Objective: To evaluate the effect of liraglutide on body weight and metabolic parameters associated with cardiovascular risk in patient with or without type 2 diabetes mellitus (DM).
Methods: A descriptive, observational and analytical study was performed. A review was conducted on clinical records from patients seen in a cardiology private practice, who received liraglutide as a weight -reducing supplement, combined with prescription of several life style modifications as a 2000 kcal diet in men, or 1800 kcal diet in women and moderate daily aerobic exercise (at least 30 minutes, 5 times a week) during 3 months. Data from both, diabetic and non diabetic obese patients with body mass index (BMi) >30 kg/m2 and at least two failed weight lost attempts in previous twelve months were included. Thirty eight cases meeting these criteria were selected.
Results: An average of 4.8 kg of weight lost at the end of three months were observed (p<0.001). Further benefits in several cardiovascular and/or metabolic risk factors such as: increased c-HDL, decreased serum triglycerides and lowering of systolic blood pressure. Liraglutide showed an adequate safety profile, with only minor adverse reactions (nausea, dizziness, vomiting, diarrhea or constipation), which disappeared within the first two weeks of treatment.
Conclusion: The effectiveness and security of liraglutide as an supplement for weight loss were demonstrated. Liraglutide also showed beneficial effects improving metabolic risk factor that leads to cardiovascular disease
Introduction
Overweight and obesity are 2 major health problems in our country, because of their prevalence, as well as the risk they generate in the development of metabolic and cardiovascular diseases (these constitute the leading cause of death in our country). According to the 2012 National Health and Nutrition Survey, 26 million adult Mexicans were over-weight and 22 million were obese.1 Because of the multiple variables that play a role in the genesis of obesity, this condition creates a challenge for health professionals that can be difficult to overcome. Different drugs have been used as supplements in the handling of this disease; however, their use has been limited, and in some cases discontinued due to the adverse reactions generated, which in some cases has been fatal.2
Glucagon-like peptide-1 (GLP-1) analogues are drugs developed for type 2 diabetes mellitus (DM) management.3,4 Liraglutide is the second medication approved by the Food and Drug Administration (FDA).5 One of its advantages is leading to less hypoglycemia events compared with other oral antidiabetics.6-8 in addition to lower glucose levels, we are able to observe a decrease in body weight9 and an improvement in insulin secretion in patients who receive this medication.10 Other studies have reported the decrease in cardiovascular risk factors.11 The mechanism of action of GLP-1's incretin is well defined. It is produced in ileocolic C cells stimulating pancreatic secretion of insulin by interaction with G protein.12,13 At a gastrointestinal level, liraglutide decreases acid gastric secretion and delayed gastric emptying by antral stimulation. Moreover, it inhibits propulsion of pylorus, and decreases motility in the digestive tract after food intake. It also induces early satiety through a mechanism in central nervous system wich is not yet specified, which is thought to be AMPc stimulation.12,13 Furthermore, the possibility of the activation of neuronal receptors at the satiety center in the hypotalamus at the arcuate nucleus, has been suggested, as well as the inhibition of the solitary tract of the brain stem. Altogether, the diverse mechanisms mentioned favor a reduction in the caloric intake, which could result in weight loss.12,13
The objective of this study is to evaluate the effect of liraglutide on body weight and metabolic parameters associated with cardiovascular risk, in patients with or without type 2 DM.
Materials and methods
We conducted a descriptive, observational, analytical study, in which clinical files of patients cared for from July 2011 to June 2012, in private cardiology practices in Monterrey, N. L., Mexico were reviewed. We selected those who were being prescribed liraglutide (from 0.6 to 1.8 mg every 24 hours) in addition to follow a diet of 2000 kcal for men and 1800 kcal for women, as well as the indication to perform moderate exercise for 30 minutes at least 5 times a week. We selected those who met the following criteria: having received liraglutide treatment for at least a period of 3 months, having registered the following variables at the beginning of the study and following 3 months of treatment: Body weight, body mass index (BMi, calculated using the following formula: weight in kilograms/square of body length in meters), blood pressure measured with an aneroid monitor, plasma glucose after fasting, lipid profile, glycosylated hemoglobin and hepatic function tests measured with basic and routine laboratory techniques, body fat percentage obtained through impedanciometry using a Tanita® innerscan, mention of presence or absence of cardiovascular or digestive symptoms, and medication dosage prescribed.
Administered liraglutide dosage was 0.6 mg a day during the first week, which was gradually increased to 1.2 mg and up to 1.8 mg a day according to tolerance and requirement. In addition, we prescribed metformin to 14 of 15 diabetic patients and 13 of the 23 non-diabetic patients.
For statistical analysis we utilized measures of central tendency and dispersion. In order to compare quantitative variables we used the Student's T-test for both groups and analysis of variance when we analyzed more than 2. We used the statistical software SPSS® Statistics version 19.0. We consider a p < 0.05 as significant.
Results
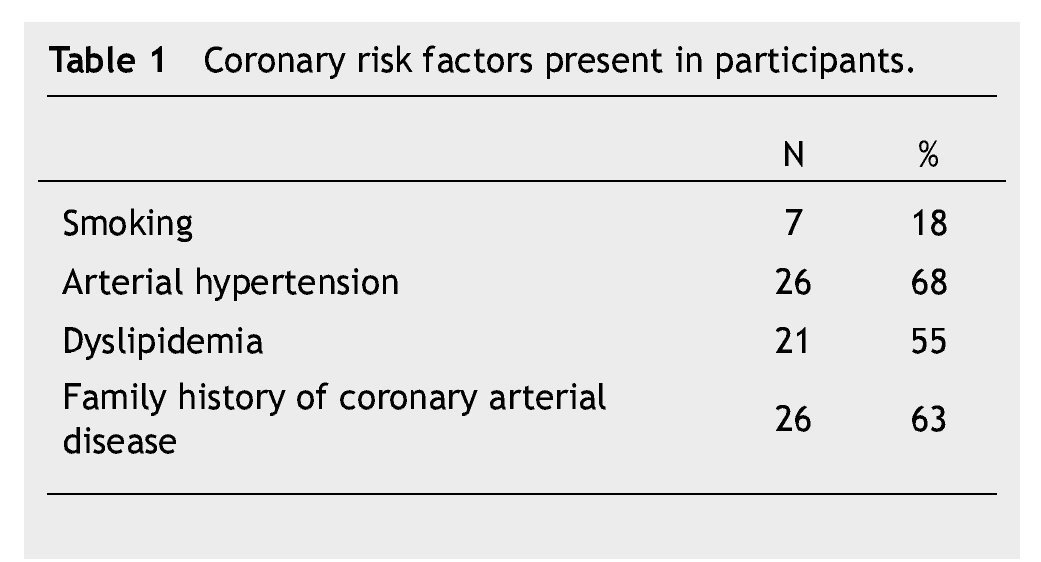
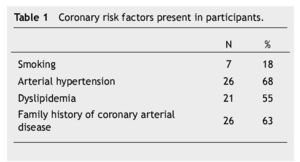
Thirty eigth cases met the inclusion criteria. There were 23 (60%) males and 15 (40%) females, with an average age of 56 ± 10 years. Fifteen patients (40%) were under type 2 DM treatment. Coronary risk factor detected are listed in Table 1.
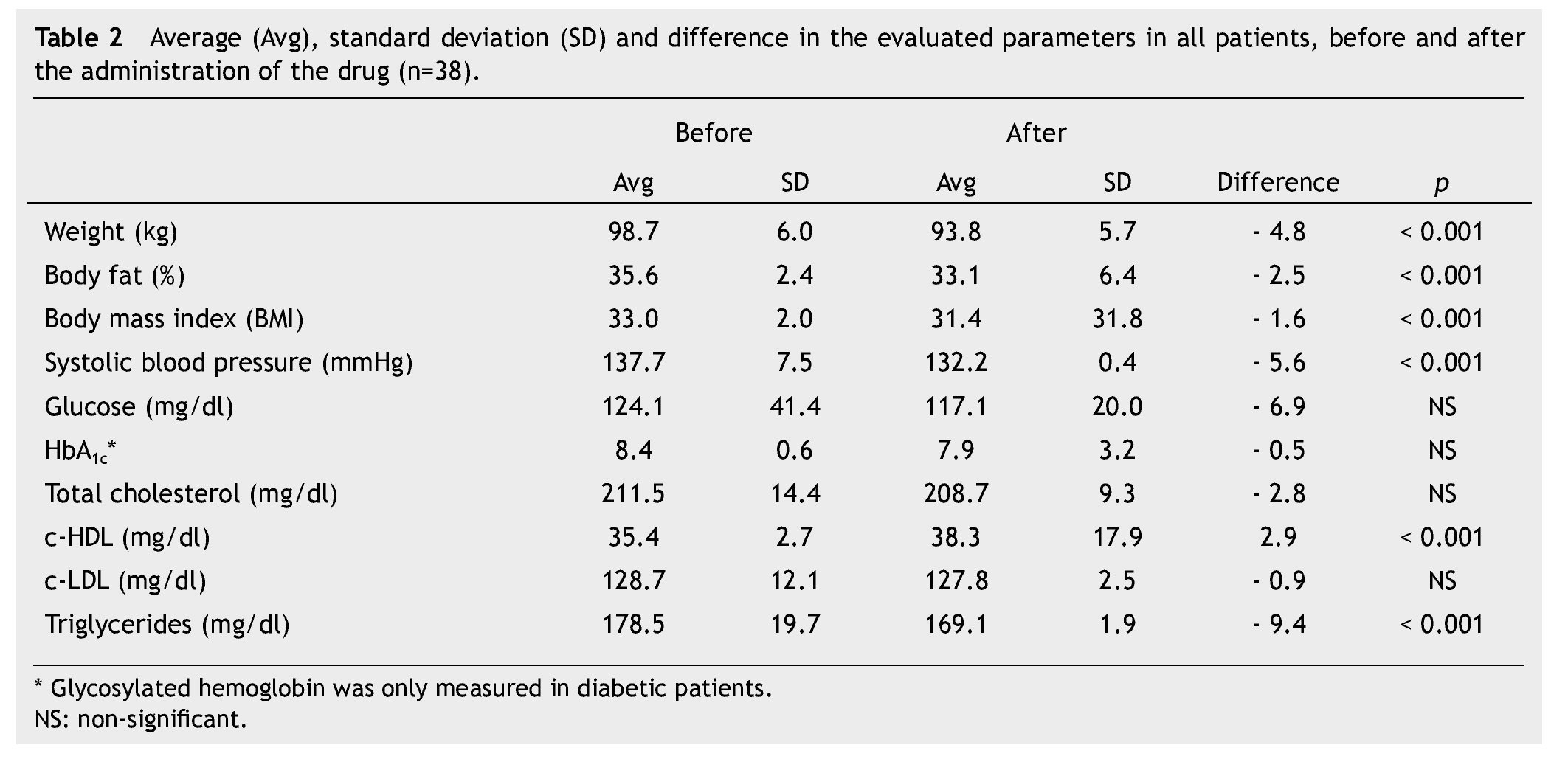
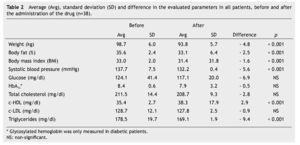
Average weight loss at the end of 3 months of treatment was 4.8 kg in 38 patients (p < 0.001), which was significant. These results, as well as the rest of the evaluated parameters are shown in Table 2.
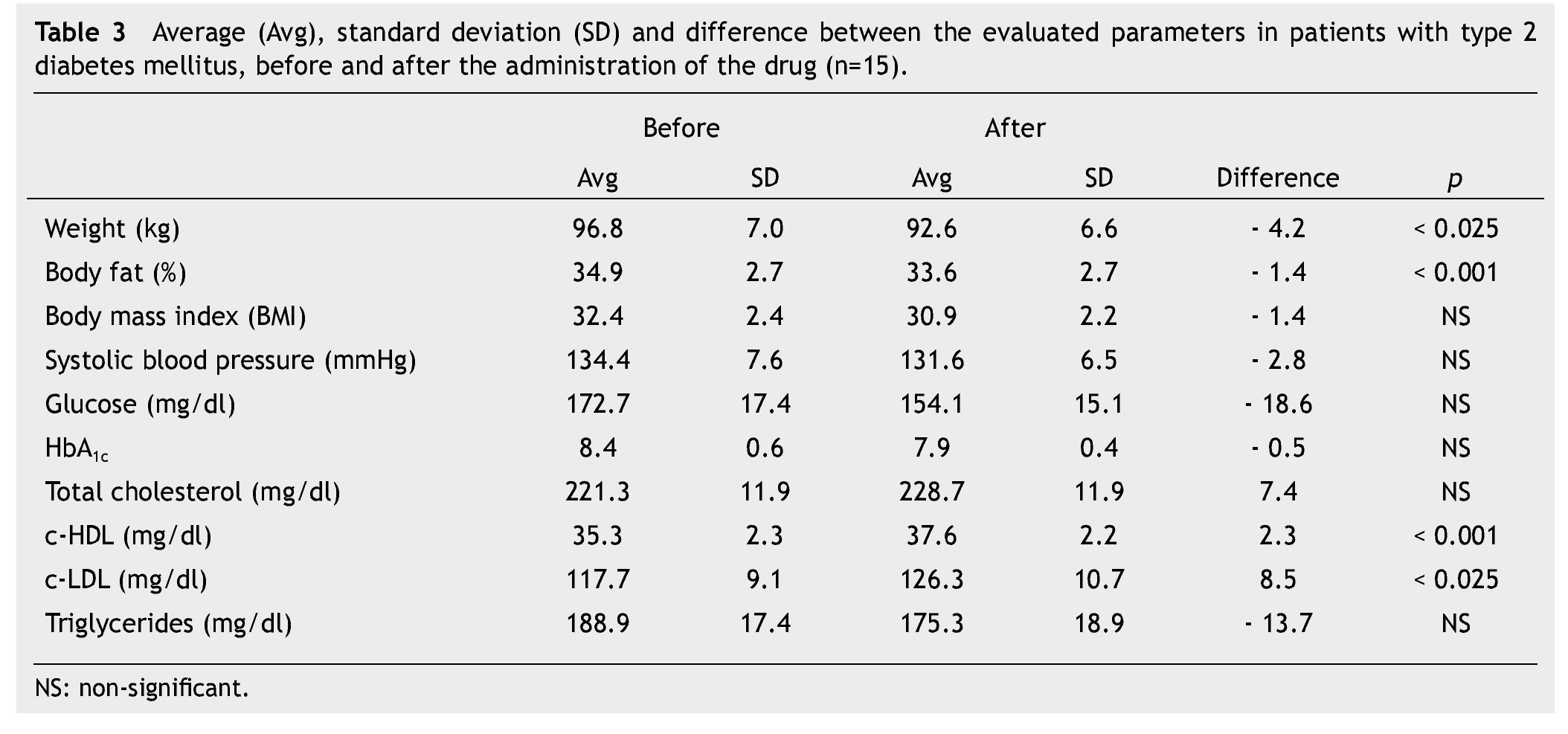
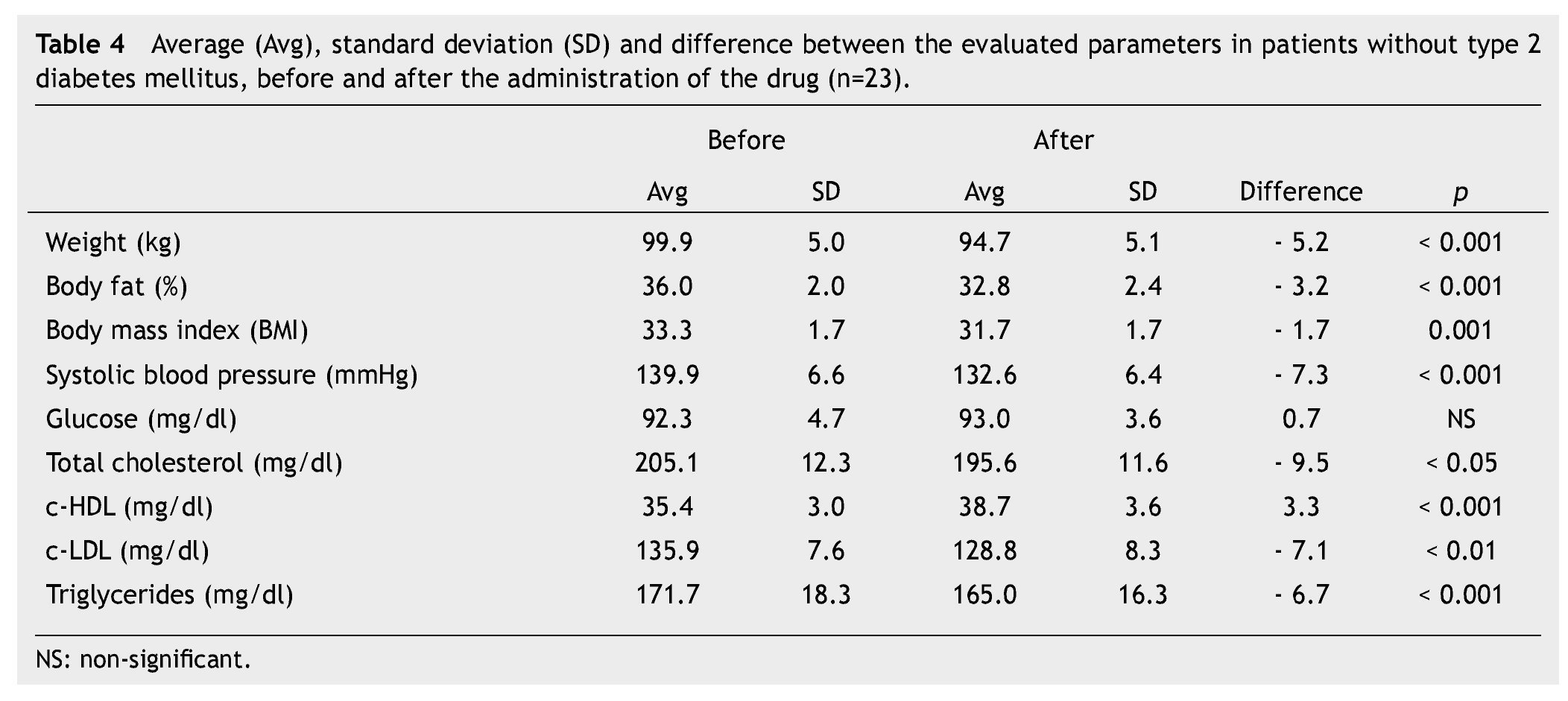
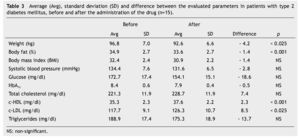
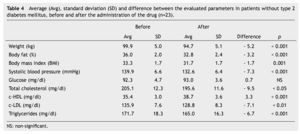
We observed a significant average weigth loss of 4.2 Kg in diabetic patients and 5.2 in non-diabetic; futhermore, an improvement in several evaluated metabolic parameters (Tables 3 and 4). The analysis of variance displayed a non-significant difference in the behavior between subgroups in weight reduction, BMi, body far percentage, serum triglycerides, total cholesterol and c-LDL; differences found in systolic arterial pressure and c-LDL were significant.
Six patients (16%) reported an adverse event of moderate intensity and relative frequency during the first 2 weeks of treatment, particularly nausea, dizziness, vomiting, diarrhea and constipation; headaches and cramps occurred less frequently. There were no reports of hypoglycemia episodes or hepatic enzyme alterations (TGO, TGP). Patients with heart diseases did not display data of cardiac decompensation.
Discussion
Different studies and trials in which liraglutide has been administered, have proven the usefulness of this medication in weight loss in obese patients with type 2 DM, as well as in patients without this condition. Weight losses between 7 to 9 kg in 20 weeks have been reported, obtaining best results when using 3 mg of liraglutide a day.14-18 it has been proven superior when compared to placebo (2.8 kg) and orlistat (4.4 kg).11
In this study, the obtained weight loss was slightly lower than that reported, which can be attributed to a smaller dose and a shorter duration of treatment.
An important parameter to evaluate weight loss is body fat percentage, because body fat at the abdominal level constitutes a risk factor for the development of metabolic syndrome. A publication reports a 1.2-1.9% decrease in body fat.18 in the present study we observed that at the end of 3 months of treatment, body fat had lowered 2.5% on average, which was slightly greater than that reported.
An improvement of different metabolic parameters when using liraglutide by itself, or in combination with other glucose-lowering drugs has been reported. Previous studies showed reductions of up to 1.7% on glycosylated hemoglobin, a decrease in triglycerides of 22 mg/dl, and in fasting plasma glucose of up to 43.2 mg/dl.11,14,15
in this study, we are able to see that glycosylated hemoglobin and triglycerides values decreased even if it was to a smaller degree than that reported.
A study had shown the reduction of systolic blood pressure of 6.1 mmHg on average.11
In our study the reductions in blood pressure were slightly superior to those reported in the literature.
Diverse side effects have been reported. Over 10% of the patients have presented gastrointestinal discomfort (nausea, vomiting and diarrhea); consequently, dose should be titrated in order to avoid the main adverse gastrointestinal effects.19 From 1% to 10% of patients developed upper tract respiratory infections, headaches and high levels of anti-liraglutide antibodies.19,20 it has not been possible to demonstrate the relationship between liraglutide and pancreatitis, thyroid C-cell hyperplasia, and thyroid papillary carcinoma.21,22
In our study, 16% of the patients showed minor adverse gastrointestinal reactions (i.e. nausea and diarrhea), which disappeared within the first two weeks of treatment. There were no hypoglycemia or pancreatitis cases, which could be related to the selection of patients or the sample size.
The study has several limitations: is a retrospective, descriptive, and non-randomized design, with a small sample size, as well as a short time frame.
Conclusions
The effectiveness of liraglutide as supplement for weight loss was demonstrated in patients with a BMi greater than 30 kg/m2, with or without type 2 DM, with at least 2 failed attempts at losing weight in the last 12 months, successfully losing an average weight of 4.8 kg in 3 months of treatment. In the case of type 2 DM patients, we obtained an improvement in glycosylated hemoglobin, fasting glucose, blood pressure and triglycerides, which theoretically shows a beneficial effect improving metabolic risk factors that lead to cardiovascular disease. The drug's safety profile was adequate, with minimal adverse effects.
It is important to note that the present study was not a controlled clinical study. Results are based on real life private office patients.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Received: November 2012;
Accepted: April 2014
* Corresponding author:
Cardiolink Educación.
1813 Hidalgo W. St. Obispado, Monterrey, N. L., Mexico.
Telephone: +52 (81) 4040 1554.
E-mail address: eliasgarcia.doc@gmail.com (E. A. García-Cantú).