacial and vertebral anomalies. Axis malformation is microtia, more often on the right side of 3:2. It may be associated with mandibular hypoplasia and vertebral malformations. It is more frequent in males and in twin pregnancies.
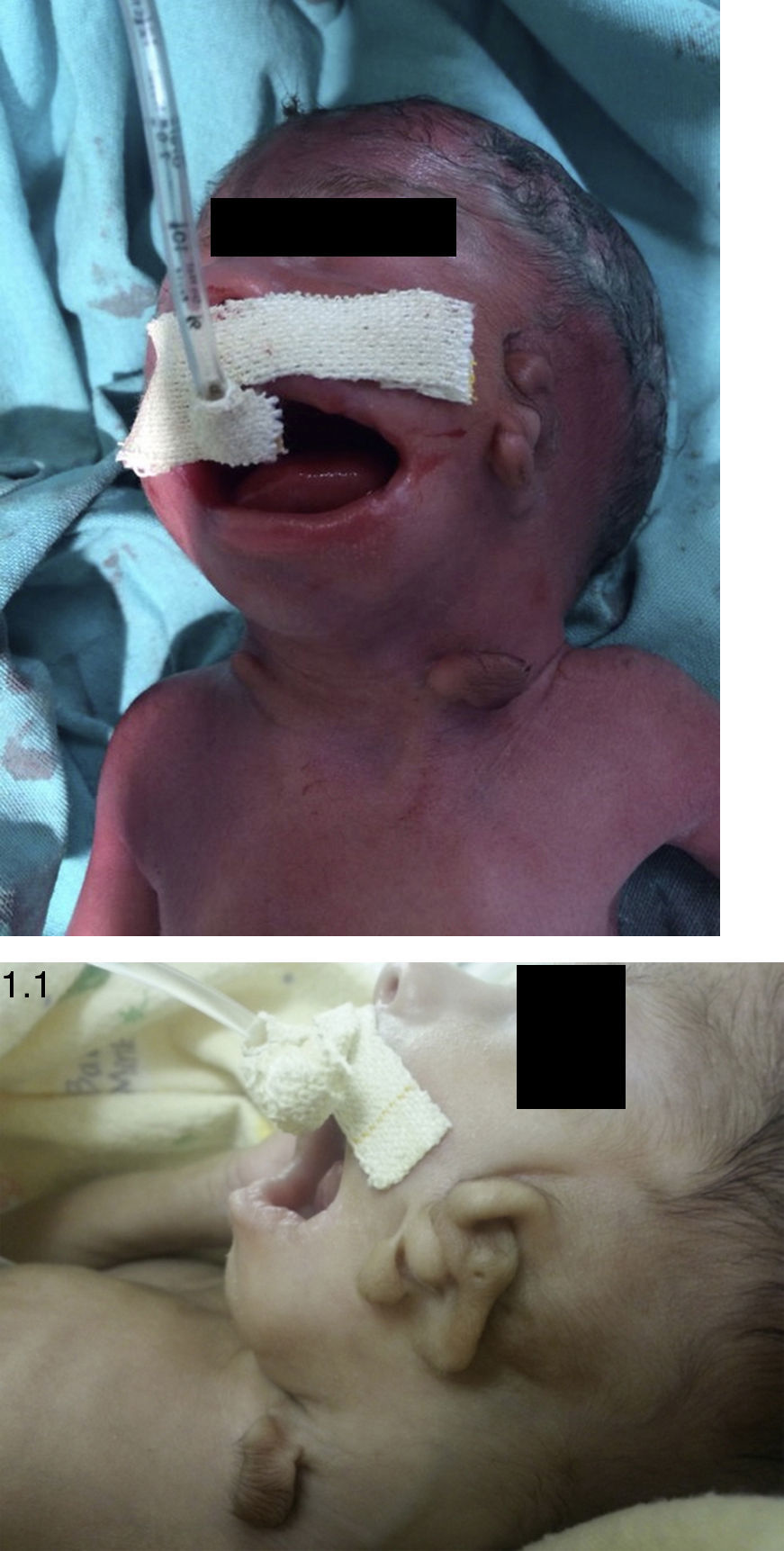
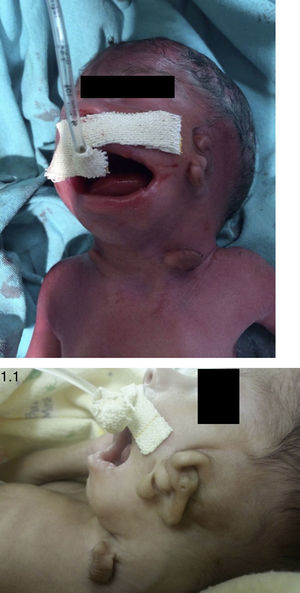
Clinical caseNewborn male, preterm, of 29.5 weeks gestational age, twin product, second twin pregnancy, dichorionic and diamniotic, born by cesarean section, which presented hemifacial microsomia, microtia of the left Tanzer 3 and the right auricle of low implantation with a backward rotation, left appendices and macrostomia. A thoracoabdominal X-ray found hemivertebrae in the cervical and dorsal area, which discussed genetic performing diagnosis of the hemifacial microsomia spectrum. An ear TAC is done, the bone atresia of the left ear meeting at the level of the left ear without evidence of tympanic membranes and with a dysplastic oscicular chain attached to the lateral wall of the attic. Discharged at 78 days of chronological age with 6 days of age, corrected with the consultation of neonatal high-risk follow-up.
ConclusionFacial asymmetry must be widely evaluated in patients with microtia, including deliberate search of renal, cardiac and spinal-level conditions, in order to diagnose pathologies such as the spectrum of hemifacial microsomia early.
The spectrum of hemifacial microsomia, or facio-auriculo-vertebral spectrum, is a complex of craniofacial and vertebral anomalies first described by Goldenhar in 1952.1 Axis malformation is microtia, and it may even be the only manifestation. Nevertheless, it can be found linked to mandibular hypoplasia and vertebral malformations.1 Microtia is a malformation characterized by the absence of some parts of the outer ear, and in some cases the entire ear. It can include the external hearing conduct and may be unilateral or bilateral.1 The unilateral form occurs in between 79 and 93% of cases.1
It has an incidence of 1:500 to 1:3000 live births, is more frequent in males at a 3:2 ratio, and has a dominant autosomal inheritance in 1–2% and a sporadic inheritance in 98%.1,2 It is the result of a defect in blastogenesis involving the first and the second pharyngeal arch, at approximately 30–45 days of gestation, with the formation of the first arch, which contributes to the formations of the structure of the face, both mandibular and maxillary portions, as well as the auricular pavilions.1–3 From the dysmorphologic point of view it is classified as a disruption. Among prenatal risk factors, there are: multiple pregnancies, anemia, advanced maternal age, threatened miscarriage, diabetes mellitus type 1 and 2 and medications such as isotretinoin. Twin pregnancy increases the risk of congenital malformations 2.47 times.4
Among the milder cases, preauricular appendices or isolated microtia may occur, whereas in the most severe cases, macrostomia and epibulbar dermoid, also known as the Goldenhar syndrome, occur.1,2 Hemifacial microsomia appears with a frequency of 20–65%. Regarding microtia, the right side is the most frequently affected, with a 3:2 ratio. Auricular malformations have a frequency of 65–99%, including preauricular appendices (with 40%).1,2,4,5
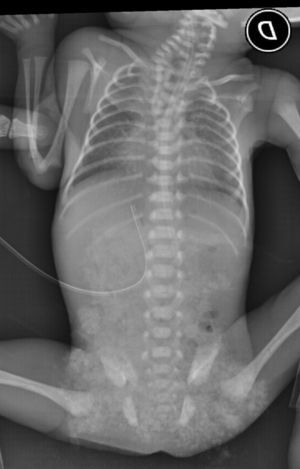
Axial skeleton alteration is limited to the cervical region and occasionally to the thoracic vertebrae, including hemivertebrae in 30%, renal malformations in 1–10% and cardiac malformations in 14–47%.6,7
There are known malformations associated with the spectrum, such as cleft palate, tracheoesophageal fistula, finger and hand anomalies and pulmonary hypoplasia.8–10
Case presentationA male, newborn, preterm of 29.5 weeks of gestational age, twin product, twin number 2, the mother 23 and the father 24 years old, non-blood relatives, without exposure to teratogens, product of a third gestation. It was a dichorionic and diamniotic spontaneous twin pregnancy, with proper prenatal care, and went through the first trimester with threatened miscarriage. There is a premature rupture of membranes at week 29.4 of gestational age, a dosage of pulmonary maturation inductors and the patient is granted conservative management after not presenting preterm labor. However, 7h after rupture there is fetal distress caused by type 2 decelerations of twin number 2, thus interrupting the pregnancy via C-section. The product is obtained with an Apgar score 5/8, a weight of 1110g, size 39cm, and a 27-cm head circumference, requiring admittance to the Neonatal Intensive Care Unit. Twin number 1, male born with a weight of 1290g, size 39cm, a 27.5cm head circumference and an Apgar score of 8/9, had no dysmorphias detected during physical examination and no bone defects in imaging studies, thus, no genetic studies are performed. He dies after 5 days of life outside the womb due to prematurity-related complications; hyaline membrane disease grade 2, pulmonary hemorrhage and late sepsis, we requested an autopsy from the parents; however, they declined.
During twin no. 2's physical examination, a wide anterior fontanelle is detected, broad nasal bridge, hemifacial microsomia, anteverted nares, left microtia Tanzer 3, low implantation of right auricular pavilion with backwards rotation, bilobed left preauricular appendices, bilateral fifth finger clinodactyly, bilateral cryptorchid, macrostomia, pointed palate, micrognathia without evidence of epibulbar dermoid (Fig. 1).
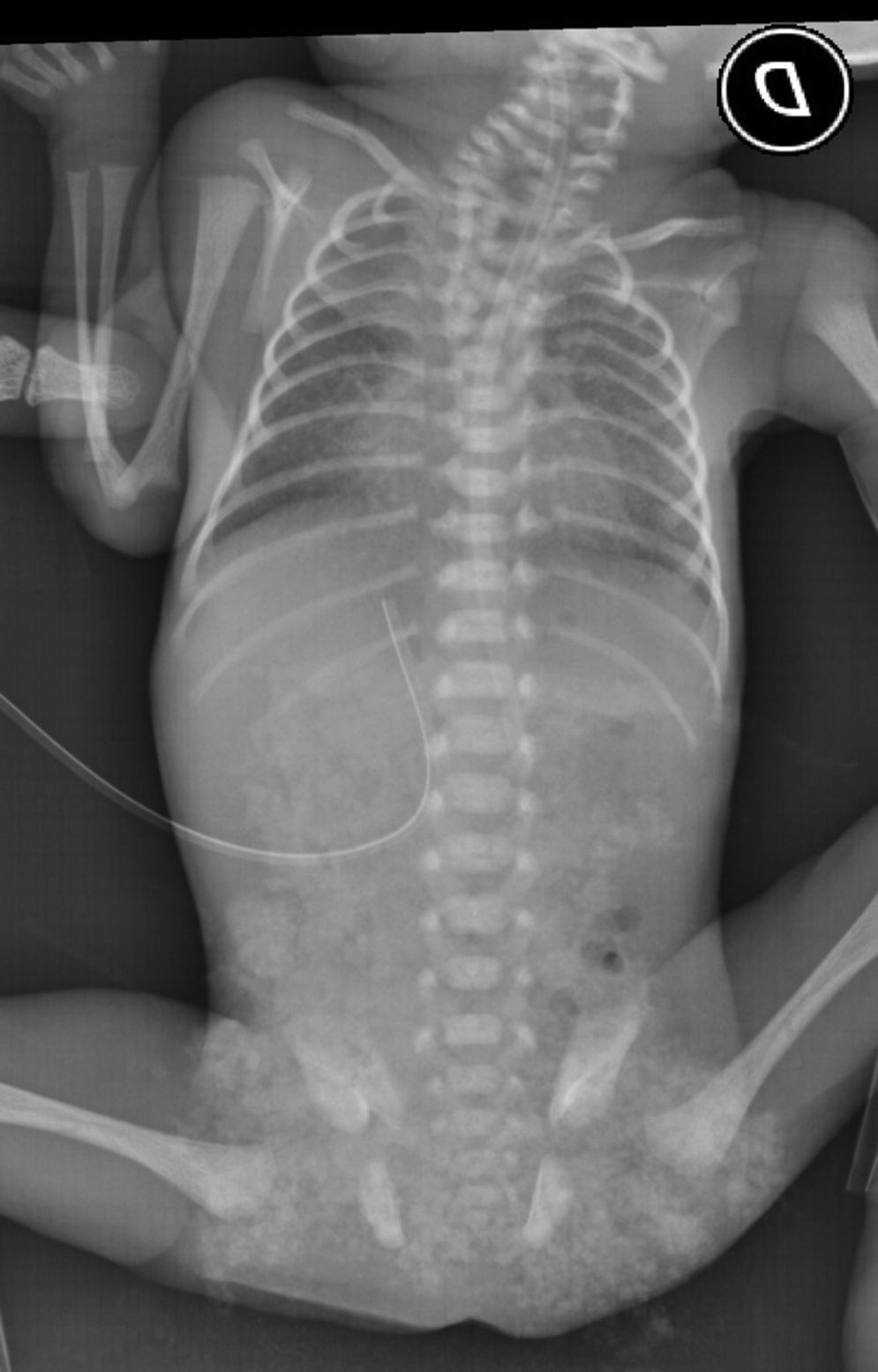
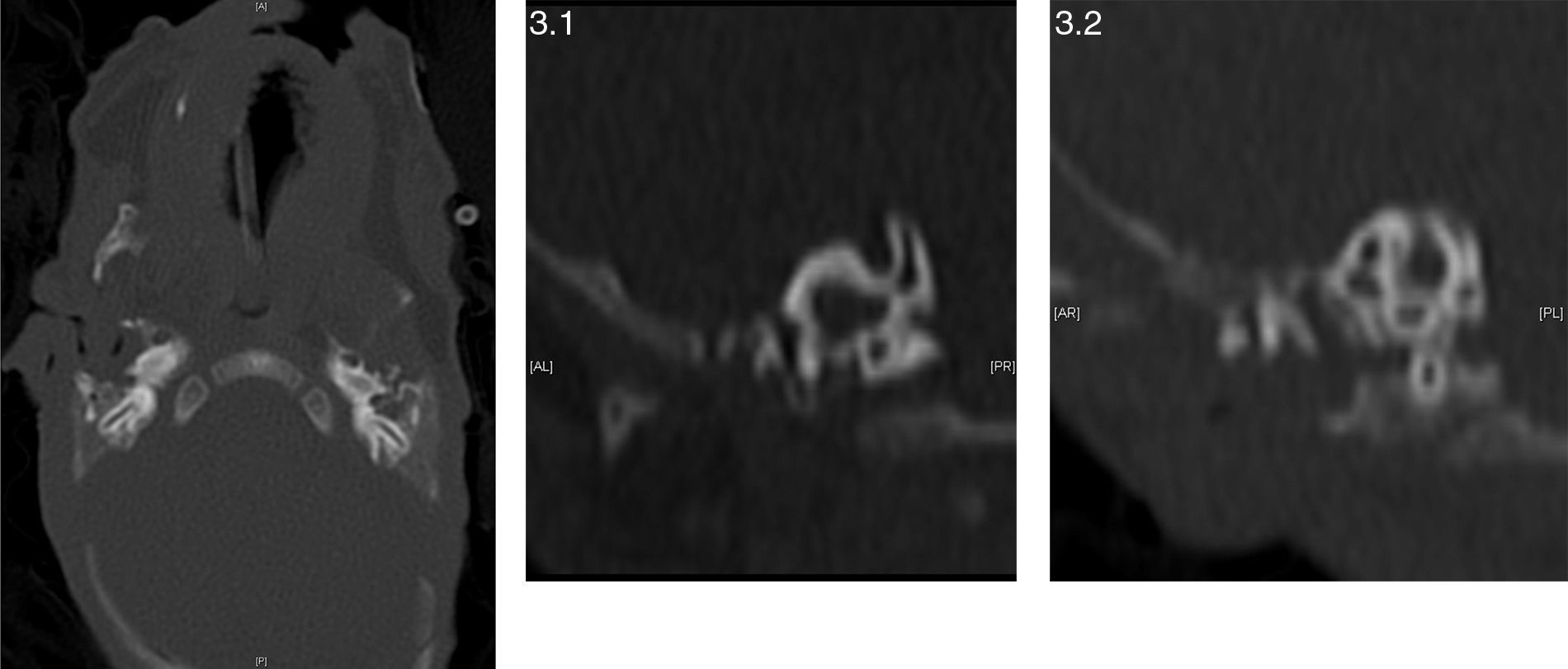
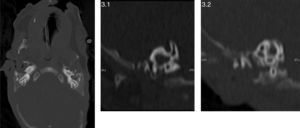
A thoracoabdominal X-ray is taken, observing hemivertebrae in cervical and dorsal areas, in addition to hyaline membrane disease grade 2 (Fig. 2) requiring 3 doses of pulmonary surfactant and mechanical ventilation, accomplishing continuous positive nasal airway pressure and successful extubation. Given the findings observed during physical and radiological examinations the Genetic Service is brought in. They obtain the parents’ genetic clinical history, and as a part of the approach of a dysmorphic patient a karyotype is performed, where a chromosomal complement of 46, XY is found corresponding to a male without major numeric or structural alterations in addition to performing follow-up during internment, giving a diagnosis of spectrum of hemifacial microsomia. A renal ultrasound is performed, as well as a transfontanellar ultrasound and an echocardiogram without pathological findings, an assessment by ophthalmology is requested without finding the presence of epibulbar dermoid or any other pathological finding during examination. The newborn is admitted to the Neonatal Intermediate Care Unit for growth and development, maternal training and attachment through the “kangaroo technique”. An ear CAT scan is performed, which reports a lack of development of the right ear and pneumatization of mastoid air cells, external hearing canal, tympanic membrane and ossicular chain without alterations, normal internal ear structures; at a left-ear level, atresia of the left ear canal without evidence of tympanic membrane and dysplastic ossicular chain attached to the attic's lateral wall (Fig. 3). The internal ear structures were normal. There is a lack of development and pneumatization of mastoid air cells. He was discharged at 78 days of chronological age, 6 days corrected age, with a weight of 1570g with follow-up in high risk neonatal consultation in order to continue with the monitoring of neurological growth and development.
DiscussionIt is described in the literature that the risk of congenital malformations increases 2.47 times in twin pregnancies; the spectrum of hemifacial microsomia is more frequent in dichorionic and diamniotic twin pregnancies, according to a study conducted at the National Institute of Perinatology on discordance of congenital defects in newborns of multiple pregnancies, this being our patient's case. Some of the risk factors found in this case include being male, a twin product and having a background of threatened miscarriage in the first trimester of pregnancy.11,12 We are talking about discordant dichorionic and diamniotic twins, which we assume are dizygotic because only one of them was affected; nevertheless we do not have the proper evaluation to define a dizygotic or monozygotic origin of the twins, which was not performed when twin number 1 died. It is also described in the literature that monochorial twins show a higher discordance frequency regarding congenital anomalies; in fact they are considered to be concordant only in 9–18% of the cases.13,14 However, the risk of congenital defects in monozygotic twins is 10 times greater in comparison to the general population and dizygotic twins.15 As of 1988, with the high amount of published cases worldwide, a concordance for this spectrum of 20% was considered for monozygotic twins and much lower for dizygotic, also weighing in environmental factors, a situation that has been confirmed due to alterations found such as vascular disruptions occurring in the stapedial and internal carotid arteries in monozygotic twins.16,17
Because most cases of spectrum of hemifacial microsomia occur as an isolated case (where the occurrence is in just one individual in the family), its low penetrance and unknown etiology, the risk of recurrence given is an empiric 2–3%.
The presence of hemifacial microsomia, macrostomy and microtia without the presence of an external hearing canal stands out in this case, because it turns out to be a relevant defect since it is commonly found in this spectrum. Different from what is described in the literature, the microtia found in the patient was on the left side, without finding any cardiac, renal and at-brain-level malformations which are common cause of morbimortality.
Within the external follow-up of these patients, special attention must be placed on the affection in speech development, the presence of pharyngeal and/or laryngeal anomalies and velopalatal insufficiency, and a dental assessment must be performed in addition to craniofacial defects which require surgical correction prior to conducting an imaging study or esthetic corrections during the assessment of appendices and microtia and the evaluation of visual and hearing acuity. These measures do not help nor strengthen the diagnosis; nevertheless, they are evaluations conducted with the purpose of assessing the patient's capability at a sensorial and functional level.
There are few reported cases of the spectrum of hemifacial microsomia in preterm newborns who accomplish a successful evolution, because of the conditions related to prematurity or alterations caused by the spectrum. Thus it is important to highlight the state of prematurity of 29.5 weeks of gestational age and being a product of a twin pregnancy in our case.
Conflict of interestThe authors have no conflicts of interest to declare.