To assess the risks and benefits of levosimendan in acute decompensated heart failure compared to dobutamine or placebo.
MethodsPubmed, the Cochrane Central Register of Controlled Trials (CENTRAL), the European Heart Journal, and the Journal of the American College of Cardiology and Circulation were searched for randomized clinical trials of a 24h IV infusion of levosimendan compared to dobutamine or a placebo in patients ≥18 years old admitted with a diagnosis of acute decompensated heart failure of any etiology with a NYHA class III and IV heart failure, a left ventricular ejection fraction <0.35, pulmonary catheter wedge pressure >15mmHg or cardiac index <2.5ml/min/m2.
ResultsEleven clinical trials (2747 patients) met the inclusion criteria for this review. Levosimendan was associated with a significant increase in NYHA class OR 3.06 (95% CI 1.23–7.59; p=0.02), and a tendency to improve fatigue OR 1.80 1.53 (IC95% 0.99–2.39, p=0.06) and clinical improvement composite OR 1.20 (IC95% 0.99–1.46; p=0.06), as compared to dobutamine or a placebo.
ConclusionsLevosimendan in acute decompensated heart failure improves NYHA functional class, LVEF and BNP levels when compared to dobutamine or a placebo, with an increase in side effects.
Heart failure (HF) is a clinical syndrome derived from any structural alteration or cardiac function which leads to the inability of the heart to deliver oxygen at the rate metabolically active tissue require unless it does so at the expense of an increase in ventricular inflow pressure.1,2 Thus, HF has been divided into heart failure with preserved ejection fraction or diastolic heart failure (HFpEF), and heart failure with reduced ejection fraction or systolic heart failure (HFrEF), although it would also always be possible to differentiate it based on the time of onset or evolution (acute or chronic), severity, clinical stage or symptom direction (anterograde or retrograde) whichever predominates in the syndrome. HFpEF prevalence varies from 40 to 71%; nonetheless, this depends on the great variability of the ejection fraction cut-off criteria to classify it.1,2 Close to 1–2% of adult population have HF, with a prevalence which may be greater than 10% in people over 70 years of age.3
Heart failure decreases quality of life by increasing morbidity and mortality due to frequent hospitalizations caused by acute decompensation or intensification. It can also be the main cause of hospitalization in individuals over 65 years of age. This not only represents an increase in the cost of heart failure treatment, but it is also a prognosis marker in the course of the disease, since mortality linked to acute decompensation may be up to 10% at 60 days, with a rate of new hospitalizations in the following 6 months of 50% and a mortality of 30% at 1 year.2,4,5
Acute decompensated heart failure is defined as the sudden or gradual onset of the signs and symptoms of heart failure, which requires an unplanned visit to the doctor's office or the emergency unit, or in some cases, hospitalization. Regardless of the precipitating causes of the exacerbation, the universal finding in decompensated HF is the rise in intracavitary filling pressure, with the resulting systemic and pulmonary congestion.6
There is no widely accepted nomenclature for HR syndromes which require hospitalization. “Acute HF” or “acute HF syndrome” or “acutely decompensated HF” have been used indistinctly. Even though the latter term has a greater acceptance, its main limitation is that it does not distinguish between a first timer or a previously stable HF with acute worsening. Patients who are hospitalized due to HF may be classified depending on their etiology in those with acute myocardial ischemia, accelerated arterial hypertension, acutely decompensated HF, cardiogenic shock and decompensated right heart failure.2
Most patients with decompensated HF admitted to the hospital suffer a worsening of their chronic HF. Between 15 and 20% represent new HF diagnoses. Close to 50% have an ejection fraction ≤40%. This group has a high prevalence of the atheromatous coronary disease (60%), fibrillation or atrial flutter (30–46%), valvular heart disease (44%) and dilated cardiomyopathy (25%), all according to the chronicity of the subjacent disease.7
Treatment goals for decompensated HF are an improvement of symptoms and hemodynamic deterioration and the reduction of morbidity and mortality. Modern treatments have improved prognosis, reducing the risk of a new hospitalization by up to 30–50%, with significant changes in mortality.8 In this sense, calcium sensitizers, unlike adrenergic agonists, increase myocardial contractility with a minimal energy consumption and a lower risk of arrhythmias, with an improvement in mortality and hemodynamic parameters.9,10 Levosimendan, an agent in the group of calcium sensitizers, has inotropic and vasodilator (inodilator) effects which improve myocardial work without a change in oxygen consumption. This is produced by the opening of ATP-dependent K+ channels in the myocyte and smooth vascular muscle cells, thus causing vasodilation with pre-charge and post-charge reduction and an increase in coronary flow.11 Moreover, it has a positive chronotropic effect caused by the increase of Ca2+ sensitivity on behalf of contractile proteins, a characteristic which determines an increase in myocardial force without alteration, ventricular diastolic relaxation or induced cell death.12,13
There are different publications comparing Levosimendan with other inotropic drugs which have been proven to show an improvement in mortality, hemodynamic parameters and biochemical parameters in favor of levosimendan; however, not much is known about the change in parameters of symptom improvement as the main objective of HF treatment. The main objective of this systemic review and meta-analysis is to evaluate whether or not levosimendan offers a benefit in symptomatic improvement versus dobutamine and/or a placebo.
MethodsPatientsAdults ≥18 years of age, admitted to the hospital due to decompensated heart failure of any etiology, in the NYHA (New York Heart Association) III or IV functional class, a left ventricular ejection fraction (LVEF) <0.35, a pulmonary wedge pressure (PWP)≥15mmHg or a cardiac index <2.5L/min/m2.
Included studiesRandomized clinical trials (control or placebo) with a priori well-defined primary outcome which did not have more than a 10% loss during follow up.
InterventionIntravenous infusion of levosimendan for 24h, compared with placebo and/or dobutamine.
Objectives- •
Primary: Clinical improvement of dyspnea, fatigue, NYHA functional class and combined terminal point of clinical improvement (dyspnea, fatigue, and NYHA functional class).
- •
Secondary: Improvement of hemodynamic parameters (LVEF, heart index or cardiac output [CO]) and pulmonary wedge pressure, brain natriuretic peptide (BNP), total mortality, adverse effects and the use of rescue treatment, defined as use of positive inotropes or intravenous vasodilators, which were not part of the analyzed medications (i.e. dopamine or norepinephrine).
Duplicated trials, trials which were in a language other than Spanish or English, trials that did not have relevant information to measure the primary outcome, non-randomized clinical trials and articles that were not available in full text format were excluded.
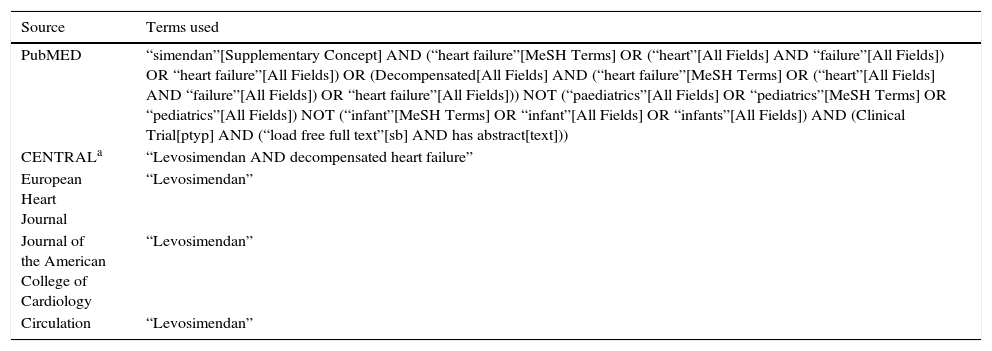
Search parameters to identify the studiesRelevant clinical trials were found by electronic search in MEDLINE (1966 to August 2013), in the platforms of Pubmed, EBSCOhost, ProQuest, the Cochrane Central Registry of Clinical Trials (CENTRAL), the European Heart Journal, the Journal of the American College of Cardiology and Circulation, the Mexican Journal of Cardiology, the Mexican Cardiology Archives and the Spanish Journal of Cardiology. No non-indexed sources were searched. The search terms can be found in Table 1. The search was limited to human subjects, with languages limited to Spanish and English, as well as those conducted by a single author and ending on August 30, 2013.
Search terms.
| Source | Terms used |
|---|---|
| PubMED | “simendan”[Supplementary Concept] AND (“heart failure”[MeSH Terms] OR (“heart”[All Fields] AND “failure”[All Fields]) OR “heart failure”[All Fields]) OR (Decompensated[All Fields] AND (“heart failure”[MeSH Terms] OR (“heart”[All Fields] AND “failure”[All Fields]) OR “heart failure”[All Fields])) NOT (“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields]) NOT (“infant”[MeSH Terms] OR “infant”[All Fields] OR “infants”[All Fields]) AND (Clinical Trial[ptyp] AND (“load free full text”[sb] AND has abstract[text])) |
| CENTRALa | “Levosimendan AND decompensated heart failure” |
| European Heart Journal | “Levosimendan” |
| Journal of the American College of Cardiology | “Levosimendan” |
| Circulation | “Levosimendan” |
Two authors reviewed the abstracts to identify potential clinical trials. If the summary met the inclusion criteria, the study was considered for a full-text review. Full-text articles were extracted and evaluated for validity and quality by both authors to determine if they met the eligibility criteria specified. When one of these was unclear, the study was not accepted for review.
StatisticsMeta-analytic techniques were used to increase the power of hypothesis testing and also to obtain information not available in individual trials. Using the effect methods for each model, we calculated the value of their statistical association and their corresponding chi-square. The chi-square of homogeneity, calculated as the difference between the total chi-square and the association of the chi-square, allowed us to measure the degree of homogeneity among the association values to be evaluated. The results were expressed as relative risk, odds ratio or difference from the standardized mean, with a 95% confidence interval. To carry out the statistical calculations and the meta-analytical graphs, we used Review Manager version 5.2, which provided us with the Cochrane Collaboration.14
Level of significanceAssociation: Only the least significant p-value of the association test should be taken into consideration; for the conclusion, the level of significance was proposed at p=0.05.
Heterogeneity: Because statistical tests lack the power to measure heterogeneity in a meta-analysis, a level of statistical significance of p=0.10 was used.
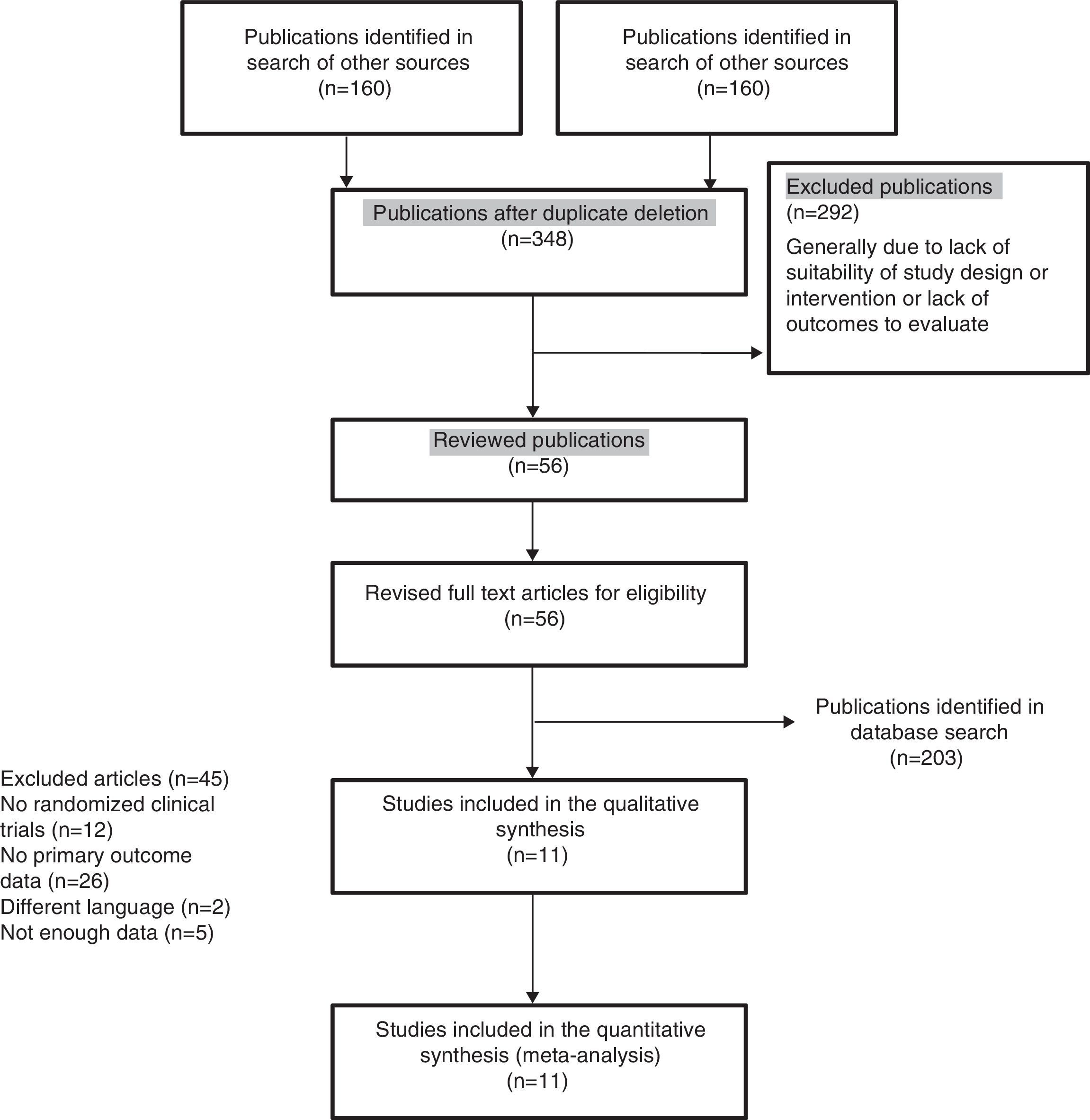
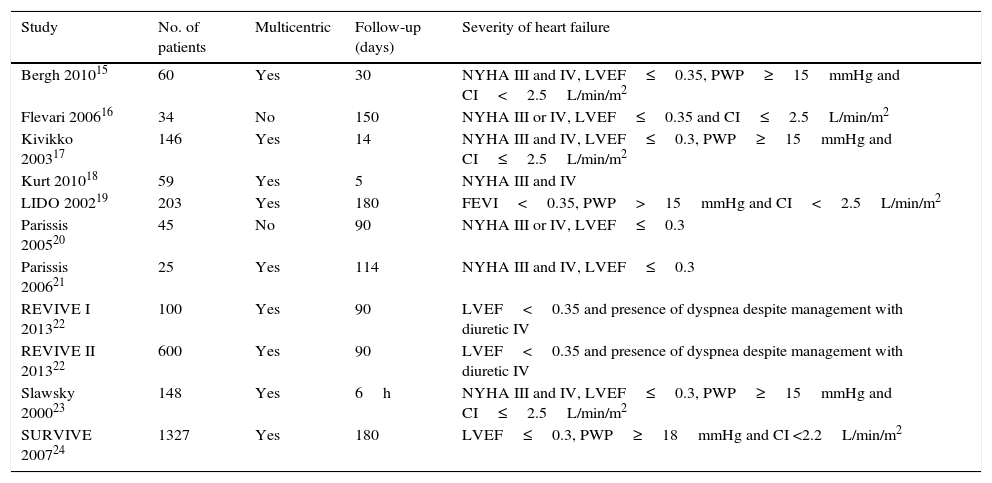
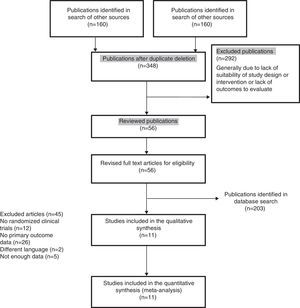
ResultsDescription of the studiesThe main features of the included studies and patients are summarized in Tables 2 and 3.15–24 The description of the data of each included trial can be consulted in Tables 5–15.15–24 Of the 363 references to potential clinical trials initially selected, only 11 randomized clinical trials met the inclusion criteria and were included in the qualitative and quantitative analysis, including a total of 2747 patients in the analysis (Fig. 1). The reasons for excluding the 45 full-text references are summarized in Table 16.31–75
Characteristics of included trials.
| Study | No. of patients | Multicentric | Follow-up (days) | Severity of heart failure |
|---|---|---|---|---|
| Bergh 201015 | 60 | Yes | 30 | NYHA III and IV, LVEF≤0.35, PWP≥15mmHg and CI<2.5L/min/m2 |
| Flevari 200616 | 34 | No | 150 | NYHA III or IV, LVEF≤0.35 and CI≤2.5L/min/m2 |
| Kivikko 200317 | 146 | Yes | 14 | NYHA III and IV, LVEF≤0.3, PWP≥15mmHg and CI≤2.5L/min/m2 |
| Kurt 201018 | 59 | Yes | 5 | NYHA III and IV |
| LIDO 200219 | 203 | Yes | 180 | FEVI<0.35, PWP>15mmHg and CI<2.5L/min/m2 |
| Parissis 200520 | 45 | No | 90 | NYHA III or IV, LVEF≤0.3 |
| Parissis 200621 | 25 | Yes | 114 | NYHA III and IV, LVEF≤0.3 |
| REVIVE I 201322 | 100 | Yes | 90 | LVEF<0.35 and presence of dyspnea despite management with diuretic IV |
| REVIVE II 201322 | 600 | Yes | 90 | LVEF<0.35 and presence of dyspnea despite management with diuretic IV |
| Slawsky 200023 | 148 | Yes | 6h | NYHA III and IV, LVEF≤0.3, PWP≥15mmHg and CI≤2.5L/min/m2 |
| SURVIVE 200724 | 1327 | Yes | 180 | LVEF≤0.3, PWP≥18mmHg and CI <2.2L/min/m2 |
NYHA: New York Heart Association functional class, LVEF: left ventricular ejection fraction, PWP: pulmonary wedge pressure, CI: cardiac index.
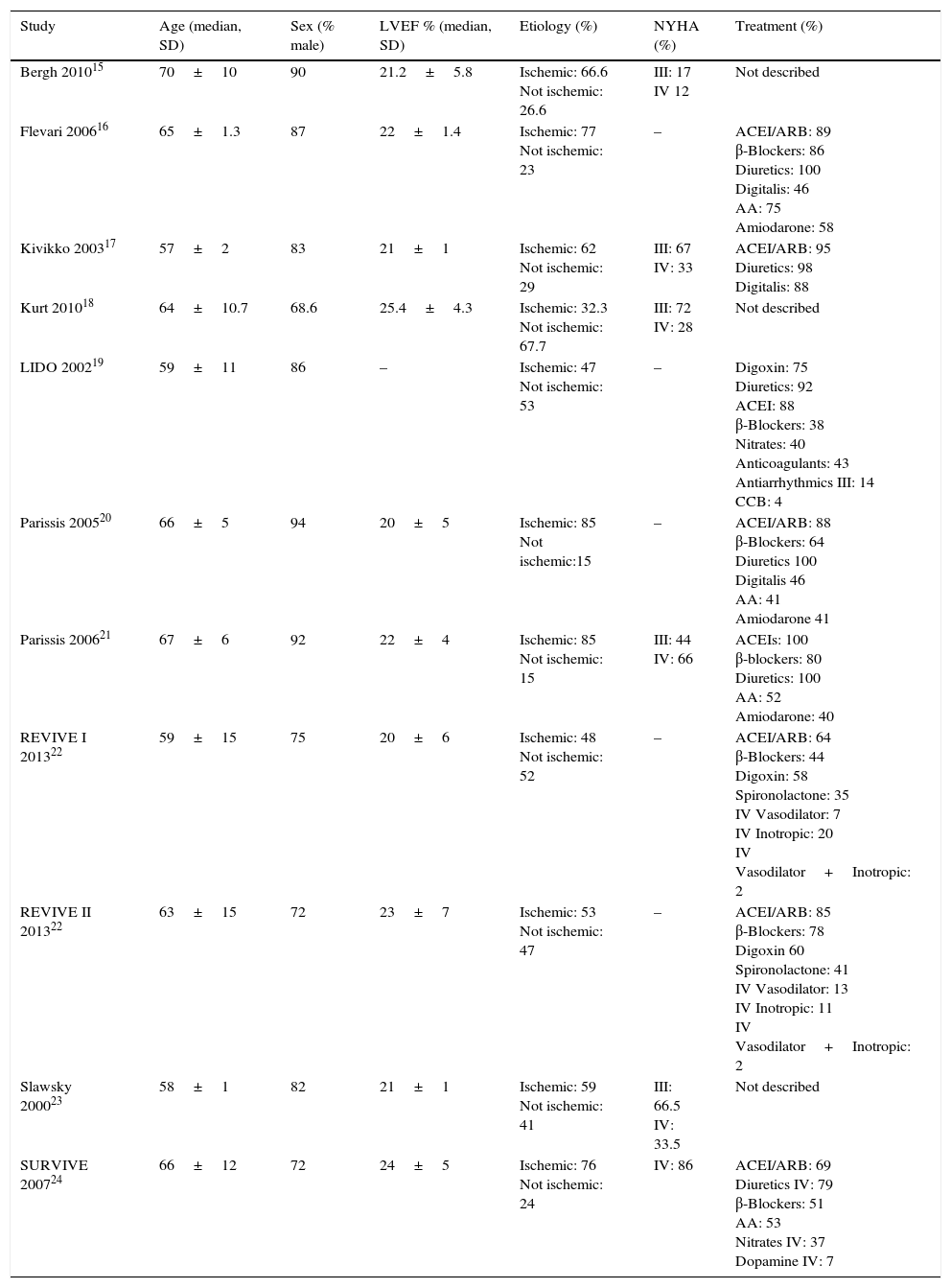
Characteristics of patients included in the trials.
| Study | Age (median, SD) | Sex (% male) | LVEF % (median, SD) | Etiology (%) | NYHA (%) | Treatment (%) |
|---|---|---|---|---|---|---|
| Bergh 201015 | 70±10 | 90 | 21.2±5.8 | Ischemic: 66.6 Not ischemic: 26.6 | III: 17 IV 12 | Not described |
| Flevari 200616 | 65±1.3 | 87 | 22±1.4 | Ischemic: 77 Not ischemic: 23 | – | ACEI/ARB: 89 β-Blockers: 86 Diuretics: 100 Digitalis: 46 AA: 75 Amiodarone: 58 |
| Kivikko 200317 | 57±2 | 83 | 21±1 | Ischemic: 62 Not ischemic: 29 | III: 67 IV: 33 | ACEI/ARB: 95 Diuretics: 98 Digitalis: 88 |
| Kurt 201018 | 64±10.7 | 68.6 | 25.4±4.3 | Ischemic: 32.3 Not ischemic: 67.7 | III: 72 IV: 28 | Not described |
| LIDO 200219 | 59±11 | 86 | – | Ischemic: 47 Not ischemic: 53 | – | Digoxin: 75 Diuretics: 92 ACEI: 88 β-Blockers: 38 Nitrates: 40 Anticoagulants: 43 Antiarrhythmics III: 14 CCB: 4 |
| Parissis 200520 | 66±5 | 94 | 20±5 | Ischemic: 85 Not ischemic:15 | – | ACEI/ARB: 88 β-Blockers: 64 Diuretics 100 Digitalis 46 AA: 41 Amiodarone 41 |
| Parissis 200621 | 67±6 | 92 | 22±4 | Ischemic: 85 Not ischemic: 15 | III: 44 IV: 66 | ACEIs: 100 β-blockers: 80 Diuretics: 100 AA: 52 Amiodarone: 40 |
| REVIVE I 201322 | 59±15 | 75 | 20±6 | Ischemic: 48 Not ischemic: 52 | – | ACEI/ARB: 64 β-Blockers: 44 Digoxin: 58 Spironolactone: 35 IV Vasodilator: 7 IV Inotropic: 20 IV Vasodilator+Inotropic: 2 |
| REVIVE II 201322 | 63±15 | 72 | 23±7 | Ischemic: 53 Not ischemic: 47 | – | ACEI/ARB: 85 β-Blockers: 78 Digoxin 60 Spironolactone: 41 IV Vasodilator: 13 IV Inotropic: 11 IV Vasodilator+Inotropic: 2 |
| Slawsky 200023 | 58±1 | 82 | 21±1 | Ischemic: 59 Not ischemic: 41 | III: 66.5 IV: 33.5 | Not described |
| SURVIVE 200724 | 66±12 | 72 | 24±5 | Ischemic: 76 Not ischemic: 24 | IV: 86 | ACEI/ARB: 69 Diuretics IV: 79 β-Blockers: 51 AA: 53 Nitrates IV: 37 Dopamine IV: 7 |
SD: standard deviation, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association functional class, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, AA: aldosterone antagonist, IV: intravenous.
As in most of the studies on HF we analyzed, the inclusion criteria were that patients be in the NYHA III or IV functional class, with an LVEF below 35% measured by two-dimensional echocardiography or radionuclide ventriculography, a PWP>15mmHg and a cardiac index<2.5L/min/m2. The mean age of the patients was 63±4.2 years, 81.9±8.8% were men and the mean LVEF was 21±1.7%. The etiology of heart failure was ischemic in 62.9±16.9% and non-ischemic in 35.7±17.4%. The most common concomitant treatments were ACE inhibitors, angiotensin II receptor antagonists (ARBs), β-blockers and diuretics.
Risk of bias in included studiesWe included 7 double-blind clinical trials and 4 open-label studies.16,18,20,21 The randomization method was described in 2 clinical trials involving 1530 patients (55.6% of all patients).19,24 The primary outcome was described in 4 trials, including 2230 patients (79.7% of all patients).19,22,24
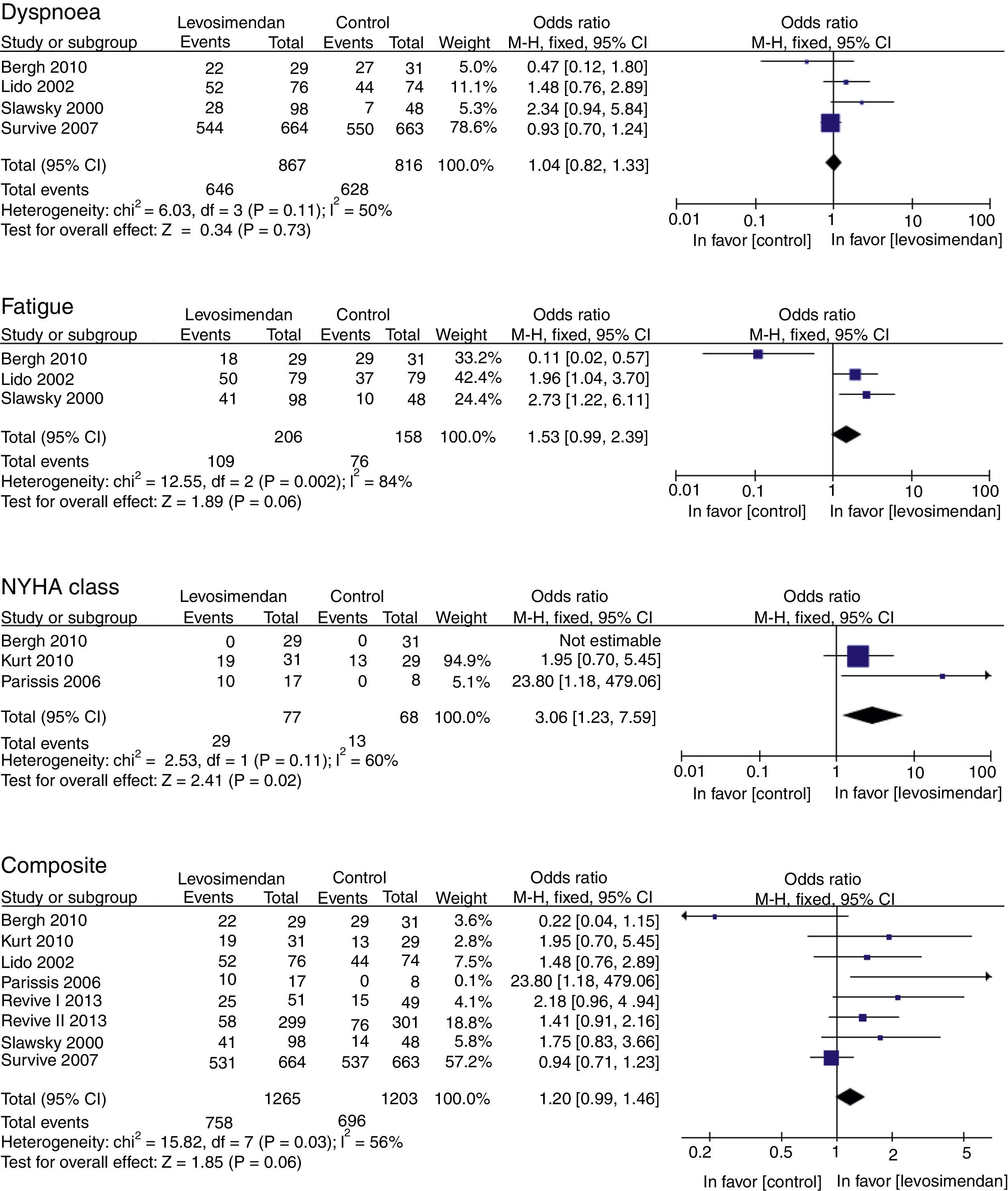
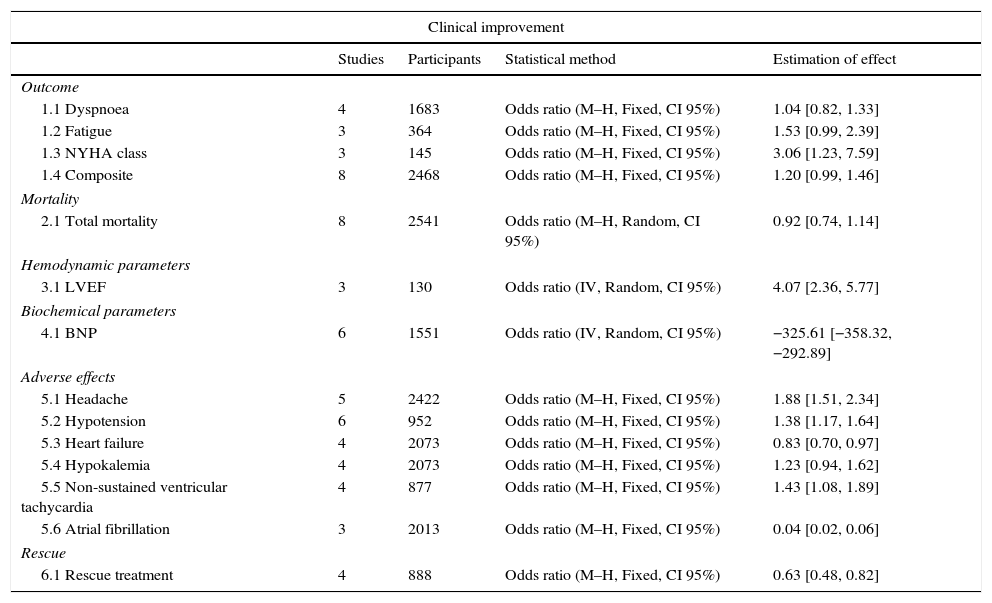
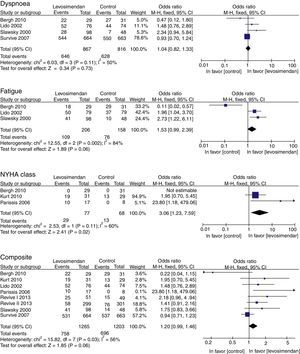
Results of the meta-analysisOutcomes of clinical improvementThere was no difference in dyspnea (RM 1.04, 95% CI 0.82–1.33, p=0.73), although there was a tendency to reduce fatigue (RM 1.53, 95% CI 0.99–2.39, p=0.06). There was a significant improvement in the NYHA functional class with levosimendan compared to that with placebo or dobutamine (RM 3.06, 95% CI 1.23–7.59, p=0.02), although at the combined endpoint of clinical improvement, there was only a marginal reduction of 20% (RM 1.20, IC 95% 0.99–1.46, p=0.06) (Table 4). There was substantial heterogeneity (p<0.10) in all primary outcomes (Fig. 2).
Levosimendan vs control comparisons.
| Clinical improvement | ||||
|---|---|---|---|---|
| Studies | Participants | Statistical method | Estimation of effect | |
| Outcome | ||||
| 1.1 Dyspnoea | 4 | 1683 | Odds ratio (M–H, Fixed, CI 95%) | 1.04 [0.82, 1.33] |
| 1.2 Fatigue | 3 | 364 | Odds ratio (M–H, Fixed, CI 95%) | 1.53 [0.99, 2.39] |
| 1.3 NYHA class | 3 | 145 | Odds ratio (M–H, Fixed, CI 95%) | 3.06 [1.23, 7.59] |
| 1.4 Composite | 8 | 2468 | Odds ratio (M–H, Fixed, CI 95%) | 1.20 [0.99, 1.46] |
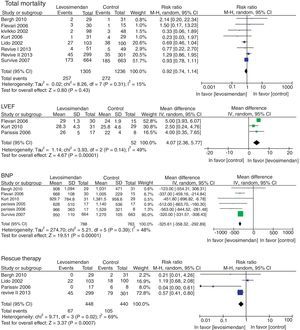
| Mortality | ||||
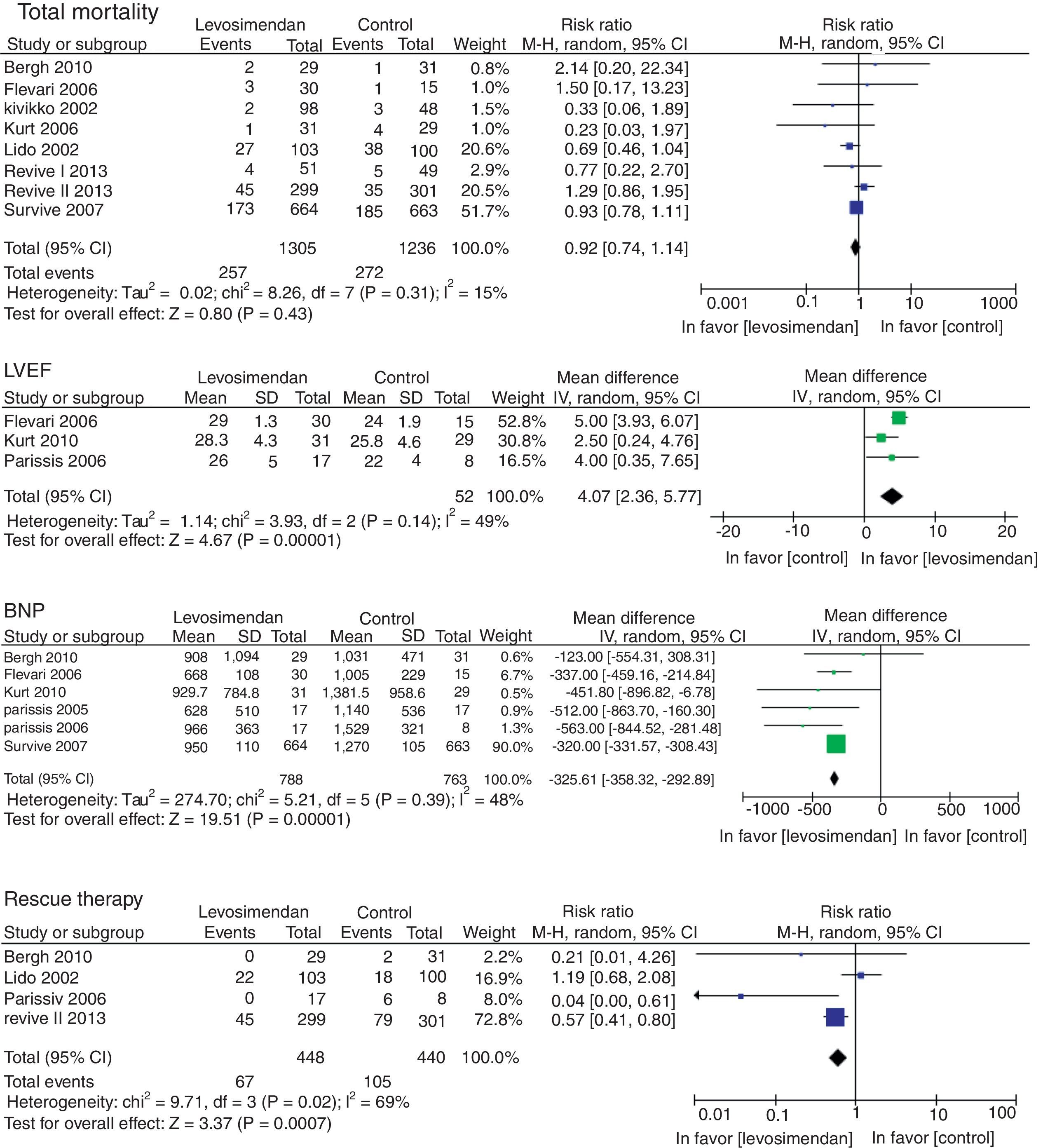
| 2.1 Total mortality | 8 | 2541 | Odds ratio (M–H, Random, CI 95%) | 0.92 [0.74, 1.14] |
| Hemodynamic parameters | ||||
| 3.1 LVEF | 3 | 130 | Odds ratio (IV, Random, CI 95%) | 4.07 [2.36, 5.77] |
| Biochemical parameters | ||||
| 4.1 BNP | 6 | 1551 | Odds ratio (IV, Random, CI 95%) | −325.61 [−358.32, −292.89] |
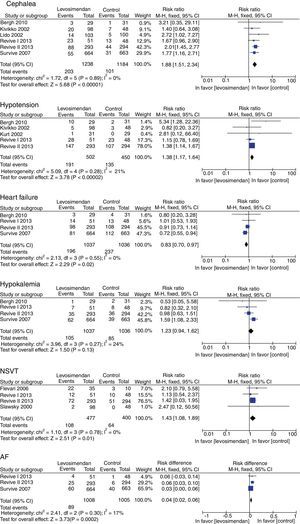
| Adverse effects | ||||
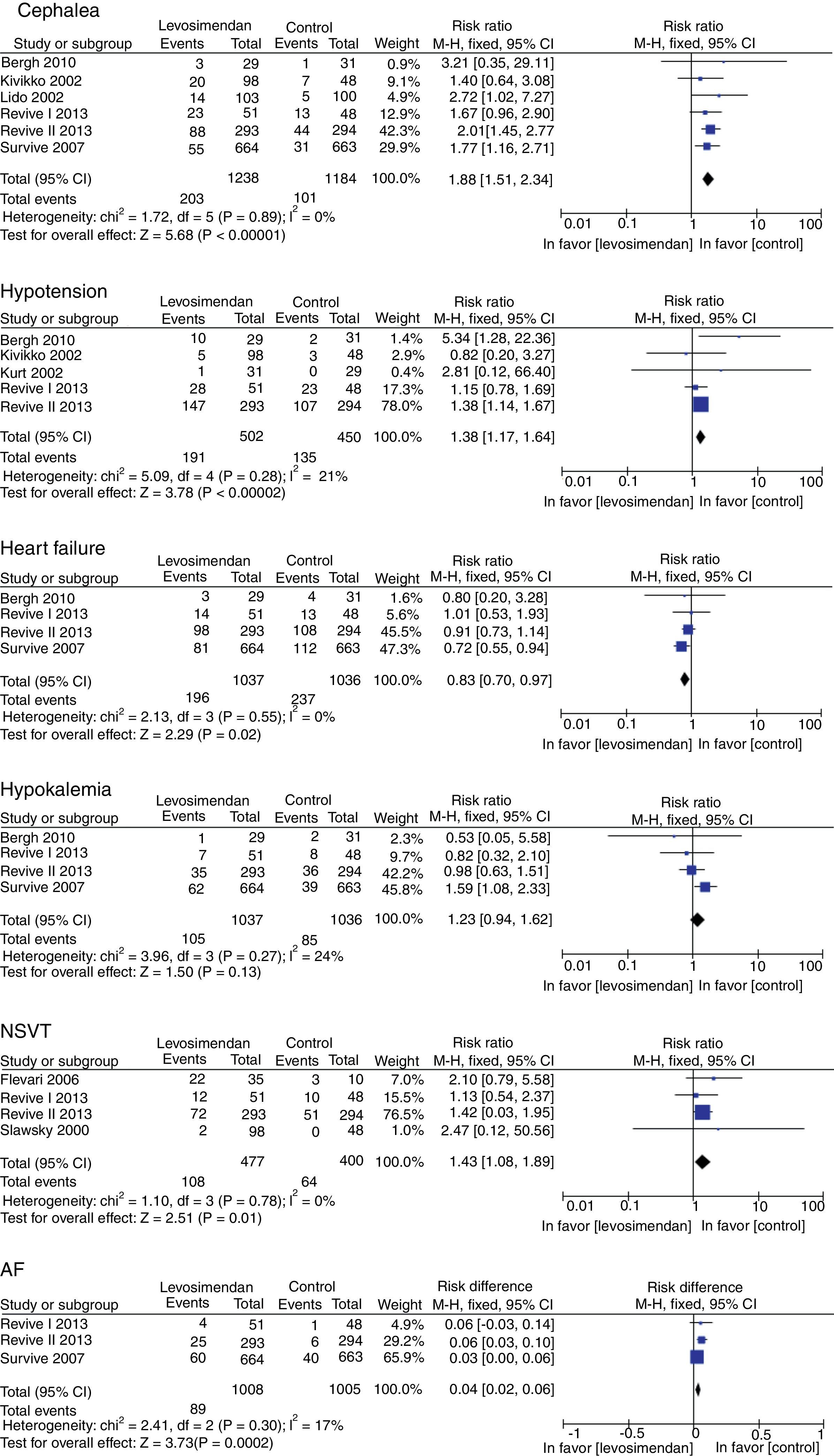
| 5.1 Headache | 5 | 2422 | Odds ratio (M–H, Fixed, CI 95%) | 1.88 [1.51, 2.34] |
| 5.2 Hypotension | 6 | 952 | Odds ratio (M–H, Fixed, CI 95%) | 1.38 [1.17, 1.64] |
| 5.3 Heart failure | 4 | 2073 | Odds ratio (M–H, Fixed, CI 95%) | 0.83 [0.70, 0.97] |
| 5.4 Hypokalemia | 4 | 2073 | Odds ratio (M–H, Fixed, CI 95%) | 1.23 [0.94, 1.62] |
| 5.5 Non-sustained ventricular tachycardia | 4 | 877 | Odds ratio (M–H, Fixed, CI 95%) | 1.43 [1.08, 1.89] |
| 5.6 Atrial fibrillation | 3 | 2013 | Odds ratio (M–H, Fixed, CI 95%) | 0.04 [0.02, 0.06] |
| Rescue | ||||
| 6.1 Rescue treatment | 4 | 888 | Odds ratio (M–H, Fixed, CI 95%) | 0.63 [0.48, 0.82] |
M–H: Mantel–Haenszel, CI: confidence interval, IV: instrumental variable.
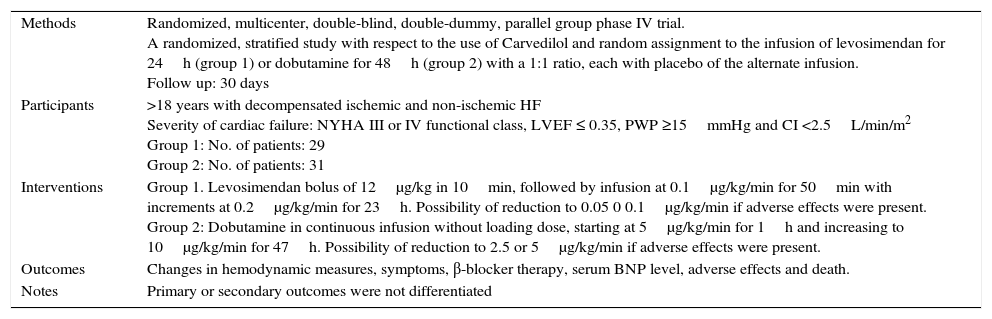
Characteristics of a study by Bergh, 2010.a
| Methods | Randomized, multicenter, double-blind, double-dummy, parallel group phase IV trial. A randomized, stratified study with respect to the use of Carvedilol and random assignment to the infusion of levosimendan for 24h (group 1) or dobutamine for 48h (group 2) with a 1:1 ratio, each with placebo of the alternate infusion. Follow up: 30 days |
| Participants | >18 years with decompensated ischemic and non-ischemic HF Severity of cardiac failure: NYHA III or IV functional class, LVEF ≤ 0.35, PWP ≥15mmHg and CI <2.5L/min/m2 Group 1: No. of patients: 29 Group 2: No. of patients: 31 |
| Interventions | Group 1. Levosimendan bolus of 12μg/kg in 10min, followed by infusion at 0.1μg/kg/min for 50min with increments at 0.2μg/kg/min for 23h. Possibility of reduction to 0.05 0 0.1μg/kg/min if adverse effects were present. Group 2: Dobutamine in continuous infusion without loading dose, starting at 5μg/kg/min for 1h and increasing to 10μg/kg/min for 47h. Possibility of reduction to 2.5 or 5μg/kg/min if adverse effects were present. |
| Outcomes | Changes in hemodynamic measures, symptoms, β-blocker therapy, serum BNP level, adverse effects and death. |
| Notes | Primary or secondary outcomes were not differentiated |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “Patients…were randomly allocated…in a 1:1 ratio” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information |
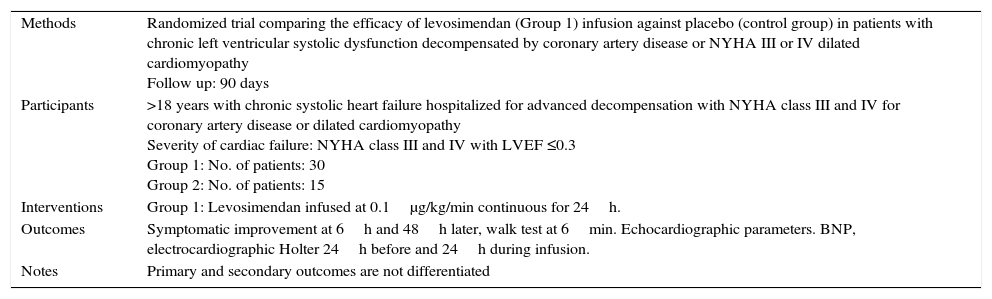
Characteristics of a study by Flevari, 2006.a
| Methods | Randomized trial comparing the efficacy of levosimendan (Group 1) infusion against placebo (control group) in patients with chronic left ventricular systolic dysfunction decompensated by coronary artery disease or NYHA III or IV dilated cardiomyopathy Follow up: 90 days |
| Participants | >18 years with chronic systolic heart failure hospitalized for advanced decompensation with NYHA class III and IV for coronary artery disease or dilated cardiomyopathy Severity of cardiac failure: NYHA class III and IV with LVEF ≤0.3 Group 1: No. of patients: 30 Group 2: No. of patients: 15 |
| Interventions | Group 1: Levosimendan infused at 0.1μg/kg/min continuous for 24h. |
| Outcomes | Symptomatic improvement at 6h and 48h later, walk test at 6min. Echocardiographic parameters. BNP, electrocardiographic Holter 24h before and 24h during infusion. |
| Notes | Primary and secondary outcomes are not differentiated |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “Patients were randomized (in a 2:1 design)” Comment: Not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information |
| Blinding (performance bias) | High risk | Comment: It is not said if there was blinding, but the outcome can be influenced by the absence of it. |
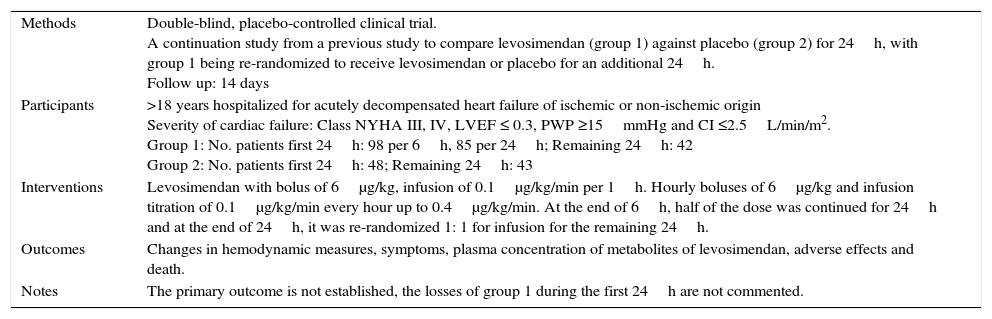
Characteristics of a study by Kivikko, 2003.a
| Methods | Double-blind, placebo-controlled clinical trial. A continuation study from a previous study to compare levosimendan (group 1) against placebo (group 2) for 24h, with group 1 being re-randomized to receive levosimendan or placebo for an additional 24h. Follow up: 14 days |
| Participants | >18 years hospitalized for acutely decompensated heart failure of ischemic or non-ischemic origin Severity of cardiac failure: Class NYHA III, IV, LVEF ≤ 0.3, PWP ≥15mmHg and CI ≤2.5L/min/m2. Group 1: No. patients first 24h: 98 per 6h, 85 per 24h; Remaining 24h: 42 Group 2: No. patients first 24h: 48; Remaining 24h: 43 |
| Interventions | Levosimendan with bolus of 6μg/kg, infusion of 0.1μg/kg/min per 1h. Hourly boluses of 6μg/kg and infusion titration of 0.1μg/kg/min every hour up to 0.4μg/kg/min. At the end of 6h, half of the dose was continued for 24h and at the end of 24h, it was re-randomized 1: 1 for infusion for the remaining 24h. |
| Outcomes | Changes in hemodynamic measures, symptoms, plasma concentration of metabolites of levosimendan, adverse effects and death. |
| Notes | The primary outcome is not established, the losses of group 1 during the first 24h are not commented. |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “…patients were randomized 1:1” Comment: Not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information |
| No loss report (attrition bias) | Unclear risk | Quote: “98 patients received levo for 6h and 48 received placebo. After 6h, 85 patients in the levo group were continued for a total of 24h on open-label Levo” Comment: There is insufficient information on 13/98 patients in the levosimendan group prior to the new randomization |
| Lack of data (reporting bias) | High risk | Comment: The complete results of the dyspnea and fatigue outcome are not described |
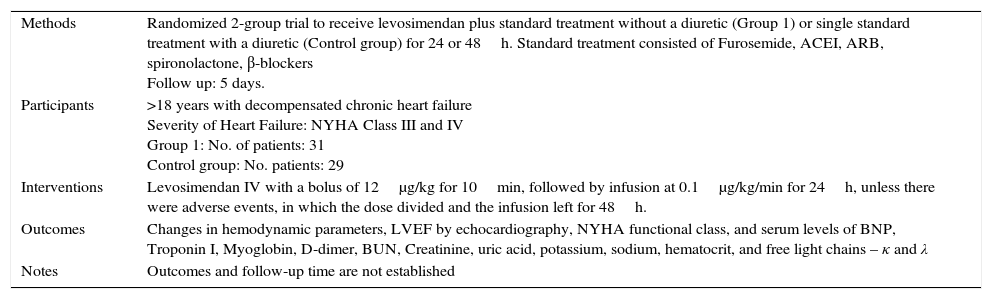
Characteristics of a study by Kurt, 2010.a
| Methods | Randomized 2-group trial to receive levosimendan plus standard treatment without a diuretic (Group 1) or single standard treatment with a diuretic (Control group) for 24 or 48h. Standard treatment consisted of Furosemide, ACEI, ARB, spironolactone, β-blockers Follow up: 5 days. |
| Participants | >18 years with decompensated chronic heart failure Severity of Heart Failure: NYHA Class III and IV Group 1: No. of patients: 31 Control group: No. patients: 29 |
| Interventions | Levosimendan IV with a bolus of 12μg/kg for 10min, followed by infusion at 0.1μg/kg/min for 24h, unless there were adverse events, in which the dose divided and the infusion left for 48h. |
| Outcomes | Changes in hemodynamic parameters, LVEF by echocardiography, NYHA functional class, and serum levels of BNP, Troponin I, Myoglobin, D-dimer, BUN, Creatinine, uric acid, potassium, sodium, hematocrit, and free light chains – κ and λ |
| Notes | Outcomes and follow-up time are not established |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “Randomized into two groups” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information |
| Blinding (performance bias) | Unclear risk | Comment: No comment, probably not performed |
Characteristics of a study by LIDO, 2002.a
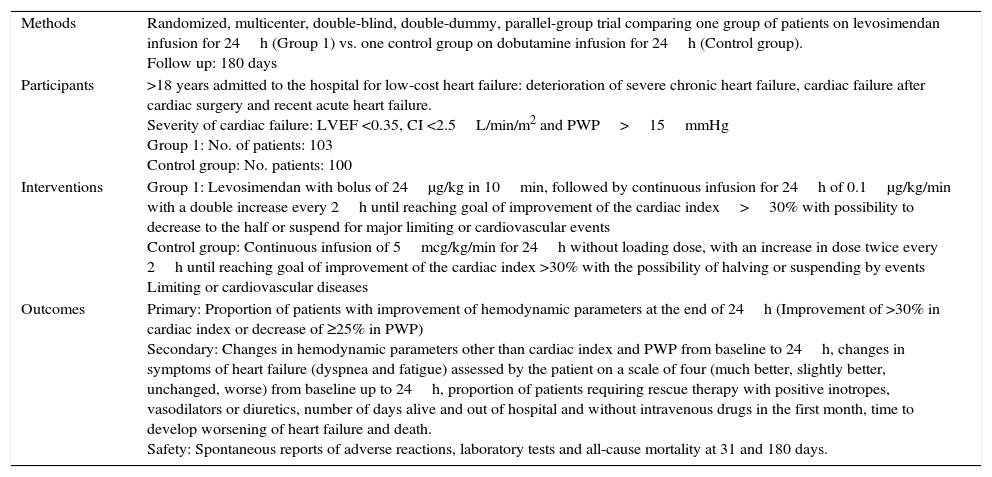
| Methods | Randomized, multicenter, double-blind, double-dummy, parallel-group trial comparing one group of patients on levosimendan infusion for 24h (Group 1) vs. one control group on dobutamine infusion for 24h (Control group). Follow up: 180 days |
| Participants | >18 years admitted to the hospital for low-cost heart failure: deterioration of severe chronic heart failure, cardiac failure after cardiac surgery and recent acute heart failure. Severity of cardiac failure: LVEF <0.35, CI <2.5L/min/m2 and PWP>15mmHg Group 1: No. of patients: 103 Control group: No. patients: 100 |
| Interventions | Group 1: Levosimendan with bolus of 24μg/kg in 10min, followed by continuous infusion for 24h of 0.1μg/kg/min with a double increase every 2h until reaching goal of improvement of the cardiac index>30% with possibility to decrease to the half or suspend for major limiting or cardiovascular events Control group: Continuous infusion of 5mcg/kg/min for 24h without loading dose, with an increase in dose twice every 2h until reaching goal of improvement of the cardiac index >30% with the possibility of halving or suspending by events Limiting or cardiovascular diseases |
| Outcomes | Primary: Proportion of patients with improvement of hemodynamic parameters at the end of 24h (Improvement of >30% in cardiac index or decrease of ≥25% in PWP) Secondary: Changes in hemodynamic parameters other than cardiac index and PWP from baseline to 24h, changes in symptoms of heart failure (dyspnea and fatigue) assessed by the patient on a scale of four (much better, slightly better, unchanged, worse) from baseline up to 24h, proportion of patients requiring rescue therapy with positive inotropes, vasodilators or diuretics, number of days alive and out of hospital and without intravenous drugs in the first month, time to develop worsening of heart failure and death. Safety: Spontaneous reports of adverse reactions, laboratory tests and all-cause mortality at 31 and 180 days. |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Low risk | Quote: “Assigned randomly… in blocks of four according to a computer-generated code” Comment: Probably was performed |
| Allocation concealment (selection bias) | Low risk | Quote: “Treatment allocation and size of the randomization blocks were concealed from the investigators” Comment: Probably was performed |
| Blinding (performance bias) | Low risk | Quote: “Double-blind, double dummy” Comment: Probably was performed |
Characteristics of a study by Parissis, 2005.a
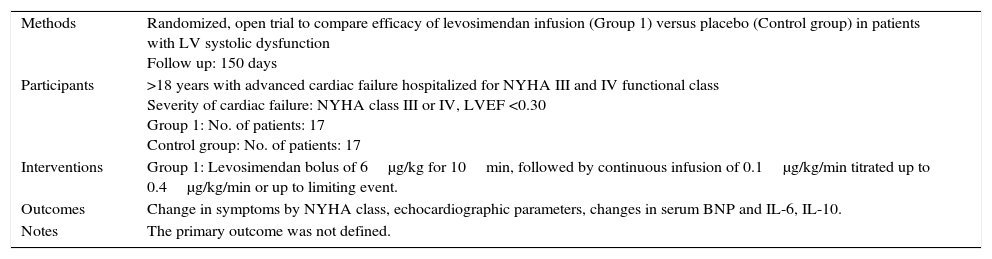
| Methods | Randomized, open trial to compare efficacy of levosimendan infusion (Group 1) versus placebo (Control group) in patients with LV systolic dysfunction Follow up: 150 days |
| Participants | >18 years with advanced cardiac failure hospitalized for NYHA III and IV functional class Severity of cardiac failure: NYHA class III or IV, LVEF <0.30 Group 1: No. of patients: 17 Control group: No. of patients: 17 |
| Interventions | Group 1: Levosimendan bolus of 6μg/kg for 10min, followed by continuous infusion of 0.1μg/kg/min titrated up to 0.4μg/kg/min or up to limiting event. |
| Outcomes | Change in symptoms by NYHA class, echocardiographic parameters, changes in serum BNP and IL-6, IL-10. |
| Notes | The primary outcome was not defined. |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “…patients were randomized immediately after their hospital admission…” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information |
| Blinding (performance bias) | High risk | Quote: “open-label study” Comment: The outcome can be influenced by the absence of blinding |
Characteristics of a study by Parissis, 2006.a
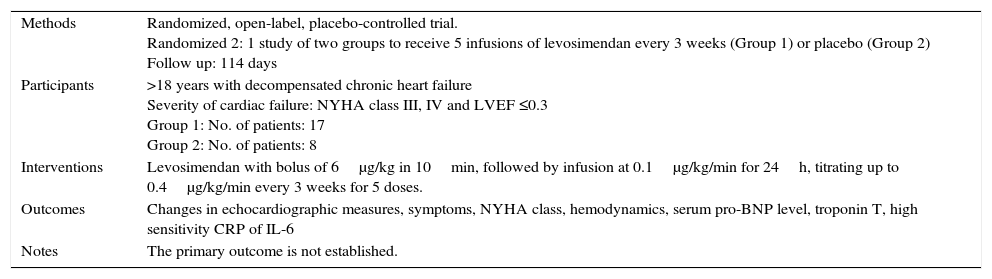
| Methods | Randomized, open-label, placebo-controlled trial. Randomized 2: 1 study of two groups to receive 5 infusions of levosimendan every 3 weeks (Group 1) or placebo (Group 2) Follow up: 114 days |
| Participants | >18 years with decompensated chronic heart failure Severity of cardiac failure: NYHA class III, IV and LVEF ≤0.3 Group 1: No. of patients: 17 Group 2: No. of patients: 8 |
| Interventions | Levosimendan with bolus of 6μg/kg in 10min, followed by infusion at 0.1μg/kg/min for 24h, titrating up to 0.4μg/kg/min every 3 weeks for 5 doses. |
| Outcomes | Changes in echocardiographic measures, symptoms, NYHA class, hemodynamics, serum pro-BNP level, troponin T, high sensitivity CRP of IL-6 |
| Notes | The primary outcome is not established. |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “Randomly allocated 2:1” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information, probably not performed |
| Blinding (performance bias) | High risk | Quote: “Open-label study” Comment: The outcome can be influenced by the absence of blinding |
Characteristics of a study by REVIVE I, 2013.a
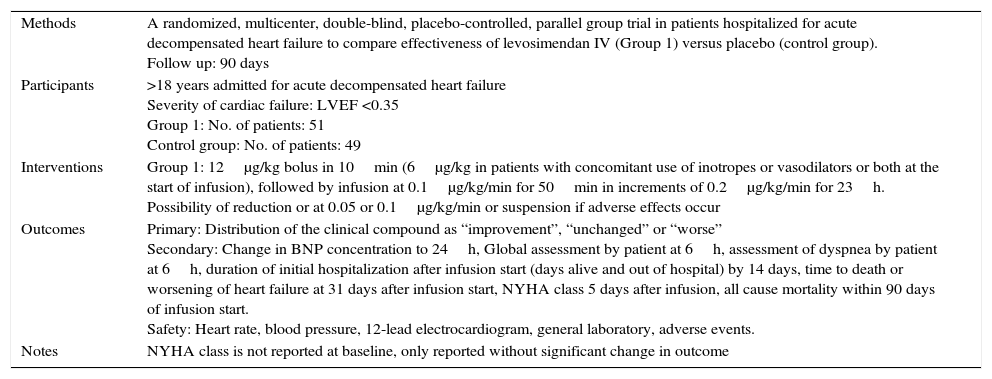
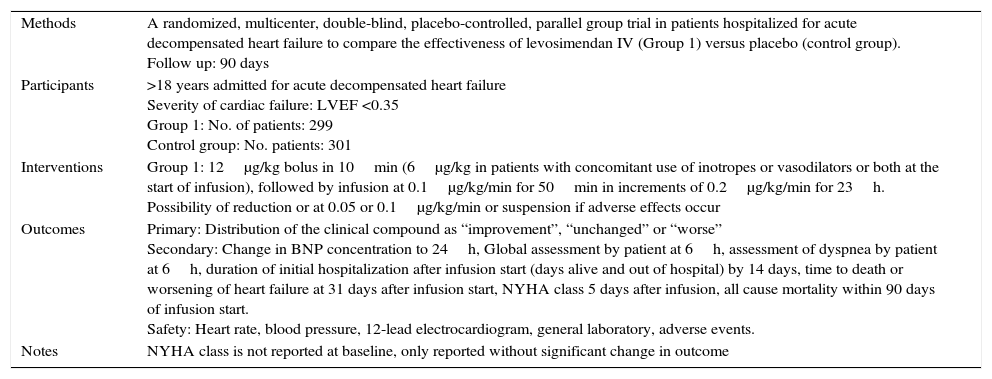
| Methods | A randomized, multicenter, double-blind, placebo-controlled, parallel group trial in patients hospitalized for acute decompensated heart failure to compare effectiveness of levosimendan IV (Group 1) versus placebo (control group). Follow up: 90 days |
| Participants | >18 years admitted for acute decompensated heart failure Severity of cardiac failure: LVEF <0.35 Group 1: No. of patients: 51 Control group: No. of patients: 49 |
| Interventions | Group 1: 12μg/kg bolus in 10min (6μg/kg in patients with concomitant use of inotropes or vasodilators or both at the start of infusion), followed by infusion at 0.1μg/kg/min for 50min in increments of 0.2μg/kg/min for 23h. Possibility of reduction or at 0.05 or 0.1μg/kg/min or suspension if adverse effects occur |
| Outcomes | Primary: Distribution of the clinical compound as “improvement”, “unchanged” or “worse” Secondary: Change in BNP concentration to 24h, Global assessment by patient at 6h, assessment of dyspnea by patient at 6h, duration of initial hospitalization after infusion start (days alive and out of hospital) by 14 days, time to death or worsening of heart failure at 31 days after infusion start, NYHA class 5 days after infusion, all cause mortality within 90 days of infusion start. Safety: Heart rate, blood pressure, 12-lead electrocardiogram, general laboratory, adverse events. |
| Notes | NYHA class is not reported at baseline, only reported without significant change in outcome |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “…patients were randomly assigned…” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information |
| Blinding (performance bias) | Low risk | Quote: “double-blind” Comment: Was probably performed |
| No complete report (report bias) | Low risk | Quote: “The NYHA functional class at 5 days was not significantly different between treatment groups (p=0.196). Comment: The complete data were not commented to be analyzed in the meta-analysis |
Characteristics of a study by REVIVE II, 2013.a
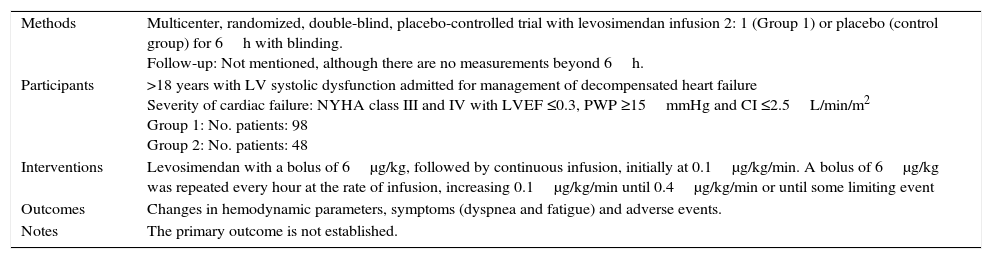
| Methods | A randomized, multicenter, double-blind, placebo-controlled, parallel group trial in patients hospitalized for acute decompensated heart failure to compare the effectiveness of levosimendan IV (Group 1) versus placebo (control group). Follow up: 90 days |
| Participants | >18 years admitted for acute decompensated heart failure Severity of cardiac failure: LVEF <0.35 Group 1: No. of patients: 299 Control group: No. patients: 301 |
| Interventions | Group 1: 12μg/kg bolus in 10min (6μg/kg in patients with concomitant use of inotropes or vasodilators or both at the start of infusion), followed by infusion at 0.1μg/kg/min for 50min in increments of 0.2μg/kg/min for 23h. Possibility of reduction or at 0.05 or 0.1μg/kg/min or suspension if adverse effects occur |
| Outcomes | Primary: Distribution of the clinical compound as “improvement”, “unchanged” or “worse” Secondary: Change in BNP concentration to 24h, Global assessment by patient at 6h, assessment of dyspnea by patient at 6h, duration of initial hospitalization after infusion start (days alive and out of hospital) by 14 days, time to death or worsening of heart failure at 31 days after infusion start, NYHA class 5 days after infusion, all cause mortality within 90 days of infusion start. Safety: Heart rate, blood pressure, 12-lead electrocardiogram, general laboratory, adverse events. |
| Notes | NYHA class is not reported at baseline, only reported without significant change in outcome |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “…patients were randomly assigned…” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information |
| Blinding (performance bias) | Low risk | Quote: “double-blind” Comment: Was probably performed |
| Incomplete result (reporting bias) | High risk | Quote: “The NYHA functional class at 5 days was not significantly different between treatment groups (p=0.196). Comment: The complete data were not commented to be analyzed in meta-analysis |
Characteristics of a study Slawsky 2000.a
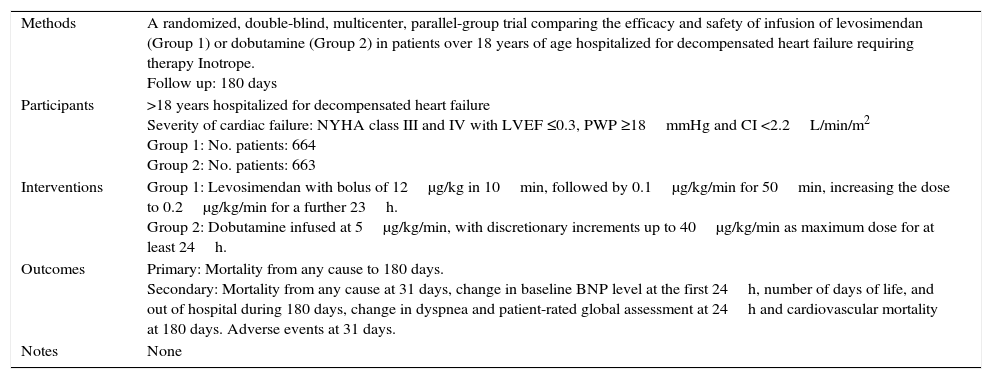
| Methods | Multicenter, randomized, double-blind, placebo-controlled trial with levosimendan infusion 2: 1 (Group 1) or placebo (control group) for 6h with blinding. Follow-up: Not mentioned, although there are no measurements beyond 6h. |
| Participants | >18 years with LV systolic dysfunction admitted for management of decompensated heart failure Severity of cardiac failure: NYHA class III and IV with LVEF ≤0.3, PWP ≥15mmHg and CI ≤2.5L/min/m2 Group 1: No. patients: 98 Group 2: No. patients: 48 |
| Interventions | Levosimendan with a bolus of 6μg/kg, followed by continuous infusion, initially at 0.1μg/kg/min. A bolus of 6μg/kg was repeated every hour at the rate of infusion, increasing 0.1μg/kg/min until 0.4μg/kg/min or until some limiting event |
| Outcomes | Changes in hemodynamic parameters, symptoms (dyspnea and fatigue) and adverse events. |
| Notes | The primary outcome is not established. |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Unclear risk | Quote: “Patients were randomized 2:1” Comment: There is not enough information |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information, probably not performed |
Characteristics of a study by SURVIVE, 2007.a
| Methods | A randomized, double-blind, multicenter, parallel-group trial comparing the efficacy and safety of infusion of levosimendan (Group 1) or dobutamine (Group 2) in patients over 18 years of age hospitalized for decompensated heart failure requiring therapy Inotrope. Follow up: 180 days |
| Participants | >18 years hospitalized for decompensated heart failure Severity of cardiac failure: NYHA class III and IV with LVEF ≤0.3, PWP ≥18mmHg and CI <2.2L/min/m2 Group 1: No. patients: 664 Group 2: No. patients: 663 |
| Interventions | Group 1: Levosimendan with bolus of 12μg/kg in 10min, followed by 0.1μg/kg/min for 50min, increasing the dose to 0.2μg/kg/min for a further 23h. Group 2: Dobutamine infused at 5μg/kg/min, with discretionary increments up to 40μg/kg/min as maximum dose for at least 24h. |
| Outcomes | Primary: Mortality from any cause to 180 days. Secondary: Mortality from any cause at 31 days, change in baseline BNP level at the first 24h, number of days of life, and out of hospital during 180 days, change in dyspnea and patient-rated global assessment at 24h and cardiovascular mortality at 180 days. Adverse events at 31 days. |
| Notes | None |
| Risk of bias | ||
|---|---|---|
| Bias | Judgment of the author | Support of the judgment |
| Random sequence (selection bias) | Low risk | Quote: “Randomization was performed by a 2-step procedure…vials were assigned a number using randomly permuted blocks…patients were randomized centrally using an interactive voice response system” Comment: Was probably performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is not enough information, probably not performed |
| Blinding (performance bias) | Low risk | Quote: “Double-blind” Comment: Was probably performed |
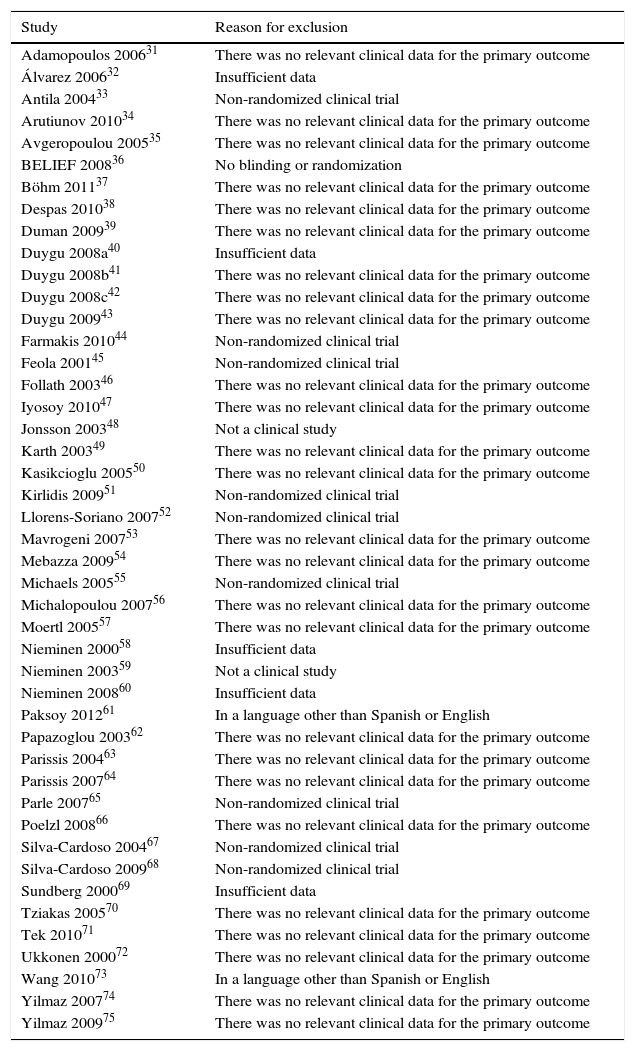
Characteristics of excluded studies.
| Study | Reason for exclusion |
|---|---|
| Adamopoulos 200631 | There was no relevant clinical data for the primary outcome |
| Álvarez 200632 | Insufficient data |
| Antila 200433 | Non-randomized clinical trial |
| Arutiunov 201034 | There was no relevant clinical data for the primary outcome |
| Avgeropoulou 200535 | There was no relevant clinical data for the primary outcome |
| BELIEF 200836 | No blinding or randomization |
| Böhm 201137 | There was no relevant clinical data for the primary outcome |
| Despas 201038 | There was no relevant clinical data for the primary outcome |
| Duman 200939 | There was no relevant clinical data for the primary outcome |
| Duygu 2008a40 | Insufficient data |
| Duygu 2008b41 | There was no relevant clinical data for the primary outcome |
| Duygu 2008c42 | There was no relevant clinical data for the primary outcome |
| Duygu 200943 | There was no relevant clinical data for the primary outcome |
| Farmakis 201044 | Non-randomized clinical trial |
| Feola 200145 | Non-randomized clinical trial |
| Follath 200346 | There was no relevant clinical data for the primary outcome |
| Iyosoy 201047 | There was no relevant clinical data for the primary outcome |
| Jonsson 200348 | Not a clinical study |
| Karth 200349 | There was no relevant clinical data for the primary outcome |
| Kasikcioglu 200550 | There was no relevant clinical data for the primary outcome |
| Kirlidis 200951 | Non-randomized clinical trial |
| Llorens-Soriano 200752 | Non-randomized clinical trial |
| Mavrogeni 200753 | There was no relevant clinical data for the primary outcome |
| Mebazza 200954 | There was no relevant clinical data for the primary outcome |
| Michaels 200555 | Non-randomized clinical trial |
| Michalopoulou 200756 | There was no relevant clinical data for the primary outcome |
| Moertl 200557 | There was no relevant clinical data for the primary outcome |
| Nieminen 200058 | Insufficient data |
| Nieminen 200359 | Not a clinical study |
| Nieminen 200860 | Insufficient data |
| Paksoy 201261 | In a language other than Spanish or English |
| Papazoglou 200362 | There was no relevant clinical data for the primary outcome |
| Parissis 200463 | There was no relevant clinical data for the primary outcome |
| Parissis 200764 | There was no relevant clinical data for the primary outcome |
| Parle 200765 | Non-randomized clinical trial |
| Poelzl 200866 | There was no relevant clinical data for the primary outcome |
| Silva-Cardoso 200467 | Non-randomized clinical trial |
| Silva-Cardoso 200968 | Non-randomized clinical trial |
| Sundberg 200069 | Insufficient data |
| Tziakas 200570 | There was no relevant clinical data for the primary outcome |
| Tek 201071 | There was no relevant clinical data for the primary outcome |
| Ukkonen 200072 | There was no relevant clinical data for the primary outcome |
| Wang 201073 | In a language other than Spanish or English |
| Yilmaz 200774 | There was no relevant clinical data for the primary outcome |
| Yilmaz 200975 | There was no relevant clinical data for the primary outcome |
There was no difference in mortality between the levosimendan group and the placebo or dobutamine groups (RR 0.92, 95% CI 0.74–1.14, p=0.43), although there was a significant improvement in LVEF (RR of 4.07, 95% RR=95% CI −358.32 to −292.89; p<0.00001), in the incidence of new episodes of heart failure (RR 0.83, 95% CI 0.70–0.97, p=0.02) and in the use of rescue treatment (RR 0.63 95% CI 0.48–082, p=0.0007) (Fig. 3). The following adverse events were more commonly observed in the levosimendan group compared to the placebo or dobutamine groups: headache (RR 1.88, 95% CI 1.51–2.34, p<0.00001), hypotension (RR 1.38, 95% CI 1.17–1.64; RR 1.43, 95% CI 1.08–1.89, p=0.01), and atrial fibrillation (RR 0.04, 95% CI 0.02–0.06, p=0.0002), as well as non-sustained ventricular tachycardia. There was no difference in the development of hypokalemia (RR 1.23, 95% CI 0.94–1.62, p=0.13) (Fig. 4).
DiscussionThere are few randomized clinical trials that report the outcomes of symptomatic improvement, and among them, the majority only report the NYHA functional class. However, it is important to note that they do not consider the limitations associated with fatigue, palpitations, dyspnea or angina, and therefore, there are no reports of the improvement of these individual symptoms in the trials. Thus, because patients with HF most often present a pivotal symptom, it is important to know if an intervention, in this case, levosimendan, will improve a specific symptom to a certain extent. In this meta-analysis evaluating the intermittent administration of levosimendan against placebo in chronic HF, we conclude that NYHA functional class ≥ 3 is similar in survivors of both groups.24 Contrary to previous findings, the present meta-analysis demonstrates a statistically significant improvement in the NYHA functional class, although only through a trend toward improvement in fatigue outcomes and the combined endpoint of clinical improvement in favor of levosimendan when compared to placebo or dobutamine. To this extent, a larger sample is required to reduce the confidence interval of the outcomes.
In a systematic review with a meta-analysis, Ribeiro, et al. concluded that there was no significant difference in mortality in patients with decompensated HF treated with a levosimendan infusion when compared to dobutamine or placebo.25 However, there are other meta-analyses that report a benefit in mortality in favor of levosimendan in critically ill patients, patients undergoing cardiac surgery with an ejection fraction <0.40 and in acute heart failure.26–29 The present meta-analysis did not show a significant difference in mortality when comparing levosimendan with placebo or dobutamine. However, an improvement in LVEF, B-type natriuretic peptide (BNP) levels and a decrease in new heart failure events and the use of salvage therapy in favor of levosimendan was shown, with a statistically significant difference.
Regarding the adverse effects analyzed, some reports of meta-analyses show no difference between levosimendan and dobutamine in hypotension, supraventricular arrhythmias, and ventricular arrhythmias.29,30
Although other systematic reviews have reported a trend toward hypotension against levosimendan and a reduction of postoperative atrial fibrillation in favor of levosimendan, in the present meta-analysis there were fewer headaches, cases of hypotension, non-sustained ventricular tachycardia, and atrial fibrillation in favor of placebo or dobutamine, with significant differences.27,28
ConclusionsThis systematic review and meta-analysis showed an improvement of the NYHA functional class and other combined points of clinical improvement, but not of the simple outcomes of dyspnea or fatigue in patients with decompensated heart failure when levosimendan was used compared to placebo or dobutamine. In addition, it demonstrated an improvement in other prognostic markers of HF, such as LVEF, serum BNP level and reduction in the use of rescue treatments during the exacerbation of HF. However, it is important to mention that the side effects associated with levosimendan may limit its use.
The main limitation of this review was the lack of standardization of the reporting of outcomes of clinical improvement, adverse effects, and follow-up times in the controlled trials used for the meta-analysis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo funding was required for the present study.
Conflict of interestThe authors declare no conflicts of interest.
Thanks to our colleagues, friends, family, Dr. Jorge Alberto Hernández Portales and Dr. Ramón Treviño Fruto, this project is possible due to your support.