To evaluate the knowledge about medications that could or could not be crushed or split among a group of patients in the Monterrey metropolitan area, and make a list of medications available in México that should not be crushed or split.
Material and methodsA descriptive, observational, transverse study was conducted using validated surveys among 950 patients undergoing medical treatment that went to clinics in the cities of San Nicolás de los Garza, Monterrey and Guadalupe of the Monterrey metropolitan area. The survey included a series of questions aimed at learning the patients’ level of knowledge regarding which drugs can be split or crushed. In order to collect the list of medications, several databases were consulted.
ResultsOf the study group, 80.3% had crushed or split a tablet prior to its administration, most of them to facilitate oral intake. Fifty four percent (54.4%) did not ask their physicians about this procedure. Seventy two (72.5%) percent considered that not all tablets should be crushed, but they did not know the exact reason why. An extensive list of medications available on the Mexican market that should not be crushed or split was presented.
ConclusionsThere is an urgent need to improve the information available regarding what dosage forms can be split or crushed in order to prevent medication errors.
According to the World Health Organization (WHO), health is defined not only as the absence of disease, but also as a state of physical, psychological and social well-being as an individual, as well as collectively. Thus, the concept of public health acknowledges the fact that health worker interventions include not only clinical services, which focus mostly on somatic and psychological aspects, but also social interventions such as production, rent distribution, consumption, housing, work, environment, etc.1
On the other hand, the irrational use of medications is also a public health concern and a major problem worldwide. This problem has been detected, and international organizations such as the WHO have developed different projects and guidelines to reduce it.2 Rational use of medications require that the patients receive the correct medications, each according to their clinical needs, as well as the correct dose and for the proper period of time, all this at the lowest possible cost for the patients and their communities.3 Public education on this subject is fundamental, since the vast majority of medications are prescribed by a physician, including dosage, frequency, length of treatment and the correct method of administration; and even if a pharmacist were able to solve all the patient's questions on common administration practices and the best hours to take the medications, it is the patient who decides whether or not to follow those instructions, basing his/her decision on a complex set of family beliefs, social, economic and health factors. The patient decides whether or not to buy the medication, take the right doses at the correct frequency, and take it as indicated or split it, especially when dealing with over-the-counter medications. Being well-informed is considered a right for the patient, but it is also an obligation for them to actively participate in the care of their well-being in conjunction with health care professionals.3,4
As a part of a pharmaceutical treatment, splitting (cutting in half) or crushing medications has been an accepted practice as a way of obtaining the prescribed dose when a specific dose is not available. For example, in pediatric or geriatric patients for whom, more often than not, the proper doses are not available on the market. Other examples include cases where there is a need to provide proper fractioned doses within a flexible regimen, when there is a need to reduce or increase the dosage in a dosing regimen, when there is a need to begin therapy with the lowest possible dose in order to avoid an incidence of adverse effects, when there is a need to adjust the therapeutic response of an individual patient, as an aid in the administration of large tablets which patients find hard to swallow whole, and as a way of saving using cheaper larger dose medications in the required proportions.5–14
However, there are pharmaceutical dosage forms which, in principle, should never be crushed. Amongst these are those that come with an enteric coating used for drugs which are inactivated by gastric acid, or that could irritate gastric mucous and used for delayed release. Other dosage forms which should never be crushed are those for sublingual administration, these are designed so that the drug dissolves quickly for a better absorption, thus reaching the bloodstream in a shorter period of time. Certain tablets with a polymeric or sugar coating which disguise unpleasant flavors and smells that avoid mucous irritation or protect active ingredients which are affected by light or humidity should also not be crushed or split, as well as effervescent or dispersible pharmaceutical dosage forms which are designed to dissolve or disperse in water before ingestion; if these are chewed, they could lose their ability to dissolve quickly, thus possibly causing a loss of dosage, in addition to presenting effervescence in the mouth if it is not dissolved in water first. Soft gelatin capsules (with liquid content) should also not be chewed or split, since the extraction of the liquid inside may lead to an incorrect dosage. In the case of medications with prolonged or extended released, if the developed system which contains the dose is destroyed, incidence of collateral side effects or the toxicity of the medication may increase when releasing a greater dose of the active ingredient.15–18 Other formulations which may present problems when crushed are medications with carcinogenic potential, not because their pharmacokinetic characteristics are modified, but because of the risk that tampering with it involves. Some active ingredients like warfarin or levothyroxine have small therapeutic windows; that is to say, if split in uneven parts and ingested, elevated doses of the medication are obtained for immediate absorption and can potentially cause toxicity, or result in a dosage which is under the therapeutic dose.5,19–23
The objective of this study was to evaluate the knowledge of a group of patients in Monterrey, Nuevo León, México and its metropolitan area has about oral medications that should not be split or crushed, and to gather commercial names of medications on the Mexican market which should not be altered.
Material and methodsAn observational, transversal, descriptive study was conducted through a previously validated survey. The surveys were applied to those patients who were on medical treatment at that moment and visited clinics and hospitals located in Monterrey, San Nicolas and Guadalupe, both cities located in the metropolitan area of Monterrey, Nuevo Leon, México. Pharmacists were in charge of conducting the survey, and the surveys were applied between July and December of 2015. All patients provided a signed consent prior to the application of the instrument. In the case of minors, their guardians provided said consent. The survey included a series of questions aimed at understanding the degree of knowledge patients had about which medications could be split and/or crushed.
The instrument was developed by experts in the area after a thorough bibliographic review. The items were then evaluated by an expert panel in the field of assistential pharmacy with a doctoral degree in Pharmacy, and finally, these evaluated questions were first applied to a small population group that was given a close follow-up to see if they fully understood each of the questions and once any situations found were corrected, a survey was applied to a pilot group close to the target population of our survey (30 patients), i.e. patients in the metropolitan area of Monterrey. Once this was done, the validated survey was applied to the population described. The responses to the survey were stored in a database and processed in Excel. Descriptive statistical analysis was performed. For the compilation of the list of medicines that should not be splitted or crushed, consultations were carried out from various sources of up-to-date information, such as scientific articles of indexed journals, as well as secondary bibliographic sources, specifically PubMed, EBSCO, and LexiComp, and tertiary sources such as books of pharmaceutical technology and dictionaries of pharmaceutical specialties, among others.
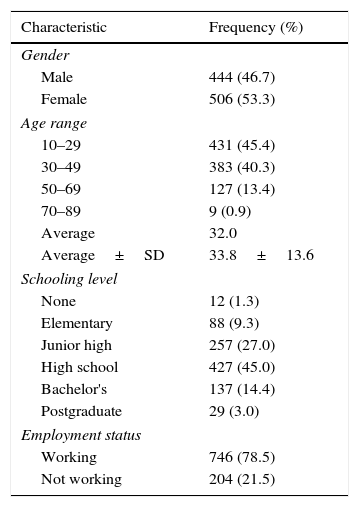
ResultsThe study involved 950 people from different communities in the metropolitan area of Monterrey, N.L., México; their demographic characteristics are shown in Table 1.
Demographics of the population.
| Characteristic | Frequency (%) |
|---|---|
| Gender | |
| Male | 444 (46.7) |
| Female | 506 (53.3) |
| Age range | |
| 10–29 | 431 (45.4) |
| 30–49 | 383 (40.3) |
| 50–69 | 127 (13.4) |
| 70–89 | 9 (0.9) |
| Average | 32.0 |
| Average±SD | 33.8±13.6 |
| Schooling level | |
| None | 12 (1.3) |
| Elementary | 88 (9.3) |
| Junior high | 257 (27.0) |
| High school | 427 (45.0) |
| Bachelor's | 137 (14.4) |
| Postgraduate | 29 (3.0) |
| Employment status | |
| Working | 746 (78.5) |
| Not working | 204 (21.5) |
N=950; SD, standard deviation.
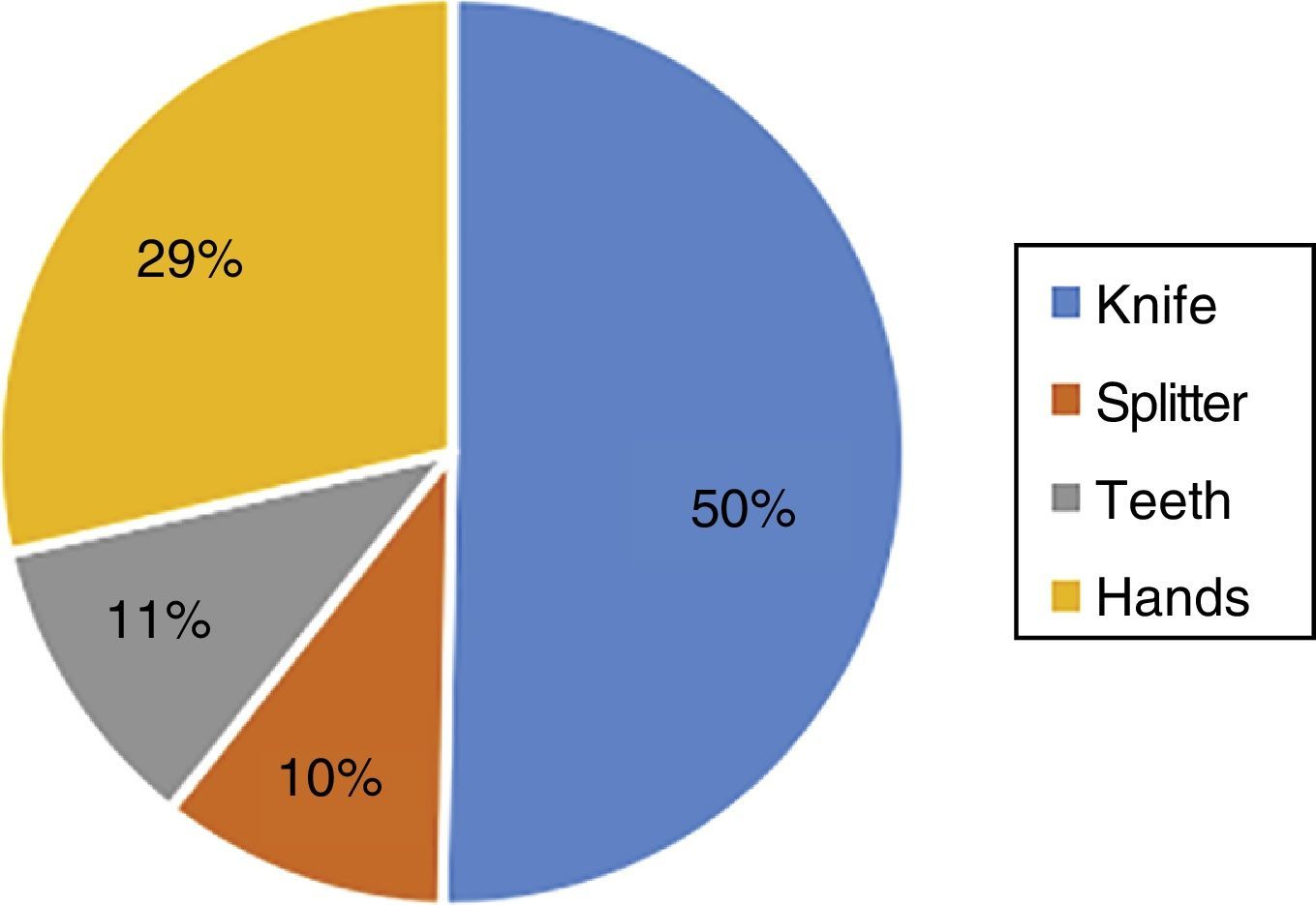
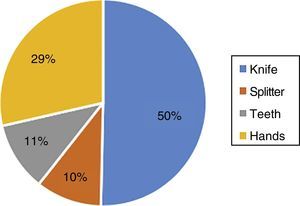
Results showed that 80.3% reported having split or crushed a tablet prior to administration, the main reason (49.8%) being to facilitate administration and swallowing, followed by an indication by the physician (20.0%), to adjust the dose (22.0%), to disguise a bad taste (7.2%), and 1.0% of the respondents mentioned another reason. The most frequently used instrument (50%) for splitting the tablets was a knife, followed by the hands (29%), the teeth (11%), and finally a splitter (10%) (Fig. 1).
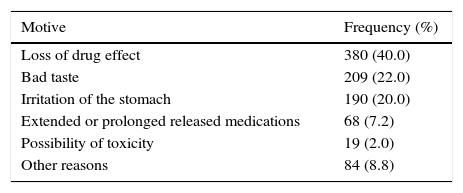
Most respondents (72.5%) considered that not all medicines can be splitted or crushed, and when questioned about why, the most common response was the fact that it could cause the drug to have no effect (40.0%); other reasons are shown in Table 2.
Further results show that 54.4% did not consult a physician before carrying out this practice. A large part of the respondents, 895 people (94.2%), were interested in knowing more about medications that could or could not be split in two or crushed, and would like to receive this information from a health professional.
Moreover, 49% of the respondents reported having had some discomfort when ingesting a split tablet, the most frequent being stomach irritation. Forty nine percent (49.6%) said that the main consequence of crushing tablets is the bitter taste that may occur and some less common ones would be: oral mucosal irritation, toxicity, dose inaccuracy and dental staining.
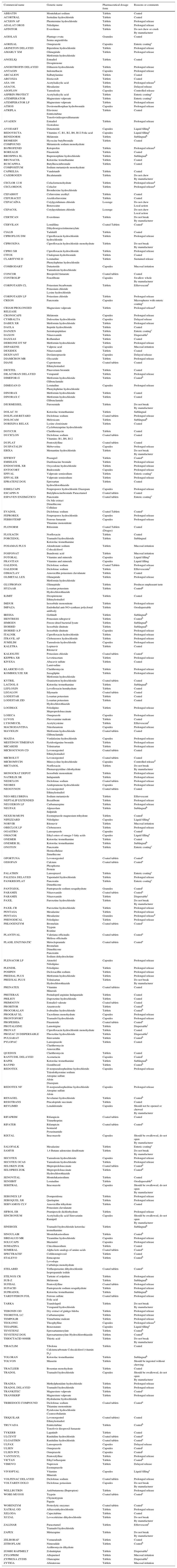
Table 3 lists orally administered medications that should not be crushed or splitted, thereby avoiding side effects, toxicity or inefficacy caused by this practice. Although the list is numerous and serves as a guide for health professionals in México, there are other medicines on the market that are not listed.19,24–26
Oral medications that should not be splitor crushed.
| Commercial name | Generic name | Pharmaceutical dosage form | Reasons or comments |
|---|---|---|---|
| ABBATIN | Montelukast sodium | Tablets | Coated |
| ACORTRAL | Sertraline hydrochloride | Tablets | Coated |
| ACXION AP | Phentermine hydrochloride | Tablets | Prolonged release |
| ADALAT OROS | Nifedipino | Tablets | Prolonged release |
| AFINITOR | Everolimus | Tablets | Do not chew or crush By manufacturer |
| AGIOLAX | Plantago ovata Senna angustifolia | Granules | Coated |
| AGRIXAL | Omeprazole | Capsules | Enteric coatingc |
| AKINETON DELAYED | Biperidene hydrochloride | Tablets | Prolonged release |
| AMARLY XM | Glimepiride Metformin hydrochloride | Tablets | Prolonged release |
| ANGELIQ | Estradiol Drospirenone | Tablets | Coated |
| ANGIOTROFIN DELAYED | Diltiazem hydrochloride | Tablets | Prolonged release |
| ANTAGIN | Indomethacin | Capsules | Prolonged release |
| ARCALION | Sulbutylamine | Tablets | Coated |
| ARCOXIA | Etoricoxib | Tablets | Coated |
| ASA 100 | Acetylsalicylic acid | Tablets | Prolonged released |
| ASACOL | Mesalazine | Tablets | Delayed release |
| ASOFLON | Tamsulosin | Capsules | Controlled release |
| ASPIRIN PROTECT | Acetylsalicylic acid | Tablets | Enteric coatingc |
| ATEMPERATOR | Magnesium valproate | Tablets | Enteric coatingc |
| ATEMPERATOR LP | Magnesium valproate | Tablets | Prolonged release |
| ATHOS | Dextromethorphan hydrobromide | Capsules | Prolonged release |
| ATRIPLA | Efavirenz Emtricitabine Tenofovirdisoproxilfumarate | Tablets | Coated |
| AVADEN | Estradiol Gestodeno | Tablets | Prolonged release |
| AVODART | Dutasteride | Capsules | Liquid fillingk |
| BEDOYECTA | Vitamins: C, B1, B2, B6, B12 Folic acid | Capsules | Liquid fillinge |
| BENEDORM | Melatonin | Tablets | Sublingualh |
| BIOMESIN COMPOUND | Hyoscine butylbromide Metamizole sodium monohydrate | Tablets | Coated |
| BI-PROFENID | Ketoprofen | Tablets | Prolonged releaseg |
| BOREALIS | Finasteride | Tablets | Coated |
| BROSPINA SL | Buprenorphine hydrochloride | Tablets | Sublingualh |
| BRUNACOL | Ketorolac tromethamine | Tablets | Coated |
| BUSCAPINA COMPOSITUM | Butylhioscinbromide Sodium metamizole monohydrate | Tablets | Coated |
| CAPRELSA | Vandetanib | Tablets | Coated |
| CASDROGEN | Bicalutamide | Tablets | Do not chew By manufacturer |
| CECLOR 12 H | Cefaclormonohydrate | Tablets | Prolonged releaseg |
| CECLORDOX | Cefaclor Bromhexine hydrochloride | Tablets | Prolonged releaseg |
| CEFABIOT | Cefuroxime axethyl | Tablets | Coated |
| CEFURACET | Axetilcefuroxime | Tablets | Coated |
| CEPACAÍNA | Cetylpyridinium chloride Benzocaine | Lozenge | Do not chew Local action |
| CEPACOL | Cetylpyridinium chloride | Lozenge | Do not chew Local action |
| CERTICAN | Everolimus | Tablets | Do not break By manufacturer |
| CERVILAN | Lomifilina Dihydroergocristinemesylate | Coated Tablets | Coatedc |
| CIALIS | Tadalafil | Tablets | Coated |
| CIPROFLOX DM | Ciprofloxacin hydrochloride hydrochloride | Tablets | Prolonged release |
| CIPROXINA | Ciprofloxacin hydrochloride monohydrate | Tablets | Do not break By manufacturer |
| CIPRO XR | Ciprofloxacin hydrochloride | Tablets | Prolonged release |
| CITOX | Citalopram hydrobromide | Tablets | Coated |
| CLARITYNE D | Loratadine Phenylephrine hydrochloride | Tablets | Sustained release |
| COMBODART | Dutasteride Tamsulosin hydrochloride | Capsules | Mucosal irritation |
| CONCOR | Bisoprolol fumarate | Coated tablets | Coated |
| CONTROLIP | Fenofibrate | Capsules | Swallow whole By manufacturer |
| CORPOTASIN CL | Potassium bicarbonate Potassium chloride Lysine hydrochloride | Tablets | Effervescentf |
| CORPOTASIN LP | Potassium chloride | Tablets | Prolonged release |
| CREON | Pancreatin | Capsules | Microspheres with enteric coatingc |
| CRIAM PROLONGED RELEASE | Magnesium valproate | Tablets | Prolonged releaseg |
| CRONOCAPS | Melatonin | Capsules | Prolonged release |
| CYMBALTA | Duloxetine hydrochloride | Capsules | Delayed release |
| DABEX XR | Metformin hydrochloride | Tablets | Prolonged release |
| DAGLA | Itopride hydrochloride | Tablets | Coated |
| DANZEN | Serratiopeptidase | Tablets | Enteric coatingc |
| DAXON | Nitazoxanide | Tablets | Dispersablei |
| DAXXAS | Roflumilast | Tablets | Coated |
| DEBEONE DT NF | Metformin hydrochloride | Tablets | Prolonged release |
| DEPAKENE | Valproic acid | Capsules | Mucosal irritation |
| DEXIDEX | Nitazoxanide | Tablets | Coated |
| DEXIVANT | Dexlansoprazole | Capsules | Delayed release |
| DIAMICRON MR | Glycazide | Tablets | Prolonged release |
| DIANE | Cyproterone Ethinylestradiol | Coated tablets | Coated |
| DICETEL | Pinaverium bromide | Tablets | Coated |
| DILACORAN DELAYED | Verapamil | Tablets | Prolonged release |
| DIMEFOR-G | Metformin hydrochloride Glibenclamide | Tablets | Coatedb |
| DIMEGAN-D | Loratadine Phenylephrine hydrochloride | Capsules | Prolonged release |
| DINORAX | Metformin hydrochloride | Tablets | Coated |
| DINORAX C | Metformin hydrochloride Glibenclamide | Tablets | Coated |
| DIURMESSEL | Furosemide | Tablets | Do not break By manufacturer |
| DOLAC 30 | Ketorolac tromethamine | Tablets | Sublingual |
| DOLFLAM-RETARD | Diclofenac sodium | Coated tablets | Prolonged release |
| DOLOCAM | Meloxicam | Tablets | Sublingualh |
| DORIXINA RELAX | Lysine clonixinate Cyclobenzaprine hydrochloride | Tablets | Coated |
| DOYCUR | Clarithromycin | Tablets | Coated |
| DUCICLON | Diclofenac sodium Vitamins: B1, B6, B12 | Coated tablets | Coated |
| DUPLAT | Pentoxifylline | Coated tablets | Coated |
| DUSPATALIN | Mebeverina | Capsules | Prolonged release |
| EBIXA | Memantine hydrochloride | Tablets | Do not break By manufacturer |
| EFFIENT | Prasugrel | Tablets | Coateda |
| EMSELEX | Darifenacine bromide | Tablets | Prolonged release |
| ENDOCODIL XR | Oxycodone hydrochloride | Tablets | Prolonged release |
| ENTOCORT | Budesonide | Capsules | Prolonged release |
| EPIVAL | Valproate semisodium | Tablets | Enteric coatingc |
| EPIVAL ER | Valproate semisodium | Tablets | Prolonged release |
| EPRATENZ DOX | Eprosartan hydrochlorothiazide | Tablets | Coated |
| ESBELCAPS | Fenproporex hydrochloride Diazepam | Capsules | Prolonged release |
| ESCAPIN-N | Butylphoscinebromide Paracetamol | Coated tablets | Coated |
| ESPAVEN ENZIMÁTICO | Pancreatin Ox bile extract Dimethicone Cellulase | Coated tablets | Enteric coatingc |
| EVADOL | Diclofenac sodium | Coated Tablets | Coatedc |
| FEPROREX | Fenproporex hydrochloride | Capsules | Prolonged release |
| FERROTEMP | Ferrous fumarate Thiamine mononitrate | Capsules | Prolonged release |
| FLONORM | Rifaximin | Coated Tablets (Dragee) | Coated |
| FLOXACIN | Norfloxacin | Tablets | Coated |
| FORCEDOL | Tramadol hydrochloride Ketorolac tromethamine | Tablets | Sublingual |
| FOSAMAX PLUS | Alendronate sodium Colecalciferol | Tablets | Mucosal irritation |
| FOSFONAT | Ibandronic acid | Tablets | Mucosal irritation |
| FOTORAL | Vitamins and minerals | Capsules | Liquid fillinge |
| FRAVITAN | Vitamins and minerals | Capsules | Liquid filling |
| GALEDOL | Diclofenac sodium | Coated Tablets | Prolonged release |
| GALEDOR | Diclofenac sodium | Tablets | Effervescentef |
| GIMACLAV | Amoxicillin potassium clavulanate | Tablets | Coated |
| GLIMETAL LEX | Glimepiride Metformin hydrochloride | Tablets | Prolonged release |
| GLUPROPAN | Glimepiride | Tablets | Produces unpleasant taste |
| HYZAAR | Losartan potassium Hydrochlorothiazide | Tablets | Coatedg |
| ILIMIT | Drospirenone Ethinylestradiol | Tablets | Coated |
| IMDUR | Isosorbide mononitrate | Tablets | Prolonged release |
| IMPAZA | Endothelial anti-NO-synthase polyclonal antibody | Tablets | Orodispersable |
| IRESSA | Gefitinib | Tablets | Sublingualh |
| ISENTRESS | Potassium raltegravir | Tablets | Coatedb |
| ISMIGEN | Freeze-dried bacterial lysate | Tablets | Sublingualh |
| ISORBID | Isosorbide dinitrate | Tablets | Sublingualh |
| ISORBID A.P | Isosorbide dinitrate | Capsules | Prolonged release |
| ITALNIK | Ciprofloxacin hydrochloride | Tablets | Prolonged release |
| ITRAVIL AP | Clobenzorex hydrochloride | Tablets | Prolonged release |
| JUMSLIM | Tamsulosin hydrochloride | Capsules | Prolonged release |
| KALETRA | Lopinavir Ritonavir | Tablets | Coated |
| KALIOLITE | Potassium chloride | Coated tablets | Coatedc |
| KEPPRA XR | Levetiracetam | Tablets | Prolonged release |
| KIVEXA | Abacavir sulfate Lamivudine | Tablets | Coated |
| KLARICID O.D. | Clarithromycin | Tablets | Prolonged release |
| KOMBIGLYZE XR | Saxagliptin Metformin hydrochloride | Tablets | Prolonged release |
| KYTRIL | Granisetron hydrochloride | Coated tablets | Coated |
| LACDOL-S | Ketorolac tromethamine | Tablets | Sublingualh |
| LEFLOXIN | Levofloxacin hemihydrate | Tablets | Coated |
| LEGALON | Silymarin | Coated tablets | Coated |
| LODESTAR | Losartan potassium | Tablets | Coated |
| LODESTAR ZID | Losartan potassium Hydrochlorothiazide | Tablets | Coated |
| LOGIMAX | Felodipino Meproprololsuccinate | Tablets | Prolonged release |
| LOSECA | Omeprazole | Capsules | Prolonged release |
| LUVOX | Fluvoxamine maleate | Tablets | Coated |
| LYSOMUCIL | Acetylcysteine | Tablets | Effervescentf |
| MACRODANTINA | Nitrofurantoin | Capsules | Prolonged release |
| MAVIGLIN | Metformin hydrochloride Glibenclamide | Coated tablets | Coated |
| MAZDA | Venlafaxine hydrochloride | Capsules | Prolonged release |
| MESTINON TIMESPAN | Pyridostigmine bromide | Tablets | Prolonged release |
| MICARDIS | Telmisartan | Tablets | Prolonged release |
| MICROGYNON CD | Levonorgestrel Ethinylestradiol | Coated tablets | Coated |
| MICROLUT | Levonorgestrel | Coated tablets | Coated |
| MICROMYCIN | Minocycline hydrochloride | Capsules | Controlled released |
| MICTASOL | Norfloxacin Phenazopyridine chlorhydrate | Tablets | Do not break By manufacturer |
| MONOCRAT DEPOT | Isosorbide mononitrate | Tablets | Prolonged release |
| NATRILIX SR | Indapamide | Tablets | Prolonged release |
| NEDICLON | Diclofenac sodium | Coated tablets | Prolonged release |
| NEOBES | Amfepramone hydrochloride | Capsules | Prolonged release |
| NEOGYNON | Levonorgestrel Ethinylestradiol | Coated tablets | Coated |
| NEO-MELUBRINA | Sodium metamizole | Tablets | Effervescent |
| NEPTALIP EXTENDED | Bezafibrate | Tablets | Prolonged release |
| NEUGERON LP | Carbamazepine | Tablets | Prolonged release |
| NEUPAX | Alprazolam Sulpiride | Tablets | Sublingualh |
| NEXIUM-MUPS | Esomeprazole magnesium trihydrate | Tablets | Coatedj |
| NIFEZZARD | Nifedipino | Capsules | Liquid fillinge |
| NORVIR | Ritonavir | Tablets | Mucosal irritation |
| OBECLOX LP | Clobenzorex | Tablets | Prolonged release |
| OGASTRO | Lansoprazole | Capsules | Coatedj |
| OMACOR | Ethyl esters of omega-3 fatty acids | Capsules | Liquid fillinge |
| ONEMER | Ketorolac tromethamine | Tablets | Coated |
| ONEMER SL | Ketorolac tromethamine | Tablets | Sublingualh |
| ONOTON | Pancreatin Hemicellulase Simethicone | Tablets | Enteric coatingc |
| OPORTUNA | Levonorgestrel | Coated tablets | Coateda |
| OSSOPAN | Calcium Phosphorus Protein | Coated tablets | Coateda |
| PALATRIN | Lansoprazol | Tablets | Enteric coatingc |
| PALEXIA DELAYED | Tapentadol hydrochloride | Tablets | Prolonged release |
| PANKREOFLAT | Pancreatin Dimethicone | Tablets | Enteric coatingc |
| PANTOZOL | Pantoprazole sodium sesquihydrate | Granules | Coatedj |
| PARAMIX | Nitazoxanide | Coated tablets | Coateda |
| PARAMIX | Nitazoxanide | Tablets | Dispersablei |
| PAXIL | Paroxetine hydrochloride | Tablets | Do not break By manufacturer |
| PAXIL CR | Paroxetine hydrochloride | Tablets | Prolonged release |
| PENTASA | Mesalazine | Tablets | Prolonged release |
| PENTASA | Mesalazine | Granules | Prolonged released |
| PHENODICAL | Felodipino | Tablets | Prolonged release |
| PHLOGENZYM | Bromelain Trypsin Routine | Coated tablets | Coatedc |
| PLANTIVAL | Valeriana officinalis Melissa officinalis | Coated tablets | Coateda |
| PLASIL ENZYMATIC | Metoclopramide Bromelain Dimethicone Pancreatin Sodium dehydrocholate | Coated tablets | Coateda |
| PLENACOR LP | Atenolol Nifedipino | Capsules | Prolonged release |
| PLENDIL | Felodipino | Tablets | Prolonged release |
| POSIPEN | Dicloxacillin sodium | Tablets | Prolonged release |
| PREDIAL PLUS | Metformin hydrochloride | Tablets | Prolonged release |
| PREDXAL PLUS | Telmisartan Hydrochlorothiazide | Tablets | Do not break By manufacturer |
| PRENATEX | Vitamins Minerals | Coated tabletss | Coated |
| PRETERAX | Perindopril arginine Indapamide | Tablets | Coated |
| PRILIGY | Dapoxetine hydrochloride | Tablets | Coated |
| PRIMOGYN | Estradiol valerate | Coated tablets | Coated |
| PROBITOR | Anastrozole | Tablets | Coatedb |
| PROCORALAN | Ivabradine hydrochloride | Tablets | Coatedb |
| PROGRAF XL | Tacrolimus monohydrate | Capsules | Prolonged release |
| PRONTOFORT | Tramadol hydrochloride | Capsules | Prolonged release |
| PROPESHIA | Finasteride | Coated tablets | Coateda |
| PROTALGINE | Lamotrigine | Tablets | Dispersablei |
| PROVAY | Ciprofloxacin hydrochloride monohydrate | Tablets | Coated |
| PROZAC 20 DISPERSABLE | Fluoxetine hydrochloride | Tablets | Dispersablei |
| PULSARAT | Simvastatin | Tablets | Coatedb |
| PYLOPAC | Lansoprazole Clarithromycin Amoxicillin | Tablets | Coated |
| QUEDOX | Clarithromycin | Tablets | Coated |
| RANTUDIL DELAYED | Acemetacin | Capsules | Coatedj |
| RAPIX | Ketorolac tromethamine | Tablets | Sublingualh |
| RAYPID | Gemfibrozil | Tablets | Coatedb |
| REDOTEX | D-norpseudoephedrine hydrochloride Triiodothyronine sodium Atropine sulfate Aloin Diazepam | Capsules | Prolonged release |
| REDOTEX NF | D-norpseudoephedrine hydrochloride Atropine sulfate Aloin | Capsules | Prolonged release |
| RENAGEL | Sevelamer hydrochloride | Tablets | Coatedb |
| RESOTRANS | Prucalopride succinate | Tablets | Coated |
| REVLIMID | Lenalidomide | Capsules | Should not be opened or chewed By manufacturer |
| RIFAPRIM | Rifampicin Trimethoprim | Coated tablets | Coated |
| RIFATER | Rifampicin Isoniazid Pyrazinamide | Coated tablets | Coateda |
| RIXTAL | Itraconazole | Capsules | Should be swallowed, do not open By manufacturer |
| SALOFALK | Mesalazine | Tablets | Enteric coatingc |
| SAMYR | 1,4 Butane adenosine disulfonate | Tablets | Do not break By manufacturer |
| SECOTEX | Tamsulosin hydrochloride | Capsules | Prolonged release |
| SECOTEX OCAS | Tamsulosin hydrochloride | Tablets | Prolonged release |
| SELOKEN ZOK | Meproprololsuccinate | Coated tablets | Coatedd |
| SELOPRES ZOK | Meproprololsuccinate Hydrochlorothiazide | Tablets | Coatedd |
| SENOVITAL | Montelukastsodium | Tablets | Coated |
| SENSIBIT | Loratadine | Tablets | Orodispersablen |
| SERITRAL | Itraconazole | Capsules | Should be swallowed, do not open By manufacturer |
| SERONEX LP | Domperidona | Tablets | Prolonged release |
| SEROQUEL XR | Quetiapine | Tablets | Prolonged release |
| SERVAMOX CLV | Amoxicillin trihydrate Potassium clavulanate | Tablets | Coated |
| SIFROL ER | Pramipexole dichlorhydrate | Tablets | Prolonged release |
| SINCRONIUM | Acetylsalicylic acid Simvastatin Ramipril | Capsules | Should be swallowed, do not open By manufacturer |
| SINERGIX | Tramadol hydrochloride ketorolac tromethamine | Tablets | Sublingualh |
| SINGULAIR | Montelukastsodium | Tablets | Coatedb |
| SIRDALUD MR | Tizanidine hydrochloride | Capsules | Prolonged release |
| SOLUCAPS | Mazindol | Capsules | Prolonged release |
| SOMAZINA | Citicolinasodium | Tablets | Coated |
| SOMERAL | Alpha keto analogs of amino acids | Coated tablets | Coateda |
| SPECTRACEF | Cefditorenpivoxil | Tablets | Coated |
| STALEVO | Entacapone Levodopa Carbidopa monohydrate | Tablets | Coatedb |
| STELABID | Trifluoperazine dihydrochloride Isopropamide iodide | Coated tablets | Coateda |
| STILNOX CR | Tartrate of zolpidem | Tablets | Prolonged release |
| SUB-Z | Melatonin | Tablets | Sublingualh |
| SUFISAL | Pentoxifylline | Coated tablets | Prolonged release |
| SUPACID | Pantoprazole sodium sesquihydrate | Tablets | Prolonged release |
| SUPRADOL | Ketorolac tromethamine | Tablets | Sublingualh |
| TARDYFERON FOL | Ferrous sulfate Folic acid | Coated tablets | Prolonged release |
| TARKA | Trandolapril Verapamil hydrochloride | Tablets | Do not break By manufacturer |
| TEBONIN OD | Dry extract of ginkgo biloba | Tablets | Prolonged release |
| TEGRETOL LC | Carbamazepine | Tablets | Prolonged release |
| TEMPOLIB | Trimebutine maleate | Tablets | Prolonged release |
| TEOLONG | Theophylline | Capsules | Prolonged released |
| TESAPERL | Benzonatato | Capsules | Liquid fillinge |
| TEVETENZ | Eprosartanmesylate | Tablets | Coatedb |
| TEVETENZ DOX | Eprosartanmesylate Hydrochlorothiazide | Tablets | Coatedb |
| THIOCTACID 600HR | Thiotic acid | Tablets | Do not break By manufacturer |
| TIBACLIM | Tibolona Calciumcarbonate Colecalciferol (vitamin D3) | Tablets | Coated |
| TOLORAN | Ketorolac tromethamine | Tablets | Sublingualh |
| TOLVON | Miaserin | Tablets | Should be ingested without chewing |
| TRACLEER | Bosentan monohydrate | Tablets | Coated |
| TRADOL | Tramadol hydrochloride | Capsules | Should be swallowed, do not open By manufacturer |
| TRADEA | Methylphenidate hydrochloride | Tablets | Prolonged release |
| TRADOL DELAYED | Tramadol hydrochloride | Tablets | Prolonged release |
| TRANKITEC | Magnesium valproate | Tablets | Coatedc |
| TRANSKRIP | Magnesium valproate Hydralazine hydrochloride | Tablets | Prolonged release |
| TRIBEDOCE COMPOUND | Diclofenac sodium Thiamine mononitrate Pyridoxine hydrochloride Cyanocobalamin | Coated tablets | Coateda |
| TRIQUILAR | Levonorgestrel Ethinylestradiol | Coated tablets) | Coated |
| TRUVADA | Emtricitabine Tenofovir disoproxil fumarate | Tablets | Coatedb |
| TYKERB | Lapatinib | Tablets | Coated |
| ULCEVIT | Ranitidine hydrochloride | Coated tablets | Coateda |
| ULGASTRIN | Ranitidine hydrochloride | Coated tablets | Coateda |
| ULPAX | Lansoprazole | Capsules | Delayed release |
| ULSEN | Omeprazole | Capsules | Coatedj |
| ULSEN PCS | Omeprazole | Capsules | Coatedj |
| VANTOXYL | Pentoxifylline | Tablets | Prolonged release |
| VICTAN | Ethyl loflazepate | Tablets | Coatedb |
| VIMOVO | Naproxen Esomeprazole | Tablets | Delayed release |
| VIVIOPTAL | Vitamins Minerals | Capsules | Liquid fillinge |
| VOLFENAC DELAYED | Diclofenac sodium | Coated tablets | Prolonged release |
| VOLTAREN DOLO | Diclofenac potassium | Capsules | Do not break By manufacturer |
| WELLBUTRIN | Anfebutamona (Bupropion) | Tablets | Prolonged release |
| WOBE-MUGOS | Trypsin Chymotrypsin Papain | Coated tablets | Coatedc |
| WOBENZYM | Proteolytic enzymes | Coated tablets | Coatedc |
| XATRAL OD | Alfuzosinhydrochloride | Tablets | Prolonged release |
| XELODA | Capecitabine | Tablets | Coated |
| XUZAL | Levocetirizine dihydrochloride | Tablets | Do not break By manufacturer |
| ZALDIAR | Paracetamol Tramadol hydrochloride | Tablets | Effervescentf |
| ZAPEX | Mirtazapine | Tablets | Do not break By manufacturer |
| ZELBORAF | Vemurafenib | Tablets | Coated |
| ZITROFLAM | Nimesulide Azithromycin dihydrate | Tablets | Coatedb |
| ZOMIG RAPIMELT | Zolmitriptan | Tablets | Dispersablei |
| ZYLOPRIM | Allopurinol | Tablets | Mucosal irritation |
| ZYPREXA ZYDIS | Olanzapine | Tablets | Dispersablei |
| ZYTIGA | Abiraterone | Tablets | Mucosal irritation |
Covered with sugar: masks smells and/or unpleasant flavors, protects photosensitive or easily oxidizable drugs.
Coated with polymer film: masks odors and/or unpleasant flavors, protects photosensitive or easily oxidizable drugs, allows a prolonged release, depending on the coating polymer.
Entericcoating: used for drugs that are destroyed by gastric acidity, for drugs that irritate the stomach mucose and for delayed release. They release their active substance into the small intestine.
Capsules of soft gelatin (with liquid content): The extraction of the liquid from the interior can lead to an incorrect dosage.
Effervescent: designed to dissolve in water before ingesting, if chewed they lose their ability to dissolve quickly, and may present effervescence in the mouth if not dissolved in water beforehand.
Prolonged or extended release: If the developed system containing the dose is destroyed, the incidence of side effects or the toxicity of the drug is increased by releasing a higher dose.
Sublingual: intended to the drug dissolves quickly, obtains a better absorption and reaches the bloodstream in a short time.
Our study reveals that a large proportion of patients (80.3%) who have used tablets as a pharmaceutical form of drug administration have splitted them or crushed them, unlike the study by Quinzler et al., in which 49% of its study population split at least one drug.8 The latter results resemble a study carried out in the Netherlands at five community pharmacies, where 31% of tablet prescriptions were found to have been modified prior to administration; that is, they were splitor crushed because the prescribed dose needed to be reduced; 30% were split on the initiative of the patient, 13% because of the ease of administration and 17% because the patient chose to take a lower dose.20 In the present study, about 50% of the patients stated that the main reason was for ease of administration, while 22% stated that their reason was dose adjustment. Despite the differences in the results of the cited research and the present study, according to Rodenhuis et al., there is an important need for patients to cover these two aspects.20
Although the practice of splitting tablets is common both in outpatient settings and in hospitals and nursing homes, little is known about these patterns.27 In this study, most of the people interviewed used a knife to split the tablets, followed by using the hands, then the teeth and finally a tablet cutter. Studies indicate that the use of these cutters to facilitate the division of tablets is rare, since their use is not common because they are not available in all pharmacies.8 The study by Quinzler et al. reports that 16.3% of patients had problems with tablet division, of which 70.1% solved it using a knife or other sharp object, and only 19.4% used a cutter to split the medication.8
The practice of crushing or splitting tablets by patients can lead to health problems, since not all tablets are suitable for this purpose. Splitting prolonged or extended-release tablets may result in increased side effects and a compromise in effectiveness, given the uncontrolled release of the active ingredient, or the latter may be impaired if it is contained in enteric coated tablets or has the potential to irritate the stomach.6–8,28–32
On the other hand, it is generally considered that if the manufacturer marks a dividing line on the tablet, that means that it is fit to split. However, this is not always the case, since there is a degree of inaccuracy in breaking them due to their shape, size or type of coating which results in pieces of different sizes that can lead to fluctuations in the administered dose, especially if this occurs with narrow therapeutic window drugs, such as digoxin and warfarin, unlike drugs with broad therapeutic ranges and long half-lives, in which dose fluctuations are unlikely to be clinically significant.6,7,33 In the case of psychotropic drugs, the convenience of splitting tablets may result from clinical observation, since for patients like the elderly the prescription of complete tablets can result in over sedation in some cases. Thus, it is recommended that, if they are going to be divided by the patient, also consider the possible physical limitations, and that pharmacists give appropriate counseling regarding the necessary tools to do it the right way.34,35
Other studies reveal positive aspects of the practice of breaking tablets, such as the economic benefit that both the patient and health institutions generate by reducing their costs by up to 50%, due to the fact that the costs of the drugs frequently decrease when the dose is increased, independently of this.11,13,36
The decision to split tablets should be made after reviewing the relevant considerations. The following recommendations can be used as a guide.6,26
- •
Use liquid dosage forms of the same medicines. If they are not available, consult your pharmacist to determine if extemporaneous (compounding) preparations can be made. Occasionally, the injectable form of the medication can be used by placing the appropriate amount of medication in a liquid as a juice. This should be done after consulting the pharmacist to ensure there are no compatibility problems or changes in absorption of the active ingredient.
- •
Check the product information before recommending that it be broken or crushed.
- •
Use a tablet cutter to improve accuracy. However, patients should be instructed in their proper use.
- •
Advise patients about the proper storage of tablet fragments.
In conclusion, we found that there is an urgent need to improve information about which pharmaceutical forms can be splitted, divided or crushed. The work of pharmacists and health professionals is critical in educating patients about how prescribed drugs should be administered, as well as providing relevant information and indications, with emphasis on aspects related to their use, administration, and conservation, with the goal of avoiding medication errors. Pharmaceutical companies should more clearly label presentations of drugs so that patients can recognize those that should not be splitted or crushed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo financial support was provided.
Conflict of interestThe authors declare that they have no conflict of interest.
To the hospitals for the facilities provided for the application of the surveys.