The increased susceptibility of diabetic patients to Mycobacterium tuberculosis infection is not clearly understood. Cytokines produced by the host during the infection against the mycobacteria wall components may help to elucidate this relation. For this study, four groups were considered: healthy controls, patients with type 2 diabetes mellitus (DM), patients with pulmonary tuberculosis and patients with type 2 DM infected with M. tuberculosis. Mononuclear cells were isolated from peripheral blood and cultured to obtain adherent cells. The selected cells were stimulated with apolar lipids of M. tuberculosis. Proinflammatory and anti-inflammatory cytokines production were measured by ELISA. Anti-inflammatory cytokines production, IL-10 and IL-4, were increased in DM patients with tuberculosis. On the other hand, IFNg production was found to be lower in these patients. In conclusion, apolar lipids of M. tuberculosis are inducing an anti-inflammatory environment; which could be associated with type 2 DM patients' susceptibility to M. tuberculosis infection.
A pesar del extenso conocimiento que se tiene sobre diabetes mellitus (DM) tipo 2 y tuberculosis, aún no se han logrado elucidar los mecanismos involucrados en la incrementada susceptibilidad, que poseen los pacientes diabéticos de contraer tuberculosis. Las citocinas producidas durante la infección contra los componentes de la micobacteria podrían ser la clave para descifrar esta relación. En este estudio se formaron cuatro grupos de estudio: controles sanos, pacientes con tuberculosis pulmonar, pacientes con DM tipo 2 y pacientes con DM tipo 2 con tuberculosis pulmonar. Células mononucleares fueron aisladas de sangre periférica y cultivadas para la obtención de células adherentes. Más tarde, dichas células fueron estimuladas con una concentración determinada de lípidos apolares de M. tuberculosis. La producción de citocinas proinflamatorias y antiinflamatorias fue establecida mediante ELISA. Se observó un aumento significativo en la producción de citocinas antiinflamatorias, IL-10 y IL-4, en los pacientes diabéticos infectados con M. tuberculosis. Por otro lado, los niveles de IFNg fueron significantemente menores en estos pacientes. En conclusión, los resultados sugieren que los lípidos apolares de M. tuberculosis están induciendo un ambiente antiinflamatorio, que podría estar asociado con el mayor riesgo que poseen los pacientes diabéticos de contraer tuberculosis.
Introduction
Tuberculosis is one of the main causes of illness and death worldwide. In 2006, about 9.2 million cases of people infected with Mycobacterium tuberculosis were reported by the World Health Organization and is expected to kill more than 70 million people over the next two decades.1,2 Diabetes mellitus (DM) is a metabolic disorder that weakens the immune system and is considered an important predisposing factor for tuberculosis. Recently, studies have shown that between 10 to 30% of the patients with tuberculosis suffer from type 2 DM as well.3 Cohort studies demonstrated that people with type 2 diabetes have 3-fold risk of developing active tuberculosis.4 Even when these diseases have been widely studied, the connection between them is not clear. Cytokines produced by the host during M.tuberculosis infection against mycobacteria, especially wall components, may help to elucidate this link.5
During active tuberculosis, interferon gamma (IFNg) is a key cytokine that participates in macrophage activation by mediating host defenses against M. tuberculosis.6 In contrast, production of immunosuppressive molecules during active tuberculosis contributes to the establishment of chronic mycobacterial infections.7 Additionally, overproduction of interleukin 10 (IL-10) by T cells has been associated with immunosuppression and it increases susceptibility to mycobacterial infection.8
Several studies have shown evidence in support of the relationship between DM and alternated host immunity against M. tuberculosis. For instance, studies have shown that chronic diabetic mice have significantly lower production of IFN-g and interleukin 12 (IL-12) compared to euglycemic mice.7 Moreover, IL-6 is a good inducer of acute phase protein, however, Aderka et al. reported that IL-6 inhibits LPS-induced TNF-a production.9
In human models, high levels of insulin have been shown to induce a decrease in Th1 immunity on plasma cells.10 Furthermore, an in vitro comparison study of Th1 cytokines determinate that nonspecific IFN-g levels were significantly lower in people with diabetes compared to non diabetic individuals.11
The aim of this study was to elucidate if Th1 cytokine response is being inhibited by Mycobacterium tuberculosis lipids in adherent cells of diabetic patients and if it could be associated with the induction of an anti-inflammatory environment.
Materials and methods
Study Population
Sixty subjects between the ages of 18 and 60 were studied. Each one belonged into one of four groups: 15 healthy controls, 15 patients with type 2 DM, 15 patients with pulmonary tuberculosis, and 15 patients with type 2 DM and pulmonary tuberculosis. The Bioethics Committee from the School of Medicine at the Universidad Autónoma de Nuevo León approved the project, ensuring that it was in accordance with institutional Review Board criteria.
Healthy controls were TST-negative and they did not present type-2 DM or other metabolic disorder. Alternatively, type 2 diabetes patients were selected based on their glycosylated hemoglobin alfa (A1), which needed to be between 6.0 to 8.0%. Pulmonary tuberculosis patients were enrolled during the first three months of treatment and the diagnosis was based on positive sputum smear test, confirmed by a positive culture of tuberculosis. All enrolled subjects were HiV negative and did not present any other concurrent infectious disease.
The sixty individuals studied were mostly between the ages of 35 and 50 (45%), followed by 20 to 35 year old subjects (33%). In addition, 22% were above 50 years and only 2% corresponded to the ages between 18 and 20. The gender distribution was 57% male and 43% female. Detail information about the ages and gender of each study group is shown in Table 1.
This scientific approach has been done mostly in mice with larger number of subjects. However, the size of the sample for this study was determinate based in previous studies in humans in which conclusive results were achieved employing less study subjects.
Extraction of mononuclear cells from peripheral blood
Blood with EDTA was drawn from the subjects and mononuclear cells were isolated by Ficoll-diatrizoate gradient centrifugation. The cells were collected from the interphase and thoroughly washed with pyrogen free phosphate buffer (PBS). After the last wash, the cells were resuspended in RPMI 1640 medium with 10% of FBS. This solution was transferred to a culture plaque and remained in incubation for 20 hours at 37°C with 5% of CO2.
Obtaining monocyte-derived adherent cells and stimulation with M. tuberculosis apolar lipids
Using the plate adhesion technique, supernatants were carefully removed from de culture plaque. After two washes with RPMI 1640 medium, the adherent cells were detached with a scrapper and collected in an assay tube. The cells were centrifuged and the cellular pellet was resuspended in RPMI 1640 medium with 10% of FBS. Final cell concentration was adjusted to 1x106 cell/mL. Subsequently, the cells were stimulated with 10ug of Mycobacterium tuberculosis apolar lipids resuspended in RPMI 1640 medium with 10% of DMSO for 24 hours at 37°C with 5% of CO2. The mycobacteria lipids were obtained from the M. tuberculosis H37Rv biomass using the protocol reported by Arce-Mendoza A et al.12 Adherent cells were stimulated with RPMI with 10% DMSO as negative controls.
Cytokine quantification
Supernatants were collected from the cell culture and kept at -80°C until analysis. After all samples were complete, IL-4, IL-6, IL-10, IL-12p70, and IFNg cytokines were screened in duplicate by ELISA (eBioscience ELISAkits®, California). Standard curves for each cytokine was generated using reference cytokine concentrations supplied by the manufacturer. In order to obtain only the cytokine production induced by the apolar lipids, a relation between stimulated and non-stimulated samples was established.
Statistical analysis
For the statistical analysis, results were evaluated with GraphPad Prism version 4 Software with a one-way ANOVA being applied with the Tukey test, a value of p≤0.05 was considered statistically significant.
Results
Cytokines
Supernatants were divided into two groups to establish cytokine production on each study group. One group received the stimulation of lipids of M. tuberculosis and the other group only received culture medium.
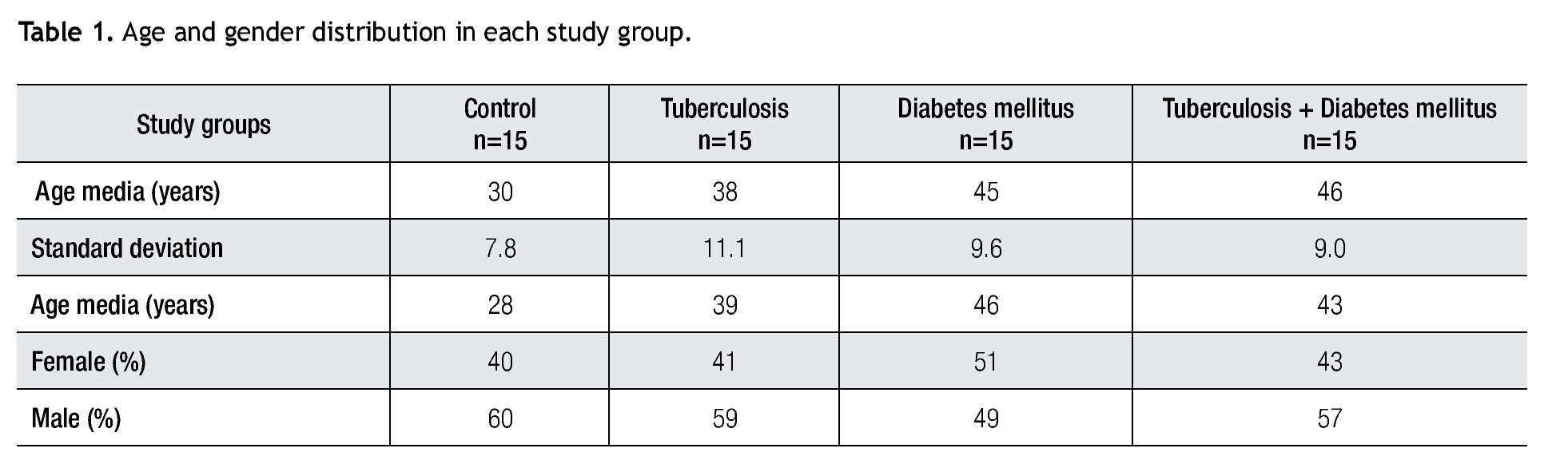
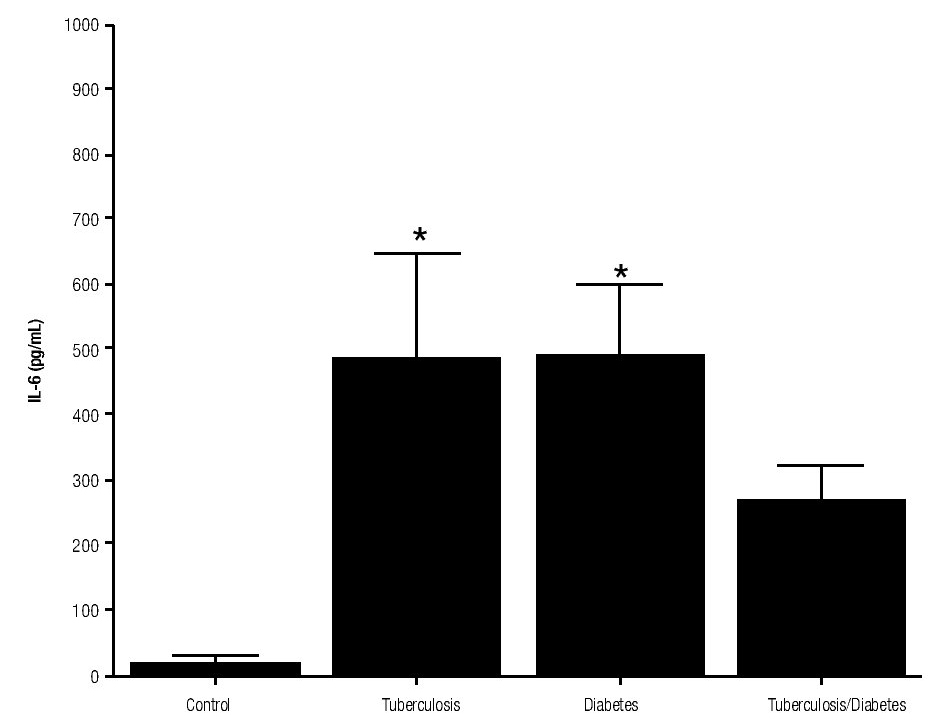
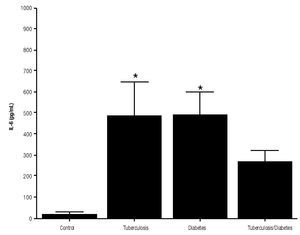
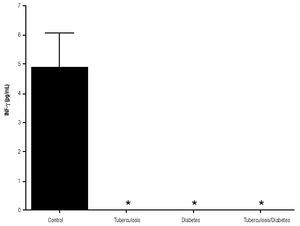
For IL-6 production analysis, the group with pulmonary tuberculosis and the group with type 2 DM showed a significant increase with respect to the control. IL-6 production was also high in the supernatants of adherent cells from patients with DM and pulmonary tuberculosis, but it was not significant (Figure 1). IFNg production presented the opposite behavior to that of IL-6. For IFNg, all groups exhibited a significant decrease with respect to the control (Figure 2). IL-12 was not detected in any study group (data not shown).
Figure 1. IL-6 Production. IL-6 levels are presented in base of its relation between stimulated and non stimulated macrophages and data shown are average±SEM. The p value of the IL-6 for the tuberculosis group was p<0.01, for the diabetes mellitus group was p<0.01.
Figure 2. INFg gamma Production. IFNg levels are presented in base of its relation between stimulated and non stimulated macrophages and data shown are average±SEM. The p value of the IFNg for the tuberculosis was p<0.05 and p<0.01 for the diabetes mellitus group.
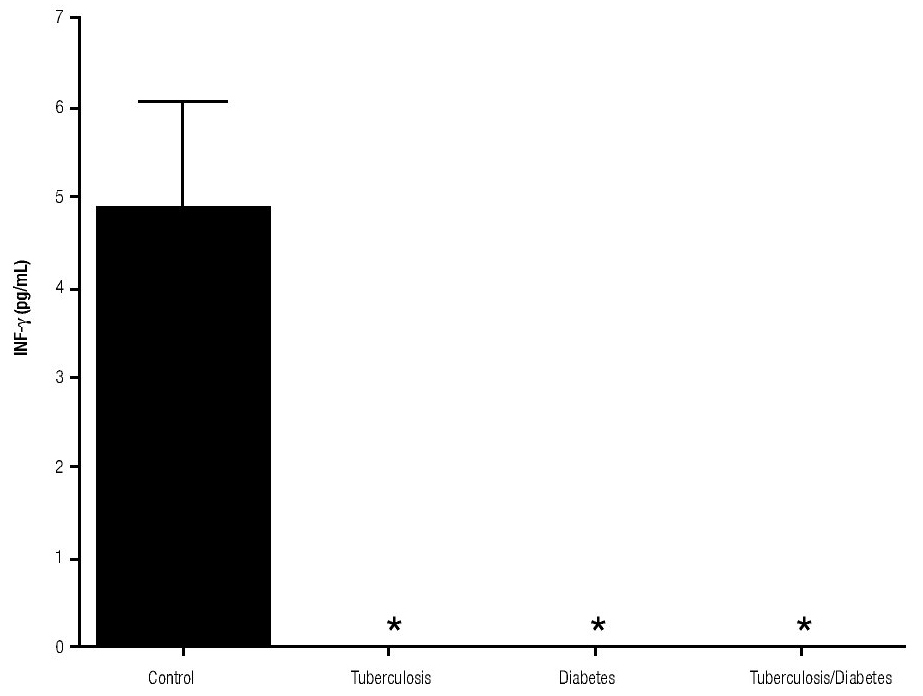
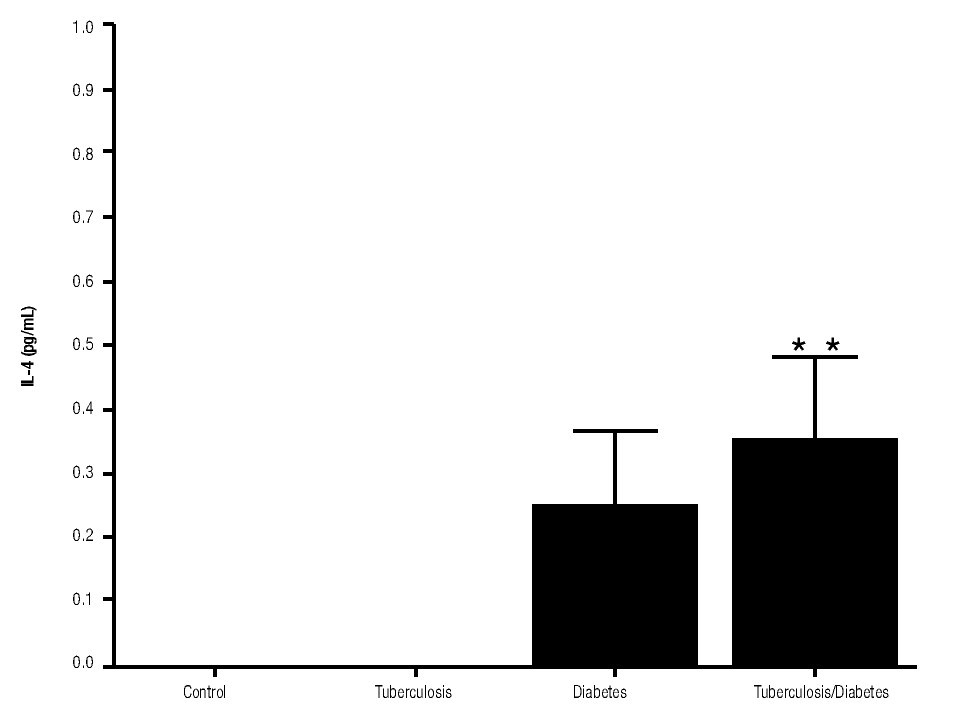
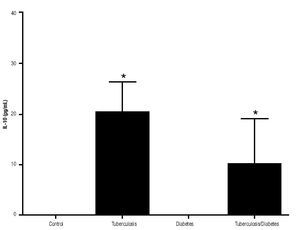
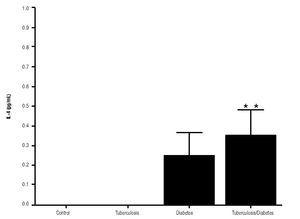
In addition, IL-10 production was significantly increased in the groups with pulmonary tuberculosis only and with type 2 DM, with respect to the control (Figure 3). The DM with pulmonary tuberculosis group was the only one that exhibited significant increase with respect to the control for the IL-4 production. Nevertheless, a significant increase of IL-4 production was also detected in the DM with pulmonary tuberculosis group with respect to the pulmonary tuberculosis group (Figure 4).
Figure 3. IL-10 Production. IL-10 levels are presented in base of its relation between stimulated and non stimulated macrophages and data shown are average±SEM. The p value of the IL-10 production for the tuberculosis group was p<0.001, for the diabetes mellitus group was p<0.05 and for the diabetes mellitus with tuberculosis was p<0.01.
Figure 4. IL-4 Production. IL-4 levels are presented in base of its relation between stimulated and non stimulated macrophages and data shown are average±SEM. The p value of the IL-4 production for the diabetes mellitus with tuberculosis group with respect to the control was p<0.05 and with respect to tuberculosis group was p<0.05.
Discussion
Diabetic patients have a higher risk of developing active tuberculosis, but the mechanism has not been elucidated yet. Therefore, we hypothesized that the Th1 cytokine response in patients with pulmonary tuberculosis and type 2 DM is inhibited by an anti-inflammatory environment induced through apolar lipids of M. tuberculosis. Studies based on animal models indicated that the production of Th1-related cytokines in response to M. tuberculosis declines in patients with DM.13,14
In order to ensure the statistical significance of the results, we based our sample size on previous human studies that have been done using similar sample sizes. Weng SF et al. presented a study, comparing diabetic and non diabetic patients with extrapulmonar tuberculosis, with comparable study groups and number of subjects.15 Furthermore, R J Al-Attiyah et al. showed almost identical study groups and size; and surprisingly also concluded that diabetic patients with tuberculosis had higher Th2 bias.16
The results of this study show that IL-10 has the capacity of downregulation IFNg production in patients with tuberculosis alone or with DM. Patients with pulmonary tuberculosis expressed higher amount of IL-10. Furthermore, our results are consistent with other findings reported by De la Barrera S et al. (2004), where IL-10 levels were increased and this increase was associated with the reduction in IFNg production in tuberculosis patients.17 Rocha-Ramirez et al. (2008) reported that M. tuberculosis apolar lipids can induce IL-10 production, especially the Beijing strain. However, we demonstrate in this study that H37Rv strain can also induce IL-10 production.18 Moreover, Higgins D et al. reported that the lack of IL-10 could be related with disease progression and a higher inflammatory environment in the lung.19 In these experiments, IFNg levels were lower in all the studied patients in comparison to healthy subjects. The high susceptibility of diabetic patients to M. tuberculosis could be in part explained by lower IFNg production. It is well known, that IFNg is considered as a key cytokine in the cell-mediated immunity response against intracellular bacteria by inducing nitric oxide production and increasing MHC-II molecules.11 In addition, IL-10 by itself can inhibit IFNg functions, like macrophages activation, antigen presentation, among others.
IL-6 production was significantly increased in pulmonary tuberculosis patients and in patients with diabetic mellitus. Besides, other studies have shown data suggesting that the lipids of M. tuberculosis are potent inducers of IL-6 gene regulation or transcription (use one of the two words) in peripheral blood monocytes.20,21 Previous studies with LPS confirmed an inhibition of TNFa production by IL-6 in cultured human peripheral blood monocytes. 9 Further investigations of these cells will help to clarify if IL-6 has a role in assisting or interfering in the host defense mechanisms.
IL-4 is a cytokine that plays a significant role in the differentiation of the precursor CD4+ T cell towards a Th2 phenotype. The production of IL-4 by the patients with diabetic mellitus and tuberculosis was higher in comparison with the other groups. In addition, this cytokine production was significantly superior in tuberculosis patients with respect to other patient groups. Besides the higher susceptibility of DM patients to develop tuberculosis, studies have demonstrated that the clinical conditions of diabetics with tuberculosis deteriorate faster than regular tuberculosis patients. These studies concluded that diabetic patients with tuberculosis shift to- wards Th2, which may explain the rise in the levels of IL-4 and thus, the deterioration in health of the patients.22 These increased levels of IL-4 production on diabetics patients with tuberculosis could explain the lack of significant production of IL-6 in these patients. Studies have showed that both IL-10 and IL-4 inhibit LPS-induced IL-6 mRNA and protein production by inhibiting the transcription rate of the IL-6 gene. However, the inhibitory effect of IL-4 was more pronounced than IL-10.23 Furthermore, IL-4 levels could be favoring a Th2 response even though, the most important and effective immune mechanism that is activated in the fight against M. tuberculosis is the one involving the Th1 cells. Therefore, here we demonstrate that apolar lipids of M. tuberculosis are inducing an anti-inflammatory environment by IL-10 production and favoring a Th2 response in adherent cells from type 2 DM and tuberculosis patients.
Acknowledgments
This project was supported by CONACYT Proyect No. 49545. We are grateful to Rosalía Guerrero García and Adrian Rendon for medical assistance with the patients.
Recibido: Abril 2011. Aceptado: Diciembre 2011
Corresponding author:
Alma Arce-Mendoza.
Universidad Autónoma de Nuevo León.
Gonzalitos 235 Norte, Mitras Centro.
Monterrey, N.L., Mexico. Z.P. 64460.
E-mail: aya_mayola@yahoo.com