Background
Langerhans cell Histiocytosis (LCH) is a rare disease characterized by the neoplastic proliferation of Langer-hans-type cells that express CD1a, langerin and S-100 protein with variable number of eosinophils, neutrophils and small lymphocytes.1 Gastrointestinal involvement in LCH is rare and usually associated with severe systemic disease. An optimal treatment regimen that could lead to a lasting remission or cure is not defined yet but etoposide and vinblastine are considered to be the most effective agents as monochemotherapy. Systemic treatments for refractory LCH have been unsatisfactory and usually toxic,2 however; Saven and Burian demonstrated that cladribine has major activity in these patients.3
On the other hand, imatinib mesylate (IM) is a competitive Bcr-Abl tyrosine kinase inhibitor showing activity against the platelet-derived growth factor receptor (PDGF-R), c-Kit, Abl-related gene (ARG) and their fusion proteins while sparing other kinases. In vitro studies have demonstrated that exposure of CD34+ cells to imatinib mesylate affects the differentiation and functional properties of generated dendritic cells (DC). Furthermore, IM produces concentration-dependent reduced expression levels of CD1a, CD83, HLA-DR and CD40. This finding might be of major clinical importance because IM is given continuously so far to prevent disease relapse in LCH.4 Recently, two patients suffering of both LCH and non-LCH improved with IM treatment.5, 6
Here we report the case of an adult patient with LCH who has improved considerably after treatment with cladribine followed by IM as a maintenance drug.
Case report
A 37-year-old Hispanic woman presented to our institution in 2003 with chronic diarrhea and weight loss. Her symptoms began 10 years ago with epigastric burning pain, accompanied by gastroesophageal reflux as well as nausea and vomiting. A year later she continued with greenish stools decreased in consistency, with mucus, steatorrhea and lientheria related to food intake and accompanied by abdominal cramps, fever and weight loss. Her symptoms went into remissions and exacerbations during the follow-up. Later on, hyperpigmented maculae of 1 mm to 2 mm in diameter appeared in the back and abdomen, these dots were associated with exacerbation of the diarrhea. Whipple's disease was initially diagnosed at another hospital but treatment was unsuccessful; therefore, she came to our hospital in 2003, due to persistent symptoms and severe malnourishment. Neither adenopathy, liver or splenic enlargement were detected on physical examination. Endoscopic studies of both the upper and lower gastrointestinal tract revealed the presence of friable mucosa, a diffuse pattern of paved area, the presence of huge number of polyps and pseudopolyps ranging from stomach to the 2nd portion of the duodenum, terminal ileum, colon and rectum. Biopsies were taken and revealed and histiocytic infiltration (intermediatesized cells with irregular nuclei, vesicular chromatin, and indistinct nucleoili, with mitotic activity variable) of stomach, duodenum, ileum and colon which stained positive for CD68, CD1a and S-100 and negative for Whipple's disease. Bone marrow biopsy showed infiltration by CD1a and S-100 positive cells too. These findings were consistent with LCH. The skin biopsy was LCH negative.
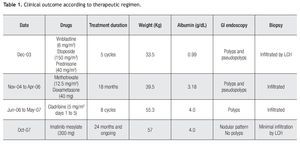
We diagnosed high-risk systemic LCH and chemotherapy was initiated in December, 2003 on a weekly basis with vinblastine (6 mg/m2), etoposide (150 mg/m2) and daily prednisone (40 mg/m2) for five cycles without any improvement. Therefore, in November, 2004 she was put on weekly methotrexate (12.5 mg/m2) and dexamethasone (40 mg), plus parenteral nutrition. The disease was subsequently stable for 18 months and her clinical conditions partially improved. Nevertheless, during the treatment tapering, symptoms reappeared while the upper and lower gastrointestinal tract as well as the bone marrow persisted infiltrated. In June, 2006 she received cladribine (5 mg/m2 days one to five) for eight cycles and there was an overall good clinical response (diarrhea disappeared), the endoscopy showed a nodular pattern in gastric mucosa with three polyps at the 2nd portion of duodenum and a large number or polyps in colon while the ileum and rectum showed a normal appearance; nevertheless the infiltration in both, the gastrointestinal tract and bone marrow persisted.
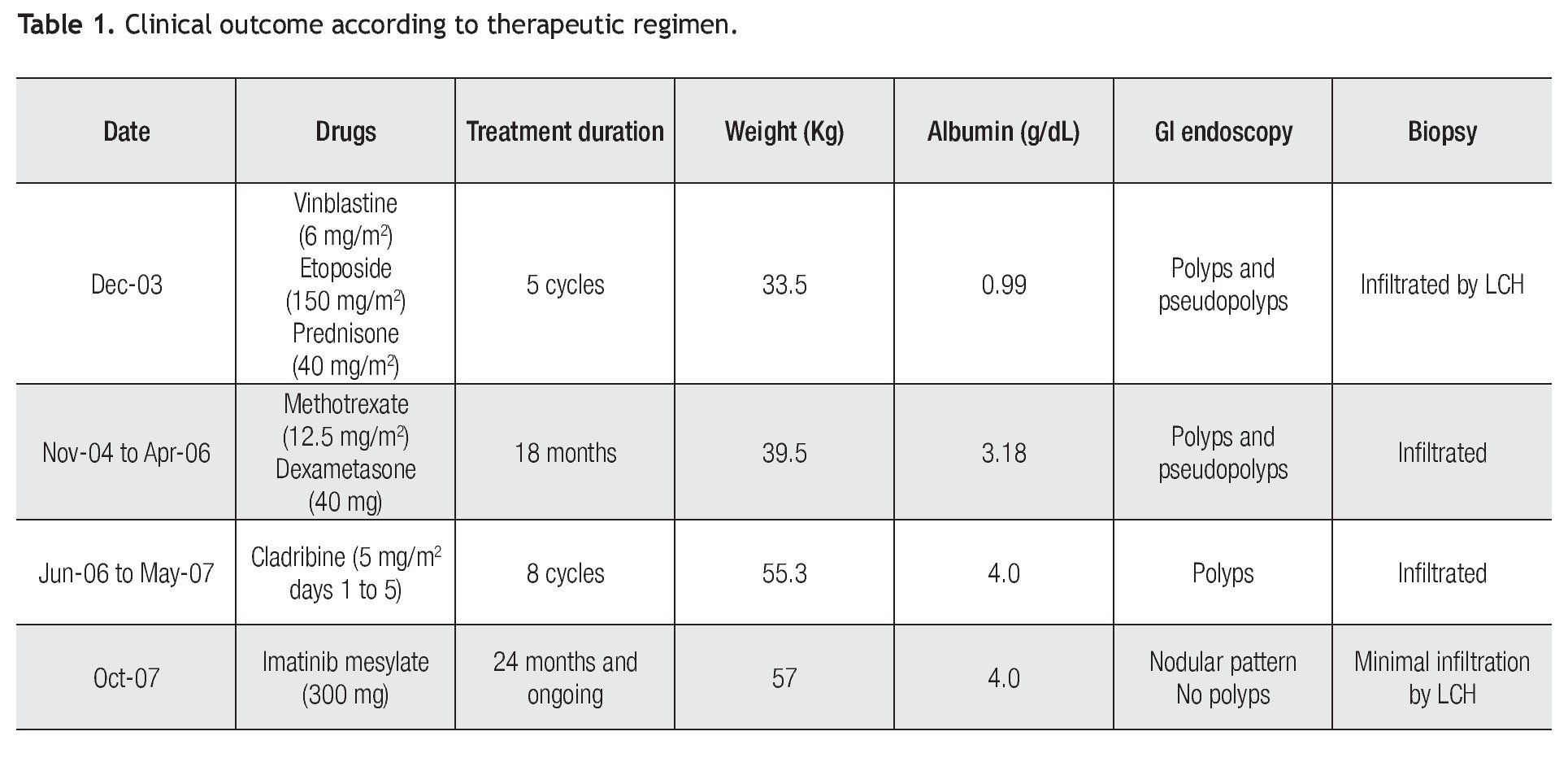
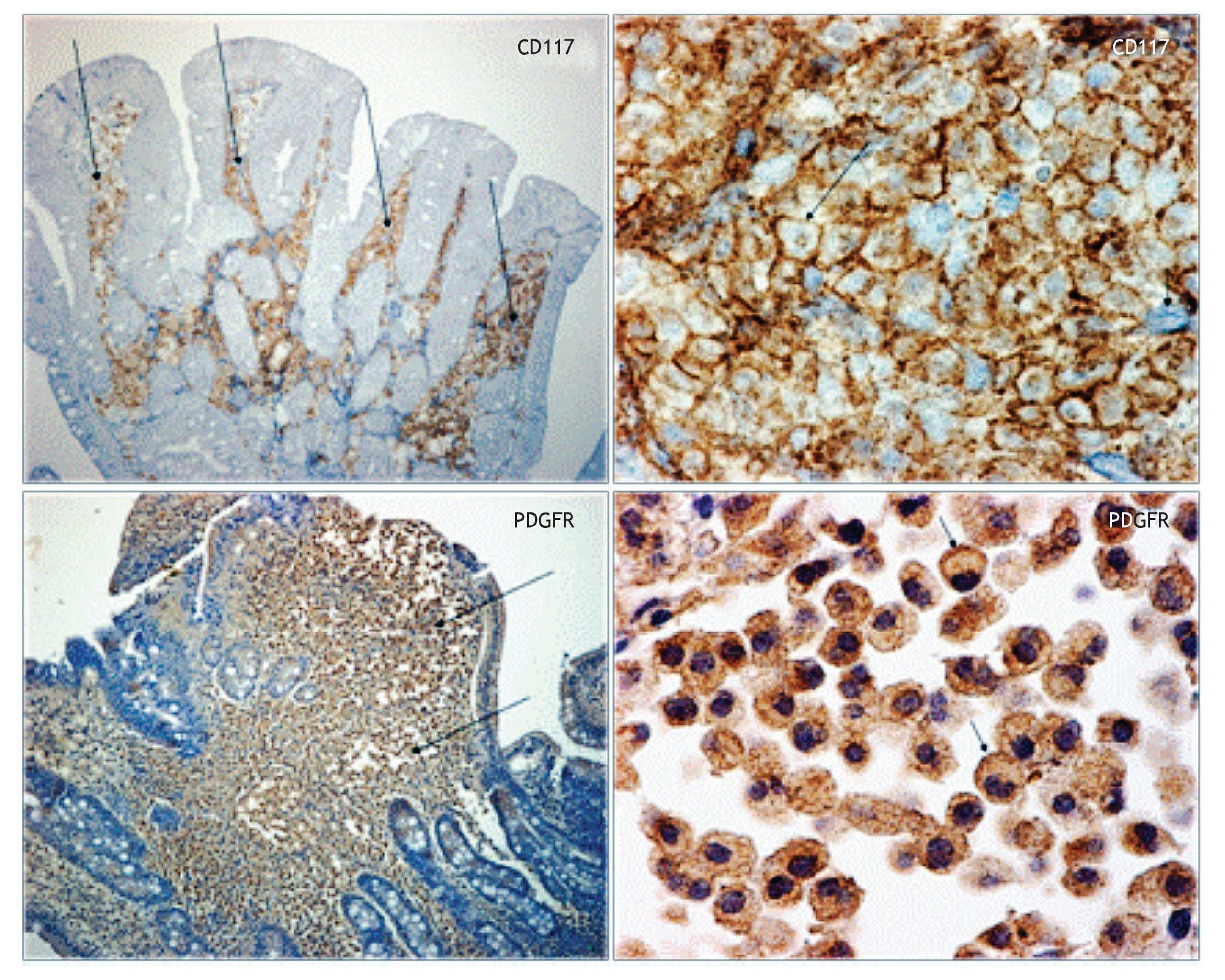
In October, 2007 we performed immunohistochemistry staining in gastrointestinal mucosal biopsy revealing CD117 positive in a pattern of membrane, (Figure 1) and PDGF-R positive with reinforcement in the cytoplasm membrane, so we decided to start Imatinib mesylate as maintenance therapy, initially at 400 mg P.O. daily but she developed a rash in upper extremities and diarrhea as adverse effects to IM, therefore, we reduced the IM dose to 300 mg/day. Currently, 24 months after initiating the IM maintenance therapy, she is in excellent clinical conditions, without complaints. Moreover, the most recent endoscopic studies showed marked improvement of the GI lesions and was corroborated by the histopathologic reports, as shown in Table 1. However, infiltrate persists in the bone marrow.
Figure 1. Immunohistochemical stain. Duodenal biopsy shows Langerhans cells strongly reacting to CD117 and to PDGFR.
Discussion
Neoplasms derived from Langerhans cells are clonal proliferations, divided into two subgroups: Langerhans cell histiocytosis and Langerhans cell sarcoma, according to the degree of atypia and clinical aggressiveness. Some reactive cases, not clonal, have been observed in the lungs of smokers. LCH can affect a single site or multiple sites in a single system or multisystem; it is characterized by cells measuring 10 μm to 15 μm, recognized by their grooved, folded, indented or lobulated nucleus with fine chromatin, inconspicuous nucleoli and thin nuclear membranes, with mitotic activity variable. The cytoplasm is moderately abundance and slightly eosinophilic. The ultrastructural hallmark is the cytoplasmic Birbeck granules, granules with a tennis racquet shape, and is 200 nm to 400 nm long and 33 nm wide, with a zipper-like appearance. This cell expresses CD1a, langerin and s-100 protein by immunohistochemistry stain.1
Gastrointestinal involvement in LCH is extremely rare and just few cases have been reported. Symptoms may include hematochezia, constipation or diarrhea and can range in severity from mild to life-threatening, whereas the diagnosis is made by endoscopic biopsy demonstrating the histological evidence of LCH. Even in patients without upper gastrointestinal tract symptoms, involvement by LCH is likely and should be treated as a multisystemic disease irrespective of the patient's clinical status. Some researchers suggest that any gastrointestinal involvement in LCH warrants aggressive evaluation and treatment as the prognosis is relatively poor.2
Two recently informed cases suggest that treatment with IM could improve the course of patients with LCH and may be considered as an alternative treatment option for refractory LCH.5,6 Our current case illustrates that multisystemic LCH can be successfully treated with, at least two more drug choices: Cladribine and, in those patients with expression of KIT and/or PDGFR, IM.
However, we have to recognize that other researchers have found inconsistent results in other forms of histiocytosis expressing PDGFR-β;7 therefore, large and multicenter studies should be considered to answer the question about the role of targeted therapy in this rare disease.
Corresponding author: Sergio Arturo Sánchez Guerrero. Puente de Piedra 150. Suite 4. Colonia Toriello Guerra. México 14050 D.F. México.
E mail:sasanche@prodigy.net.mx
Received: February 2010.
Accepted: April 2010